Abstract
Background
Somatic symptoms often co-occur with psychological symptoms but this overlap is poorly understood. Some aspects of this overlap differ in the South Asian context, but it is not clear whether this is a reporting effect or an underlying difference in experienced illness.
Methods
Home interviews were administered to 4,024 twins randomly selected from a population-based twin register in the Colombo district of Sri Lanka (the CoTASS study). These included assessments of psychological, somatic and fatigue symptoms. The data were analysed using factor analytic and quantitative genetic approaches.
Results
Confirmatory factor analysis showed that the symptoms from the three scales represented three separate dimensions, rather than all tapping into a single dimension. However, familial correlations among the data were most consistent with a common pathway model. This implies that a portion of the underlying vulnerability is common across psychological, fatigue and somatic symptoms. There were sex differences in the aetiology of this model, with shared environmental and genetic influences playing different roles in men and women.
Conclusions
There is a complex aetiological relationship between psychological, fatigue and somatic symptoms. This is similar in Sri Lanka to Western countries, but there may be a greater influence from the family environment, suggesting that care needs to be taken when generalising research findings between countries. People who complain of certain fatigue or somatic symptoms may well also have psychologicial symptoms, or may have genetic or environmental vulnerabilities to such problems.
Keywords: genetic, twin, Sri Lanka, somatic, fatigue
Psychiatric disorders are associated with both somatic symptoms and functional somatic syndromes (Simon and von Korff, 1991; Henningsen et al. 2003). This overlap is poorly understood, but it is almost certainly bidirectional (Hotopf et al. 1998). One way to disentangle the aetiology of the overlap is by using genetically sensitive study designs which may indicate whether the associations can be explained by shared genes or environments. Previous family and twin studies have assessed normal variation in symptom counts to examine the overlap between fatigue and mental health (Williamson et al. 2005), and somatic distress and mental health (Gillespie et al. 2000). These studies found considerable differentiation between the risk factors for the different sorts of symptoms, yet with a strong overlap in their presentation, and a strong familial/genetic contribution to this overlap. The existence of this overlap is consistent with co-occurrence within a broader ‘internalising’ spectrum of symptoms, with a strong genetic contribution to the co-occurrence (Kendler et al. 2003). Within-person co-occurrence suggests that somatic distress also fits into this spectrum (Krueger et al. 2003).
Research into the relationship between somatic and psychological symptoms has been focused on populations in high income countries, but it is important to widen this focus, firstly because this relationship is well recognised in developing countries (Sumathipala, 1990; Sumathipala et al. 2008a; Sumathipala et al. 2008b). Secondly, cultural differences have been reported, including more somatic presentations in South Asia; but when both somatic and psychological symptoms are directly probed, the excess of somatic symptoms disappears (Simon et al. 1996; Minhas & Nizami, 2006; Simon et al. 1999). Thus some of the excess somatic presentations may be the result of greater acceptability of reporting of somatic symptoms, or viewing them as a ‘ticket to admission’ to primary care (Simon et al. 1999), rather than representing differences in the underlying disorders. Thirdly, if much of the high burden of somatic symptoms in developing countries is related to functional somatic or psychiatric problems, then health policies which take somatic symptoms at face value risk over-medicalising distress (Patel et al. 2005). Finally, investigating the aetiology of this relationship in different geographical and cultural contexts may improve understanding of the processes at work both locally but also worldwide. For example, socioeconomic, cultural, and psychosocial contexts, as well as differences in climate and ecology, may all influence the magnitude of environmental contributions identified in a twin design.
Past research using the current large population-based twin study has suggested that environmental influences on depressive disorders may be particularly strong for men in Sri Lanka (Ball et al. 2009; Ball et al. 2010a). However, the aetiology of abnormal fatigue appeared similar to previous reports from Western countries (Ball et al. 2010b). The present study takes a dimensional approach to the co-occurrence of psychological, fatigue and somatic symptoms over a one-month period. The main aim is to compare findings with higher-income, Western countries, and with results from studies in which diagnostic (categorical) rather than dimensional approaches have been used. The main research question is: to what extent do fatigue and somatic symptoms exist independently of one another and of psychological distress, in terms of presentation and underlying aetiology?
Methods
The study received approvals from the Institute of Psychiatry, King’s College London Research Ethics Committee; the Ethical Review Committee, University of Sri Jayewardanepura; and the World Health Organisation’s Research Ethics Committee.
Study design and participants
This was a population based twin study, the twin component of the Colombo Twin And Singleton Study (CoTASS). Full details of the design and implementation of the study are described elsewhere (Siribaddana et al. 2008). Briefly, the study took place in the Colombo District of Sri Lanka, an area with population of 2.2M which includes the island’s capital, and varies from urban to semi-urban areas. We added a question to the update of the annual census, asking whether the householder knew of any twins, and identified 19,302 individual twins by this method. Of these, we randomly selected 4,387 individual twins who were at least 15 years old, and spoke sufficient Sinhala to understand the interview, to take part in the project on common mental disorders. Four thousand and twenty four (91.7%) participated, including 1,954 complete twin pairs. Some preliminary analysis also used data from 2,018 singletons, who were matched to the same local area from which the twins came (Siribaddana et al. 2008) (singleton participation rate = 87.4%). Specially trained research workers visited the subjects’ homes to interview them each separately. Interviews and questionnaires were translated in a process that aimed to retain the concepts of interest rather than a literal translation. Each component was translated at least twice independently, reviewed by a group of relevant professionals, and then by a scholar in Sinhala. Then they were trialled on lay volunteers to ensure meaning was understood (Siribaddana et al. 2008).
