Abstract
Background
Loss of finger extension is common after stroke and can severely limit hand function. Contralaterally controlled functional electrical stimulation (CCFES) is a new treatment aimed at restoring volitional finger and thumb extension. A previous pilot study showed reductions in hand impairment after 6 weeks of CCFES, but the effect did not persist after end of treatment.
Objective
This study aimed to evaluate the feasibility of achieving greater and more persistent gains with CCFES by increasing the treatment period to 12 weeks.
Methods
CCFES uses neuromuscular electrical stimulation to open the paretic hand in direct proportion to the degree of volitional opening of the unimpaired contralateral hand, which is detected by an instrumented glove. Three subjects with chronic hemiplegia participated in a 12-week CCFES treatment, which consisted of daily CCFES-assisted active repetitive hand-opening exercises and twice weekly functional task practice with CCFES.
Results
Maximum voluntary finger extension increased by 101° and 68° for subjects 1 and 2, respectively, but subject 3 had no improvement in finger extension. Box and Block score increased by 6, 15, and 7 blocks, and upper extremity Fugl-Meyer score increased by 11, 15, and 7 points for subjects 1, 2, and 3, respectively. The finger extension gains declined at the 1-month and 3-month follow-up for subjects 1 and 2, but the gains in Box and Block and Fugl-Meyer scores persisted at follow-up.
Conclusions
Greater reductions in hand impairment were achieved by extending the treatment period. The effect and its longevity may be related to baseline impairment level.
Keywords: Stroke, Hemiplegia, Contralaterally controlled functional electrical stimulation, Rehabilitation, Medical device
Loss of hand function is one of the most frequently persisting consequences of stroke and is often characterized by inability to open the hand.1,2 Although routine occupational therapy is beneficial, it remains limited in its effectiveness in restoring full independent use of the impaired upper extremity and consequently many stroke survivors never regain the function of their affected hand.
Several advanced rehabilitation techniques have emerged in recent years that have demonstrated that hand function may be improved in stroke survivors, even in those with chronic (>6 months post-cerebrovascular accident; post-CVA) hemiplegia. Some of these, such as constraint-induced movement therapy (CIT)3–5 and robot-assisted movement therapy6–8 emphasize active (patient initiated), repetitive, task-specific movement of the impaired arm and hand. Additional therapies shown to reduce upper extremity motor impairment include bilateral symmetric movement of the paretic and nonparetic upper limbs,9–12 and motor imagery or mental practice techniques,13–15 which may include the use of a mirror16,17 or virtual reality environments18–20 to help create the perception of restored motor control.
Neuromuscular electrical stimulation (NMES) has been used in numerous studies to produce repetitive movement in paretic limbs with the goal of facilitating motor relearning. Electromyography (EMG)-triggered NMES is a technique that requires subjects to produce a threshold level of EMG from either the paretic muscle itself (often wrist extensors) or another muscle to receive stimulation to the paretic muscles (wrist and finger extensors).21–26 The technique emphasizes linking motor intention (detected by the volitionally generated EMG signal) to motor response (brought by stimulation). Another use of NMES focuses on having subjects use their stimulated arm and hand to perform tasks with a prescribed regimen.27–31 In these studies, subjects triggered a stimulation sequence by hitting buttons on the device. These methods are thought to improve paretic hand function by inducing and facilitating changes in the brain.32
Contralaterally controlled functional electrical stimulation (CCFES) is a technique for restoring volitional hand opening that combines elements of several of the methods mentioned above.33 With CCFES, hand opening is produced by electrical stimulation of the finger and thumb extensor muscles. The degree of stimulated hand opening is controlled by the user; the intensity of stimulation is proportional to the degree of volitional opening of the contralateral unimpaired hand. A glove with sensors detects the opening of the unimpaired hand (Figure 1). CCFES treatment consists of using the stimulation system for a prescribed period of time to (1) perform active repetitive hand-opening exercises, and (2) practice using the affected hand to perform functional tasks.
Figure 1. Contralaterally Controlled Functional Electrical Stimulation. Volitional Opening of the Unaffected Hand Produces a Proportional Intensity of Stimulation to the Paretic Hand Extensors.
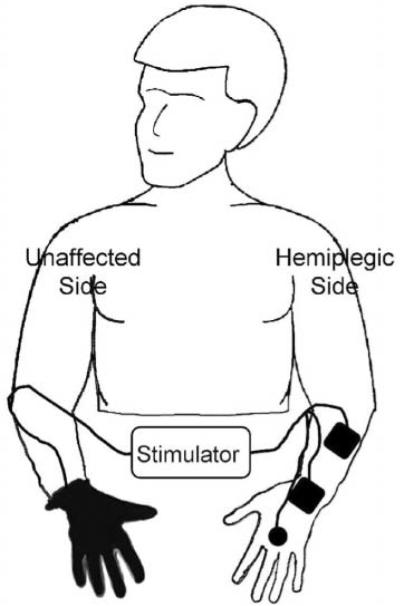
The CCFES paradigm is novel in 2 aspects: (1) it creates a stronger coupling of motor intention to stimulated motor output than other existing stroke NMES therapies by giving the user direct proportional control of the stimulus intensity, and (2) it uses movement of the unimpaired contralateral hand to modulate stimulation of the paretic hand. Previous animal and clinical studies support the hypothesis that therapies that temporally link motor intention to motor output and sensory feedback from the affected limb may facilitate neuroplastic changes leading to motor recovery.21,32,34 Furthermore, synchronizing movement of the paretic hand to the nonparetic hand may lead to facilitatory central effects similar to those demonstrated in studies of bilateral symmetric arm training.9,10,35
The purpose of our initial CCFES studies is to evaluate the feasibility of CCFES treatment to improve volitional hand opening. In our first study, 3 subjects with chronic (>6 months post-CVA) arm/hand hemiplegia used CCFES for 6 weeks.33 Gains in maximum voluntary finger extension, finger extension strength, finger-movement control (tracking task), and Box and Block score were seen in every subject. In general, the gains persisted at 1 month follow-up but diminished somewhat after 3 months.
This article reports on 3 additional subjects who participated in an amended version of the protocol. We lengthened the treatment period from 6 to 12 weeks to see if the longer treatment period would produce greater gains and if those gains would have greater longevity.
Methods
Participants
Stroke survivors were recruited from an outpatient stroke clinic and evaluated using the following inclusion criteria: (1) at least 6 months poststroke, (2) manual muscle grade of 3 or less for finger extensors, (3) adequate shoulder and elbow movement to allow volitional positioning of the hand in the workspace, (4) functional hand opening in response to electrical stimulation, and (5) cognitively capable of using the CCFES system as instructed. Individuals were ineligible if they had intramuscular botulinum toxin injections in any upper-extremity muscle within 3 months before enrollment or at any time during the study, were apraxic, had uncompensated hemineglect, or had uncompensated hemianopsia. The study protocol was approved by the hospital's institutional review board, and written informed consent was obtained from each subject.
Instrumentation
The CCFES system consists of a stimulator, a command glove, and surface electrodes (Figure 1). The custom-built multichannel programmable stimulator can deliver up to 7 independent monopolar channels (using a common anode) of biphasic current, though we use only 3 channels in the CCFES application. The stimulator is programmed to produce light and sound cues to prompt the subject to attempt to open both hands for several seconds and rest both hands for several seconds. The custom-built command glove has a sensor assembly attached on the dorsal side that consists of 3 bend sensors (Images SI Inc, 109 Woods of Arden Road, Staten Island, New York) enclosed in cloth sheaths. When the glove is opened and closed, proportional impedance changes in the sensors modulate the analog voltage input to the stimulator. This input, in turn, modulates the stimulus intensity (pulse duration) from each channel. Square (2 in × 2 in) and round (1.25 in) self-adhering pregelled electrodes are used to deliver stimulation to the extrinsic and intrinsic finger and thumb extensor muscles. The 2 sizes of electrode allow us to better target small and larger muscles in the hand and forearm.
The electrode positions and stimulus intensities that produced functional hand opening were determined for each subject. For each subject, a 2 in × 2 in electrode was placed over the dorsum of the wrist as the anode. Up to 3 additional electrodes were placed on the forearm and hand at positions to activate the finger and thumb extensors. To produce finger extension, the extensor digitorum communis (EDC) was targeted with an electrode placed on the dorsal mid-forearm. Wrist extension greater than ~40° was undesirable as it makes it difficult to use the hand in functional tasks; therefore over-activation of wrist extensors was avoided by positioning the forearm electrode distal to the wrist extensors. If the forearm electrode did not also produce thumb extension, another electrode was placed either distal to the EDC electrode to recruit the extensor pollicis longus (EPL) or at the base of the thumb to recruit the abductor pollicis brevis (AbPB). If extension of the proximal interphalangeal joints of the fingers was incomplete with EDC stimulation, a small electrode was placed on the dorsum of the hand to activate the dorsal interosseous muscles. If necessary, the extensor indicis proprius was also targeted to enhance extension of the index finger. Once the electrode positions were determined, pictures of the electrodes in their proper positions on the forearm and hand were taken and given to the participants to help them in placing the electrodes themselves at home. The stimulator was programmed so that stimulation intensity (pulse duration) on each electrode increased from a minimum (submotor threshold) when the gloved hand was lightly closed to a maximum (producing desired muscle contraction without pain) when the gloved hand was fully open. Minimum pulse durations ranged from 24 to 30 microseconds and maximum pulse durations ranged from 138 to 250 microseconds across subjects. The stimulus frequency was set at 35 Hz and pulse amplitude was set at 30 mA. A user's manual detailing how to put on and use the CCFES system at home was carefully reviewed with each subject and they demonstrated the ability to use the device as instructed in the laboratory before being sent home with it.