Interviews took place between 2006 and 2007, when Sri Lanka had been experiencing violent civil war for over 20 years. However, much of the conflict has centred in areas to the North and East of the island, far from the location of the current study. Nonetheless, a small minority (2.6%) of the participants reported directly participating in the conflict as combatants.
Measures
All participants were assessed on the Chalder Fatigue Questionnaire (Chalder et al. 1993) (CFQ). This includes 11 items assessing fatigue, and 2 assessing muscle pain, experienced over the past month (each coded 0-3).
The Bradford Somatic Inventory (Mumford et al. 1991a) (BSI) was developed using UK and Pakistani populations, by noting physical symptoms in the psychiatric case notes of patients with a clinical diagnosis of anxiety, depression, hysteria or hypochondriasis. From these, 21 items were selected that differentiated psychiatric from ‘organic’ patients (Mumford et al. 1991b); these include bodily aches and pains, dryness of throat, heart palpitations etc. These were each coded 0-2 based on experiences over the past month. Two of the items relate to tiredness and may thus represent item overlap with Chalder Fatigue Questionnaire; thus all analyses below were run using the 19 non-fatigue items from the BSI-21.
The Short Form 36 Health Survey questionnaire (SF36) (Ware, Jr. and Sherbourne, 1992) was also administered. It contains a set of 5 items that assess psychological aspects of mental health (such as: “Have you been a very nervous person?” and “Have you felt downhearted and blue?”). These are assessed over the past month, and were each coded 0-5.
For factor analysis, the items in each dimension were coded as described in the above paragraphs, and summed into three composite scales: psychological symptoms (from the 5 SF36 items), fatigue (from the 13 CFQ items) and somatic symptoms (from the 19 BSI items). For correlations and genetic analyses, the composites were standardised within sex (to remove sex effects on the variance), mean effects of sex and age was regressed out, and log transformation was used to correct for skew.
Zygosity was assessed using a validated questionnaire (Ooki et al. 1990; Sumathipala et al. 2000) administered to both twins.
Analyses
Descriptive analyses were performed in Stata v10.1.
Phenotypic similarity
All 37 items were used as ordinal factor indicators in a confirmatory factor analysis in Mplus, using one twin randomly selected from each twin pair. The WLSMV estimator was used (weighted least-squares with mean and variance adjustment). The 37 items from all three dimensions were entered together in order to examine whether a single factor best explained the correlations between items (unidimensionality) or whether they did represent three separate factors (one for each of the scales). The three factor structure was tested in the confirmatory factor analysis for two reasons. Firstly because an exploratory factor analysis on the area-matched but independent sample of non-twins (i.e., singletons, N=2,018) from the CoTASS sample had supported the existence of either three or four factors. The fourth factor represented just two items on the Chalder Fatigue Scale (relating to slips of the tongue and finding the correct word), which is too few to reliably index an additional factor. Secondly, the three questionnaires were constructed to probe different characteristics, and retaining them as closely as possible to the originals will enhance comparability with other research.
Genetic analyses
The variances of the scales were decomposed into genetic and environmental components, by means of structural equation modelling, treating the data as continuous. Raw data analysis was used in order to include twins with missing data. These models work on the basis that monozygotic (MZ) twins share all of their additive genetic influences (A) whereas dizygotic (DZ) twins on average share only half, relative to the whole population. The environmental influences are split into those shared by members of a twin pair (C) and unique to each twin in a pair (E). Multivariate models additionally estimate the A, C and E contributions to the covariance, e.g., to what extent the phenotypic overlap between psychological distress, fatigue and somatic symptoms is due to genes that influence all three. These models are based on comparisons across zygosity: where a within-trait cross-twin correlation is greater for MZ than DZ twins, we infer that genetic factors influence some of the variance in that trait; where the cross-twin cross-trait correlation (e.g. psychological distress in twin 1 correlated with somatic symptoms in twin 2) is greater in MZs than DZs, this indicates a genetic influence on the covariance between traits.
First a phenotypic saturated model was run, which simply estimates the phenotypic correlations without imposing a model structure. This was followed by three classical multivariate genetic models: the Cholesky decomposition, common pathway and independent pathway models (Neale & Maes, 2003). In the Cholesky model, there are three genetic factors, one of which accounts for all the genetic variance on the first variable, but is also allowed to influence the other two variables; the second genetic factor accounts for the remainder of the genetic influence on the second variable, and is also allowed to load onto the final variable; the final genetic factor accounts for the remainder of genetic influence on the final variable. There are corresponding factors for C and E influences. When including data from opposite-sex twin pairs, certain parameters must be constrained such that the correlation structure between latent factors is the same for men and women (although the loadings of the latent factors onto measured variables are allowed to differ), and so that the order of the variables does not influence the fit of the model (Neale et al. 2006).
The common pathway model assumes one common latent factor that loads on to each of the three observed variables. There are A, C and E paths to the common factor, and specific (residual) A, C and E paths to each of the three observed variables. The common latent variable is constrained to have a variance of 1. The independent pathway model has a common A factor, a common C factor and a common E factor, which can independently influence all three measured variables; there are also specific (residual) A, C and E factors for each measured variable. A key difference is that the independent pathway model allows different aetiological components of common vulnerability to be unrelated to one another, whereas the convergence of the common aetiological factors through the common pathway model suggests a pre-symptomatic state of vulnerability to all three measured variables. The common pathway model might thus provide support for seeing the three measured variables as indices of ‘common distress disorders’ that result from vulnerability to a higher-order general internalising factor (Henningsen et al. 2003).
The three genetic models are each nested within the saturated model, so a χ2 difference test can be used to assess the fit of these three models to the saturated model. The common pathway and independent pathway models are not nested within the Cholesky model, due to the constraints placed on the Cholesky model to make it appropriate for use with opposite-sex pairs. Therefore these models were compared using the Akaike Information Criterion (AIC).
In this paper we refer to ‘shared’ environments as the environmental influences shared within a pair of twins, and which contribute to phenotypic similarity (i.e., C); ‘common’ influences refer to any influences (A, C or E) that operate through the common factor (common to the three measured traits: psychological distress, fatigue and somatic symptoms).
Results
Complete data were available for 3,747 twin individuals (including 1,805 pairs of twins). The mean age was 33.9 years (range 15-85, standard deviation 13.4 years), further demographic information has been published elsewhere (Siribaddana et al. 2008).
Descriptive statistics
The mean summed score for psychological symptoms was 5.9 (S.E. 0.1) for men and 6.7 (0.1) for women (sex difference: t=4.60, P<0.001). This is equivalent to 76.4 (S.E. 0.4) in men, 73.3 (S.E. 0.4) in women, when using the same scoring method as a UK-based population sample, giving very similar results to this sample (Jenkinson et al. 1993) (among whom, men scored 74.8-78.0 and women scored 70.2 – 74.4). Note that a higher score using this scoring method indicates fewer psychological symptoms. The somatic symptoms indicated that 5% of respondents scored above the optimal cutoff for use as a screening tool to identify current psychiatric disorder (i.e., a score of 13+ using all 21 BSI items) (Mumford et al. 1991b); the mean score for men was 3.1 (S.E. 0.1) and for women it was 4.2 (S.E. 0.1) (sex difference: t=6.42, p<0.001). The 11 items in the Fatigue Questionnaire gave a total score of 12.3 (S.E. 0.1) for men and 12.8 (S.E. 0.1) for women (sex difference: t=5.00, P<0.001), which is very similar to the levels found in population based samples in Norway and the UK (Loge et al. 1998; Pawlikowska et al. 1994) (among whom, men scored 11.9, and women 12.6).
Correlations
The phenotypic (within-person) correlations between composite scores were, for men and women respectively, psychological distress-fatigue: 0.32 and 0.30, psychological distress-somatic: 0.28 and 0.28, fatigue-somatic: 0.51 and 0.46 (Table 1). The cross-twin correlations were higher for MZ than DZ pairs, both within-trait and cross-trait (Table 1), suggesting familial resemblance due to genetic factors, but the confidence intervals are wide. The highest MZ within-trait correlation was only 0.47, indicating a large degree of influence from nonshared environmental factors. The DZ opposite-sex (DZOS) twin pairs’ correlations were not significantly different from the DZ same sex (DZSS) twin pairs’, suggesting a lack of qualitative sex differences.
Table 1.
Correlations (95% CIs) between composite scores (within men and women, and between MZM, MZF, DZM, DZF and DZOS pairs)
| Composite scale | Cross – twin |
Within person |
|||||
|---|---|---|---|---|---|---|---|
| MZM | DZM | MZF | DZF | DZOS | Men | Women | |
| Within-trait | |||||||
| Psychological | 0.31 (0.21-0.39) | 0.24 (0.11-0.36) | 0.29 (0.21-0.37) | 0.15 (0.03-0.25) | 0.16 (0.08-0.24) | - | - |
| Fatigue | 0.31 (0.21-0.40) | 0.20 (0.08-0.32) | 0.28 (0.19-0.36) | 0.20 (0.09-0.31) | 0.12 (0.04-0.20) | - | - |
| Somatic | 0.32 (0.23-0.41) | 0.21 (0.09-0.33) | 0.47 (0.40-0.54) | 0.33 (0.24-0.42) | 0.18 (0.10-0.26) | - | - |
| Cross-trait | |||||||
| Psychological-fatigue | 0.13 (0.03-0.23) | 0.06 (−0.07-0.19) | 0.15 (0.06-0.24) | 0.07 (−0.04-0.18) | 0.08 (0.00-0.15) | 0.32 (0.27-0.36) | 0.30 (0.26-0.34) |
| Psychological-somatic | 0.17 (0.07-0.26) | 0.01 (−0.13-0.14) | 0.16 (0.08-0.24) | 0.08 (−0.02-0.18) | 0.08 (−0.01-0.16) | 0.28 (0.23-0.32) | 0.28 (0.24-0.32) |
| Fatigue-somatic | 0.27 (0.17-0.36) | 0.18 (0.06-0.30) | 0.24 (0.16-0.32) | 0.23 (0.14-0.32) | 0.09 (0.01-0.17) | 0.51 (0.47-0.54) | 0.46 (0.42-0.49) |
Confirmatory factor analysis
The 37 categorical items (5 psychological, 13 fatigue and 19 somatic) were initially all allowed to load onto a single factor. This produced a poor fit (Table 2), indicating that the items are measuring more than one underlying phenotype. Consequently, a model was run that allowed the items from the three different scales to load onto one of three factors representing each scale. This produced a good fit. The high percentage of variance accounted for, and low residual correlations, mean that composite scales of the summed items largely represent these three factors.
Table 2.
Confirmatory factor analysis (CFA) fit of 3-factor model and 1-factor model
| Model | Proportion of variance explained |
Root mean square residual |
Average absolute residual correlation |
CFI | TLI | RMSEA |
|---|---|---|---|---|---|---|
| 1. Unidimensional (CFA with 1 factor) |
35% | 0.111 | 0.092 | 0.771 | 0.872 | 0.100 |
| 2. CFA with 3 items (separating the 3 scales) |
47% | 0.050 | 0.042 | 0.932 | 0.973 | 0.046 |
Number of items: 37; number of individuals: 1,988
Genetic Models
The model fit statistics are presented in Table 3. The Cholesky, independent and common pathway models were each a good fit compared to the fully saturated phenotypic model of the data. In the Cholesky model, there was no evidence of qualitative sex differences (P>0.50), but there was a strong suggestion of quantitative sex differences (Δ2ll=16.27, df=9, P=0.061). Thus we compared the Cholesky, common pathway and independent pathway models which allowed quantitative sex differences, and selected the common pathway as the best fit on the basis of AIC (see Table 3). That is, although factor analysis indicated that the data represent three separate phenotypic factors, their aetiology can be best understood by recognising that part of their variance is explained by a common underlying vulnerability to all three sets of symptoms.