Intervention
The participants used the CCFES system to self-administer two 55-minute exercise sessions daily for 12 weeks at home. An exercise session consisted of three 15-minute sets separated by 5 minutes of rest. During a set, light and sound cues from the stimulator prompted the participant to open both hands for several seconds and then relax both hands for several seconds. The hand open/relax cycle durations were adjusted over the 12-week treatment period from 5/20 seconds open/relax to 6/16 seconds to 8/14 seconds to 8/10 seconds. The purpose of the graded duty cycle was to prevent fatigue early in the intervention and to build greater strength by more repetitions and longer duration muscle contractions as the treatment progressed.36 These stimulation duty cycles are within the range of those used in previous NMES studies.37 Our dosage of 14 hours of stimulation per week (including rests within and between sets) is substantially larger than several previous NMES studies,22,26,28,38 which used 3 to 5 hours per week but is less than more recent studies, which have used NMES doses up to 20 hours/week.21,29,30,39–41 The participants were instructed to separate the 2 exercise sessions by at least 2 hours to avoid fatigue and to write in a diary indicating when they did their sessions. The diaries were collected weekly. Along with the diaries, compliance was monitored by the data logging capability of the stimulator, which logged the date and time the unit was turned on and off and the date and time of start and completion of the sequence of audio and light cues corresponding to an exercise session.
In addition to daily home exercise sessions, the participants used the CCFES system to assist them in practicing functional tasks for 1½ hours twice a week. During the first 4 weeks, all of the task practice sessions were guided by an occupational therapist (OT) in the laboratory. During the second 4 weeks, half of the task practice sessions were guided by an OT in the laboratory and half were self-administered at home, as instructed by the OT. During the last 4 weeks, the frequency of task sessions in the laboratory was decreased to 1 every other week and the rest were self-administered at home. The laboratory sessions consisted of using the CCFES system to practice a finger-movement control task for 15 minutes, followed by practice using the stimulated paretic hand to perform several functional tasks for approximately 75 minutes. The finger-movement control task was a tracking task where an electrogoniometer on the index finger measured the degree of finger opening and closing and displayed it as a cursor on a computer screen. The task required the subject to keep the cursor between 2 parallel traces (a track) that scrolled across the screen by attempting to open and close their hand with the assistance of the CCFES system. The track had stretches of gradually increasing and decreasing slopes as well as interspersed periods of abrupt up and down changes over the course of the 15 minutes. This finger-movement control task was not practiced by the subjects at home. The functional tasks practiced in the laboratory and at home emphasized finger extension or whole hand opening. Example tasks include picking up a water bottle and pouring water, typing on a keyboard, opening a yogurt or frozen meal container, picking up and/or manipulating coins, and picking up a football.
Assessment
Assessments of upper extremity impairment were made at enrollment (0 weeks), mid-treatment (6 weeks), end of treatment (12 weeks), and at 1 (~16 weeks) and 3 months (~25 weeks) thereafter. The assessments included (1) maximum voluntary finger extension angle, (2) finger-movement control (tracking test), (3) maximum isometric finger extension moment, (4) Box and Block test, and (5) the upper-extremity portion of the Fugl-Meyer assessment of motor impairment. The participants were instructed to refrain from using the stimulator the day prior to and day of the mid-treatment and end of treatment assessments to prevent muscle fatigue or any possible short-term carryover effect from affecting the outcomes.
The maximum voluntary finger extension angle was measured with a custom-built electrogoniometer that recorded the metacarpophalangeal (MP), proximal interphalangeal (PIP), and distal interphalangeal (DIP) joints of the index finger simultaneously while the subject attempted to maximally open the paretic hand in response to a 3-second audio tone. The finger-movement tracking test was similar to the finger movement control task described earlier, except that the track was a 30-second long sinusoid of 0.1 Hz with its amplitude scaled to the middle 70% of the subject's voluntary range of motion as determined in the maximum finger extension test.42,43 The maximum isometric extension moment of the MP joint of the index finger was measured using an apparatus44 having an instrumented beam against which the subject extended the index finger of the paretic hand in response to a 3-second audio tone. The beam was positioned so that the MP joint was fixed at 30° of flexion during the measurements, while the wrist and forearm were restrained in a neutral posture. The Box and Block test45,46 is a valid and reliable measure of gross manual dexterity that requires the subject to pick up one 1-in. block at a time, lift it over a partition, and release it in a target area as many times as possible in 60 seconds. The upper-extremity Fugl-Meyer motor assessment47,48 is a valid and reliable measure of poststroke motor impairment in which subjects are asked to make specific coordinated and isolated upper limb movements, which are graded on a 3-point ordinal scale and summed to provide a score. The Box and Block test and Fugl-Meyer assessment were administered by an experienced occupational therapist; the other assessments were administered by a biomedical engineer.