Table 3.
Genetic model fit statistics
| Model | Model fit statistics | Fit statistics (compared to phenotypic saturated model) |
||||
|---|---|---|---|---|---|---|
| −2 ll | df | Δ −2ll | Δ df | P-value | Δ AIC | |
| 1. Phenotypic saturated | 55319.66 | 11259 | - | - | - | - |
| 2. Cholesky | 55396.51 | 11337 | 76.85 | 78 | 0.516 | −79.15 |
| 3. Common pathway | 55388.18 | 11336 | 68.52 | 77 | 0.744 | −85.48 |
| 4. Independent pathway | 55383.66 | 11328 | 64.00 | 69 | 0.648 | −74.00 |
Bold indicates best fitting model.
All models allow separate parameter estimates according to sex
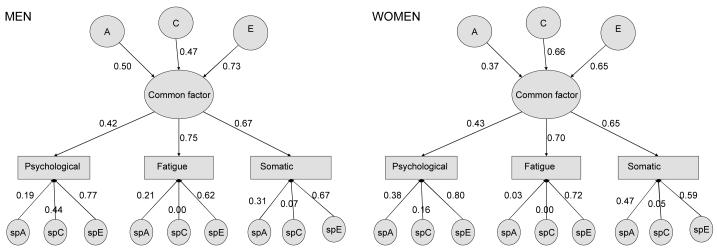
The parameters in this common pathway model could not be equated across sex (Δ-2ll =28.20, df=14, p=0.013), so we retained the model that allowed different parameters for each sex (Tables 4a and 4b, and Figure 1). In men, significant A influences came only via the latent factor; there was also a marginally significant effect of C specific to psychological distress (Δ-2ll=3.18, Δdf=1, p=0.07). This tallies with our previous finding of a possible shared environmental impact on categorically measured lifetime depressive episodes in men only (Ball et al. 2009). However in women, significant C influences came only via the latent factor, with additional significant A influences specific to psychological distress and somatic symptoms. In addition, there were significant E influences on the latent factor as well as specific E influences to all three measured variables in both men and women.
Table 4A.
Common pathway model of genetic and environmental influences: Contributions to the common factor
| Sex | Contributions to variance of common factor (95% CI) |
||
|---|---|---|---|
| A | C | E | |
| Men | 0.25 (0.01-0.60) | 0.22 (0.00-0.43) | 0.53 (0.41-0.66) |
| Women | 0.14 (0.00-0.45) | 0.44 (0.18-0.62) | 0.42 (0.32-0.54) |
Table 4B.
Common pathway model of genetic and environmental influences: Contributions to the variance in the three measured variable
| Contributions to variance (95% CI) | Overall source of variance |
|||||||
|---|---|---|---|---|---|---|---|---|
| Sex | Measured trait |
Specific A | Specific C | Specific E | Common factor |
A | C | E |
| Men | Psychological | 0.04 (0.00-0.28) | 0.19 (0.00-0.28)^ | 0.60 (0.52-0.67) | 0.18 (0.14-0.22) | 0.08 | 0.23 | 0.69 |
| Fatigue | 0.04 (0.00-0.12) | 0.00 (0.00-0.09) | 0.39 (0.30-0.48) | 0.57 (0.48-0.67) | 0.18 | 0.12 | 0.68 | |
| Somatic | 0.10 (0.00-0.17) | 0.00 (0.00-0.14) | 0.45 (0.37-0.53) | 0.45 (0.38-0.53) | 0.21 | 0.10 | 0.69 | |
|
| ||||||||
| Women | Psychological | 0.15 (0.02-0.24) | 0.03 (0.00-0.14) | 0.64 (0.57-0.72) | 0.18 (0.14-0.23) | 0.17 | 0.11 | 0.72 |
| Fatigue | 0.00 (0.00-0.06) | 0.00 (0.00-0.04) | 0.52 (0.44-0.58) | 0.48 (0.41-0.55) | 0.07 | 0.21 | 0.73 | |
| Somatic | 0.22 (0.04-0.29) | 0.00 (0.00-0.15) | 0.35 (0.29-0.42) | 0.43 (0.36-0.50) | 0.28 | 0.19 | 0.53 | |
Marginally significant, P=0.07
Figure 1. Common pathway model for men and women.
Boxes represent measured traits. Circles represent latent (unobserved) factors that influence the measured traits. A, C and E represent influences through the common factor; spA, spC and spE represent influences specific to each of the three measured traits. Arrows represent standardized paths, which are squared to indicate the magnitude (variance) of the relevant factors shown in table 4.
The latent factor had larger contributions to fatigue and somatic symptoms, accounting for 43-57% of the variance in these measured traits, but only 18% for psychological distress (Table 4B). Familial factors (A+C) were responsible for between 28% and 47% of the total variance in each trait (i.e., either directly or through the common factor), with the remainder due to nonshared environmental (E) effects. The total estimated heritability (A) for each trait was greatest for fatigue (18%) and somatic symptoms (21%) in men, and psychological (18%) and somatic symptoms (28%) in women; total C influences were greatest for psychological symptoms (23%) in men and fatigue (21%) and somatic symptoms (19%) in women. The specific E influences include measurement error specific to each trait as well as any true nonshared environmental effects; the E that influences the common factor could include measurement error that is correlated across the three traits, as well as any true nonshared environmental influences on the common factor.
The factor loadings from the common factor to the three measured traits could be equated across sex without significant deterioration in fit (Δ-2ll=2.54, df=3, P=0.47). This shows that the common factor is tapping into the same phenotypic vulnerability in men and women, despite the differences in the aetiological influences on this common factor. When focusing on the aetiological influences on the common factor, these could not be equated across sex, regardless of whether the factor loadings had already been equated across sex (Δ-2ll=8.535 for 2df, p=0.014; or Δ-2ll=9.092 for 2df, p=0.011 after having fixed the factor loadings across sex). This demonstrates that there are sex differences specifically in the aetiology of the common vulnerability.
The models were re-run using only same-sex pairs of twins. The common pathway model was again selected as the best fitting model (using a χ2 difference test), and the aetiological structure was very similar.
Discussion
This study finds that psychological, fatigue and somatic symptoms can be understood as relatively distinct dimensions (as shown by confirmatory factor analysis). These dimensions nonetheless share a large proportion of their genetic and environmental risk and/or protective factors (as shown through genetic model fitting assessing twin similarity). The prevalence and co-occurrence of these symptoms in Sri Lanka are similar to those in higher income, Western countries. The aetiological contributions also appear similar to those in other countries, but there are some contributions from shared environmental risk factors in this Sri Lankan context, which have not been reported in other countries.
Common vulnerability across psychological, fatigue and somatic symptoms
This is the first study in which a model with a single aetiological common factor (in addition to influences specific to each measured trait) has provided a good fit to a combination of psychological, fatigue and somatic symptoms. This suggests that comorbidity is partly the result of a prior state of vulnerability to all of these symptoms, rather than a process of phenotypic causation, as has been suggested by certain cohort studies (Harvey et al. 2008a; Fishbain et al. 1997).
Previous studies have suggested more aetiological differentiation between affective and sensory subdimensions of symptoms (Kato et al 2008; Williamson et al 2005; Gillespie et al 2000). In the current study, the common pathway explained a large proportion of the variance in all three symptom dimensions, but more so for fatigue and somatic symptoms than the psychological dimension. Nonetheless, the good fit of the common pathway model is consistent with commonly presenting psychological and physical distress being partially the outcomes of underlying core processes, which gives some justification to thinking of them as a group of ‘common distress disorders’ (Henningsen et al. 2003). It is important to note the considerable familial and nonfamilial contributions from scale-specific aetiological factors, which indicate that there is also much to differentiate these sets of symptoms.
Sex differences in familial influences on the common vulnerability
The common factor was more strongly influenced by genetic factors in men and shared (family) environmental factors in women, although nonshared environmental factors also played a large role in both men and women. This suggests there are some sex differences in the triggers for this common vulnerability to fatigue, somatic and psychological symptoms. This is despite similar outcomes of this common vulnerability in men and women (i.e. the factor loadings contributing to psychological, somatic and fatigue symptoms were similar for men and women). The genetic contribution to the common factor is likely to represent multifactorial biological mechanisms capable of influencing affective experiences as well as somatic sensations. This could include monoamine neurotransmitter systems, as these are thought to have roles in the generation of depression as well as pain modulation, inflammatory and immune responses and neuroendocrine responses to stress (Goldenberg, 2009). While the genetic contribution to the common factor was greatest in men, and non-significant in women, a genetic role could not be ruled out in women. Additionally there were specific genetic influences on psychological distress and somatic symptoms in women, suggesting at least a small genetic role in differentiation between different but related symptom dimensions.
The finding of shared (family) environmental effects, and their different impacts for men and women, may be specific to this Sri Lankan population rather than other previously studied populations. However, it is also possible that small shared environmental influences exist in these other populations (but are typically harder to identify than genetic and nonshared environmental influences). Shared environmental effects were seen influencing both the common liability (which was more strongly related to fatigue and somatic symptoms, but also to a lesser extent to psychological distress) in women; and as a marginally significant influence specific to psychological distress in men. These effects may operate through events or exposures that occurred while still living with the family of origin such as opportunities for education, or experiences while in the marital family (which could be selected or influenced by characteristics of the family of origin, and thus exposures would be shared across twin pairs to some extent). It is unclear why these environmental influences appear to have a stronger influence on psychological symptoms in men, and fatigue and somatic symptoms in women, but this is consistent with the greater environmental influence found for men than women in this sample when using a diagnostic assessment of depression (Ball et al. 2009).
Links to the aetiology of medically unexplained somatic symptoms
A large proportion of physical symptoms seen in the community (Kroenke, 2003) and presenting in clinics (Nimnuan et al. 2001) are medically unexplained. The current study focused on symptoms known to be associated with psychiatric rather than organic disease, and the dimensional approach gave more weight to people with multiple physical symptoms (which are less likely to be medically explicable) (Nimnuan et al. 2001). However, there were no exclusion criteria to rule out medically explained physical symptoms. The strong pattern of familial common vulnerability across symptoms identified in our study might reflect the more population-normative end of the overlap between psychiatric disorders and functional somatic syndromes. If so, the shared environmental influences could reflect risk factors for these disorders, including illness behaviours learned from parents in childhood (Hotopf, 2002). However, parent-child similarity and childhood risk factors could also be explained by genetic influences on behaviour, e.g., via perfectionist personality styles (Harvey et al. 2008b).
Nonshared (person-specific) environmental influences
Large nonshared environmental (E) influences were found for both men and women, and through both the common and specific paths. These might reflect the influence of stressful life events, which are known to be associated with depression (Tennant, 2002), fatigue (Kato et al. 2006) and other somatic problems (Tosevski & Milovancevic, 2006). But nonshared environmental influences (E) also played a large role in influencing why people experience certain symptom dimensions rather than others – these could include exposure to certain infectious agents (Moss-Morris and Spence, 2006), or different sorts of abusive experiences (Romans et al. 2002). However, where assessed retrospectively, these may be subject to recall bias, and more evidence points toward psychosocial stress having a generic influence on both psychological and a range of functional somatic outcomes (Campbell et al. 2003). And some of these apparently environmental exposures may actually be manifestations of genetic tendencies (Tennant, 2002) that lead to common vulnerability to all three symptom dimensions.
Comparisons to other countries
One aim of research using the CoTASS sample is to assess whether aetiology differs when examined in populations living in substantially different environmental and cultural contexts to those in the majority of previous twin studies. Past studies in higher-income, Western countries found stronger support for models which place a greater emphasis on influences specific to each trait, and greater differentiation between psychological and somatic symptoms or disorders (Williamson et al. 2005; Kato et al. 2008). However, because the current study has only three measured dimensions, any differentiation between the psychological, fatigue and somatic dimensions can reside in the residual influences on the sole psychological dimension. Thus the smaller influence of the common factor on psychological distress is relatively consistent with the findings from other countries. Although the current study found a sex difference in the aetiological contributions in contrast to the above studies, the extent of the overall familial influence (A+C) was similar for men and women, and A and C can be hard to tease apart because they are correlated with one another. So, the current evidence is broadly suggestive of cross-cultural similarity, but the details are hard to compare, partly due to the small number of genetically-informative studies of this kind reported in other countries.
It is interesting that the prevalence of the symptoms measured in the current study were in line with findings from population-based studies in other countries, but the proportions of people categorised with depressive or fatigue disorders (Ball et al. 2010a; Ball et al. 2010b) was lower. This suggests that the underlying distribution of symptoms is similar across countries, but those at the high end are not well detected using diagnostic tools devised in other cultures. If so, a dimensional scale of symptoms (rather than diagnostic criteria) might provide a more level playing field from which to assess cross-cultural similarity.
Limitations
The measures we used (such as the Short Form-36 Health Survey) allowed us to tap into the variation in the general population, rather than focusing on the pathological end of the spectrum. This means the results do not directly relate to clinical conditions. However, the quantitative trait hypothesis (Plomin et al. 1994) postulates that aetiological risk factors influence a continuum from normal to abnormal behaviour. So understanding the influences underlying common variability may also inform us about processes that cumulatively contribute to disorders that are at the extreme end of the spectrum. Part of the reason for this approach is that the genetic models require data on large numbers of people rather than a smaller selected group. The complexity of these models still meant we had relatively little power for certain analyses, as reflected in the wide confidence intervals in tables 4a and 4b. For example, although we found no sex differences in the ACE contributions to the common factor, this may have been due to low power.
The measures we used have been designed for population-comparative purposes rather than being specific to one population. The translation procedure was very thorough and retained meaning of the relevant concepts rather than literal translation. This enabled us to have an “etic” approach, i.e., examining similar phenomena in different cultural contexts. However, this does mean the scales may have missed “emic” phenomena, i.e., those understood from within the specific perspective of the population studied.
The results are based on the assumptions of the twin method, in particular the assumption that MZ twins are not treated more similarly than DZs purely because of their zygosity. Past studies have supported this assumption when examining psychiatric disorders (Kendler et al. 1993).
The large representative sample (the only one of its kind outside of the developed world) supports the current findings as being a valid representation for this population at this point in time. However, the results presented here do not account for potential gene-environment or gene-gene interactions within the sample, and for this reason, the results should be viewed as an overall approximation of the influences likely to be contributing.
The symptoms of all three measured dimensions come from self-reports, without any assessment to rule out physical disorders that have a medical explanation. However, the Bradford Somatic Inventory was devised to pick up symptoms that co-occur with psychiatric rather than diagnosable physical disorders. The current study contains only cross-sectional data, but genetic inheritance necessarily occurs prior to experience of psychological or physical symptoms.
Conclusion
Common underlying vulnerability predisposes to each of psychological, fatigue and somatic symptoms, although there are also risk factors specific to each. It has been suggested that somatic symptoms are especially likely to reflect (or at least co-occur with) psychological symptoms in South Asia. However, we found that the presentation and the aetiological influences (including considerable heritable contributions) on these symptoms and their overlap, are generally similar in Sri Lanka to results from higher-income, Western countries, suggesting similar underlying processes are involved.
Acknowledgements
The Wellcome Trust (grant number 069629) provided funding for the CoTASS study, and the Institute for Research and Development, Sri Lanka, provided infrastructural support. HB was supported by an ESRC research studentship, and a Foulkes Foundation fellowship. MH is funded by the South London and Maudsley NHS Foundation Trust and Institute of Psychiatry, King’s College London, National Institute of Health Research, Biomedical Research Centre.
PM has received honoraria from Eli Lilly and GSK and has acted as a consultant in the recent past for GSK and Astra Zeneca. NG has received honoraria from Sanofi-Aventis and Servier. All other authors declare that they have no potential conflicts of interest.
Reference List
- Ball HA, Siribaddana SH, Kovas Y, Glozier N, McGuffin P, Sumathipala A, Hotopf M. Epidemiology and symptomatology of depression in Sri Lanka. Journal of Affective Disorders. 2010a;123:188–96. doi: 10.1016/j.jad.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball HA, Sumathipala A, Siribaddana S, Kovas Y, Glozier N, McGuffin P, Hotopf M. Genetic and environmental contributions to depression in Sri Lanka. British Journal of Psychiatry. 2009;195:504–509. doi: 10.1192/bjp.bp.109.063529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball HA, Sumathipala A, Siribaddana SH, Kovas Y, Glozier N, McGuffin P, Hotopf M. The aetiology of fatigue in Sri Lanka and its overlap with depression. British Journal of Psychiatry. 2010b;197:106–113. doi: 10.1192/bjp.bp.109.069674. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: a biopsychosocial perspective. Biological Psychiatry. 2003;54:399. doi: 10.1016/s0006-3223(03)00545-6. [DOI] [PubMed] [Google Scholar]
- Chalder T, Berelowitz C, Pawlikowska T. Development of a fatigue scale. Journal of Psychosomatic Research. 1993;37:147–154. doi: 10.1016/0022-3999(93)90081-p. [DOI] [PubMed] [Google Scholar]
- Fishbain DA, Cutler R, Rosomoff HL, Rosomoff RS. Chronic pain-associated depression: antecedent or consequence of chronic pain? A review. The Clinical Journal of Pain. 1997;13:116–137. doi: 10.1097/00002508-199706000-00006. [DOI] [PubMed] [Google Scholar]
- Gillespie NA, Zhu G, Heath AC, Hickie IB, Martin NG. The genetic aetiology of somatic distress. Psychological Medicine. 2000;30:1051–1061. doi: 10.1017/s0033291799002640. [DOI] [PubMed] [Google Scholar]
- Goldenberg DL. The Interface of Pain and Mood Disturbances in the Rheumatic Diseases. Seminars in Arthritis and Rheumatism. 2010;40:15–31. doi: 10.1016/j.semarthrit.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Harvey SB, Wadsworth M, Wessely S, Hotopf M. The relationship between prior psychiatric disorder and chronic fatigue: evidence from a national birth cohort study. Psychological Medicine. 2008a;38:933–940. doi: 10.1017/S0033291707001900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SB, Wadsworth M, Wessely S, Hotopf M. Etiology of chronic fatigue syndrome: testing popular hypotheses using a national birth cohort study. Psychosomatic Medicine. 2008b;70:488–95. doi: 10.1097/PSY.0b013e31816a8dbc. [DOI] [PubMed] [Google Scholar]
- Henningsen P, Zimmermann T, Sattel H. Medically Unexplained Physical Symptoms, Anxiety, and Depression: A Meta-Analytic Review. Psychosomatic Medicine. 2003;65:528–533. doi: 10.1097/01.psy.0000075977.90337.e7. [DOI] [PubMed] [Google Scholar]
- Hotopf M, Mayou R, Wadsworth MEJ, Wessely S. Temporal relationships between physical symptoms and psychiatric disorder. Results from a national birth cohort. British Journal of Psychiatry. 1998;173:255–261. doi: 10.1192/bjp.173.3.255. [DOI] [PubMed] [Google Scholar]
- Hotopf M. Childhood experience of illness as a risk factor for medically unexplained symptoms. Scandinavian Journal of Psychology. 2002;43:139–46. doi: 10.1111/1467-9450.00279. [DOI] [PubMed] [Google Scholar]
- Jenkinson C, Coulter A, Wright L. Short form 36 (SF36) health survey questionnaire: normative data for adults of working age. British Medical Journal. 1993;306:1437. doi: 10.1136/bmj.306.6890.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Sullivan PF, Evengard B, Pedersen NL. Premorbid Predictors of Chronic Fatigue. Archives of General Psychiatry. 2006;63:1267. doi: 10.1001/archpsyc.63.11.1267. [DOI] [PubMed] [Google Scholar]
- Kato K, Sullivan PF, Evengσrd B, Pedersen NL. A population-based twin study of functional somatic syndromes. Psychological Medicine. 2008;1:9. doi: 10.1017/S0033291708003784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. A test of the equal-environment assumption in twin studies of psychiatric illness. Behavior Genetics. 1993;23:21–7. doi: 10.1007/BF01067551. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60:929–37. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kroenke K. Patients presenting with somatic complaints: epidemiology, psychiatric comorbidity and management. International Journal of Methods in Psychiatric Research. 2003;12:34–43. doi: 10.1002/mpr.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Chentsova-Dutton YE, Markon KE, Goldberg D, Ormel J. A cross-cultural study of the structure of comorbidity among common psychopathological syndromes in the general health care setting. Journal of Abnormal Psychology. 2003;112:437–47. doi: 10.1037/0021-843x.112.3.437. [DOI] [PubMed] [Google Scholar]
- Loge JH, Ekeberg O, Kaasa S. Fatigue in the general Norwegian population: Normative data and associations. Journal of Psychosomatic Research. 1998;45:53–65. doi: 10.1016/s0022-3999(97)00291-2. [DOI] [PubMed] [Google Scholar]
- Minhas FA, Nizami AT. Somatoform disorders: perspectives from Pakistan. International Review of Psychiatry. 2006;18:55–60. doi: 10.1080/09540260500466949. [DOI] [PubMed] [Google Scholar]
- Moss-Morris R, Spence M. To“ lump” or to“ split” the functional somatic syndromes: can infectious and emotional risk factors differentiate between the onset of chronic fatigue syndrome and irritable bowel syndrome? Psychosomatic Medicine. 2006;68:463–469. doi: 10.1097/01.psy.0000221384.07521.05. [DOI] [PubMed] [Google Scholar]
- Mumford DB, Bavington JT, Bhatnagar KS. Bradford Somatic Inventory. A multi-ethnic inventory of somatic symptoms reported by anxious and depressed patients in Britain and the Indo-Pakistan subcontinent. The British Journal of Psychiatry. 1991a;158:379–386. doi: 10.1192/bjp.158.3.379. [DOI] [PubMed] [Google Scholar]
- Mumford DB, Tareen IA, Bhatti MR, Bajwa MA, Ayub M, Pervaiz T. An investigation of ‘functional’ somatic symptoms among patients attending hospital medical clinics in Pakistan--II. Using somatic symptoms to identify patients with psychiatric disorders. Journal of Psychosomatic Research. 1991b;35:257–264. doi: 10.1016/0022-3999(91)90079-4. [DOI] [PubMed] [Google Scholar]
- Neale M, Maes H. Methodology for Genetic Studies of Twins and Families. Kluwer Academic Publishers B.V.; Dordrecht, The Netherlands: 2003. [Google Scholar]
- Neale MC, Roysamb E, Jacobson K. Multivariate Genetic Analysis of Sex Limitation and G + E Interaction. Twin Research and Human Genetics. 2006;9:481–489. doi: 10.1375/183242706778024937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimnuan C, Hotopf M, Wessely S. Medically unexplained symptoms: an epidemiological study in seven specialities. Journal of Psychosomatic Research. 2001;51:361–367. doi: 10.1016/s0022-3999(01)00223-9. [DOI] [PubMed] [Google Scholar]
- Ooki S, Yamuda K, Asaka A, Hayakawa K. Zygosity diagnosis of twins by questionnaire. Acta Geneticae Medicae et Gemellologiae. 1990;39:109–115. doi: 10.1017/s0001566000005626. [DOI] [PubMed] [Google Scholar]
- Patel V, Pednekar S, Weiss H, Rodrigues M, Barros P, Nayak B, Tanksale V, West B, Nevrekar P, Kirkwood BR, Mabey D. Why do women complain of vaginal discharge? A population survey of infectious and pyschosocial risk factors in a South Asian community. International Journal of Epidemiology. 2005;34:853–862. doi: 10.1093/ije/dyi072. [DOI] [PubMed] [Google Scholar]
- Pawlikowska T, Chalder T, Hirsch SR, Wallace P, Wright DJM, Wessely SC. Population based study of fatigue and psychological distress. British Medical Journal. 1994;308:763–766. doi: 10.1136/bmj.308.6931.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R, Owen MJ, McGuffin P. The genetic basis of complex human behaviors. Science. 1994;264:1733–9. doi: 10.1126/science.8209254. [DOI] [PubMed] [Google Scholar]
- Romans S, Belaise C, Martin J, Morris E, Raffi A. Childhood Abuse and Later Medical Disorders in Women. Psychotherapy and Psychosomatics. 2002;71:141–150. doi: 10.1159/000056281. [DOI] [PubMed] [Google Scholar]
- Simon G, Gater R, Kisely S, Piccinelli M. Somatic symptoms of distress: an international primary care study. Psychosomatic Medicine. 1996;58:481–488. doi: 10.1097/00006842-199609000-00010. [DOI] [PubMed] [Google Scholar]
- Simon GE, von Korff M. Somatization and psychiatric disorder in the NIMH Epidemiologic Catchment Area Study. American Journal of Psychiatry. 1991;148:1494–1500. doi: 10.1176/ajp.148.11.1494. [DOI] [PubMed] [Google Scholar]
- Simon GE, VonKorff M, Piccinelli M, Fullerton C, Ormel J. An international study of the relation between somatic symptoms and depression. New England Journal of Medicine. 1999;341:1329–1335. doi: 10.1056/NEJM199910283411801. [DOI] [PubMed] [Google Scholar]
- Siribaddana S, Ball H, Hewage S, Glozier N, Kovas Y, Dayaratne DARK, Sumathipala A, McGuffin P, Hotopf M. Colombo Twin and Singleton Study (CoTASS): A description of a population based twin study of mental disorders in Sri Lanka. BMC Psychiatry. 2008;8:49. doi: 10.1186/1471-244X-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumathipala A. Psychiatric disturbance in patients with multiple complaints and/or repeated consultations. Post Graduate Institute of Medicine, University of Sri Lanka; Colombo: 1990. MD Thesis. [Google Scholar]
- Sumathipala A, De SN, Siribaddana SH, Abeysingha MR, Fernando DJ. Cross-cultural adaptation and preliminary validation of a zygosity determination questionnaire for twins in Sri Lanka. Twin Research. 2000;3:205–212. [PubMed] [Google Scholar]
- Sumathipala A, Siribaddana S, Abeysingha MRN, De Silva P, Dewey M, Prince M, Mann AH. Cognitive-behavioural therapy v. structured care for medically unexplained symptoms: randomised controlled trial. British Journal of Psychiatry. 2008a;193:51–59. doi: 10.1192/bjp.bp.107.043190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumathipala A, Siribaddana S, Hewege S, Sumathipala K, Prince M, Mann A. Understanding the explanatory model of the patient on their medically unexplained symptoms and its implication on treatment development research: a Sri Lanka Study. BMC Psychiatry. 2008b;8:54. doi: 10.1186/1471-244X-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant C. Life events, stress and depression: a review of recent findings. Australian and New Zealand Journal of Psychiatry. 2002;36:173–182. doi: 10.1046/j.1440-1614.2002.01007.x. [DOI] [PubMed] [Google Scholar]
- Tosevski DL, Milovancevic MP. Stressful life events and physical health. Current Opinion in Psychiatry. 2006;19:184. doi: 10.1097/01.yco.0000214346.44625.57. [DOI] [PubMed] [Google Scholar]
- Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care. 1992;30:473–483. [PubMed] [Google Scholar]
- Williamson R, Purcell S, Sterne A, Wessely S, Hotopf M, Farmer A, Sham P. The relationship of fatigue to mental and physical health in a community sample. Social Psychiatry and Psychiatric Epidemiology. 2005;40:126–132. doi: 10.1007/s00127-005-0858-5. [DOI] [PubMed] [Google Scholar]