Results
Three stroke survivors with chronic upper extremity hemiplegia completed the 12-week CCFES treatment and 3 months follow-up. Demographic and baseline characteristics of the 3 subjects are shown in Table 1. The results for each subject are shown in Table 2 and are summarized in the paragraphs that follow.
Table 1.
Baseline Characteristics of 3 Stroke Survivors Receiving 12 Weeks of CCFES Therapy
| Subject |
|||
|---|---|---|---|
| 1 | 2 | 3 | |
| Age (years), Sex | 71, M | 58, F | 44, F |
| Time since CVA (years) | 0.8 | 2.0 | 1.4 |
| Type of CVA | Ischemic, lacunar | Ischemic, frontal/parietal | Subarachnoid hemorrhage |
| Prestroke hand dominance/hemiplegic side | R / R | R / L | R / L |
| MAS for the elbow joint | 1+ | 0 | 2 |
| Sensation | Intact | Reduced | Intact |
| Neglect | No | No | No |
| Field deficits | No | No | No |
Abbreviations: CCFES, contralaterally controlled functional electrical stimulation; CVA, cerebrovascular accident; MAS, Modified Ashworth Scale with scores 0 to 4.
Table 2.
Upper Extremity Motor Impairment Values of 3 Stroke Survivors Receiving 12 Weeks of CCFES Therapy Through 3 Months Follow-up
| Week |
|||||
|---|---|---|---|---|---|
| Subject No. | 0 | 6 | 12 | ~16 | ~25 |
| Maximum voluntary finger extension angle (degrees); avg (std) | |||||
| 1 | −98 (5.3) | −25 (1.1) | 4 (1.3) | −12 (1.4) | −57 (8.5) |
| 2 | −69 (6.8) | −21 (25.9) | −1 (2.0) | −72 (12.1) | −42 (3.0) |
| 3 | −90 (25.3) | −120 (13.9) | −159 (3.2) | −141 (22.7) | −124 (11.6) |
| Finger-movement tracking error (normalized); avg (std) | |||||
| 1 | 1.0 (.03) | .51 (.14) | .46 (.04) | .44 (.06) | .47 (.06) |
| 2 | 1.0 (.11) | .19 (.02) | .13 (.01) | .22 (.04) | .17 (.01) |
| 3 | .39 (.13) | .65 (.28) | 1.0 (.60) | .62 (.02) | .71 (.23) |
| Maximum isometric finger extension moment (N cm)a; avg (std) | |||||
| 1 | 24 (10.1) | 42 (11.1) | 63 (3.7) | 56 (6.7) | 43 (4.0) |
| 2 | 18 (5.4) | 34 (10.2) | 44 (1.5) | 13 (1.4) | 14 (1.6) |
| 3 | −1.4 (3.4) | 2.1 (4.9) | −2.5 (2.6) | 1.7 (1.9) | −1.9 (1.6) |
| Box and Block score | |||||
| 1 | 28 | 36 | 34 | 26 | 37 |
| 2 | 19 | 31 | 34 | 34 | 32 |
| 3 | 6 | 7 | 13 | 16 | 13 |
| Upper-extremity Fugl-Meyer score (max = 66) | |||||
| 1 | 33 | 41 | 44 | 39 | 44 |
| 2 | 42 | 51 | 57 | 56 | 55 |
| 3 | 32 | 35 | 40 | 40 | 33 |
Abbreviation: CCFES, contralaterally controlled functional electrical stimulation.
N cm denotes newton centimeters.
Maximum voluntary finger extension (Figure 2) progressively increased to full extension (0°) over the course of the treatment period for subjects 1 and 2. For subject 1, finger extension increased by 73° from baseline to mid-treatment and increased by another 29° by end of treatment. Subject 2 experienced an increase of 48° by mid-treatment and an additional increase of 20° by end of treatment. Subject 3 experienced a loss in maximum voluntary finger extension by 30° at mid-treatment and an additional loss of 39° by end of treatment. The gains in maximum voluntary finger extension experienced by subjects 1 and 2 did not persist at follow-up.
Figure 2. Sum of Joint Angles of the Index Finger (Metacarpophalangeal + Proximal Interphalangeal + Distal Interphalangeal) During Maximum Voluntary Finger Extension.
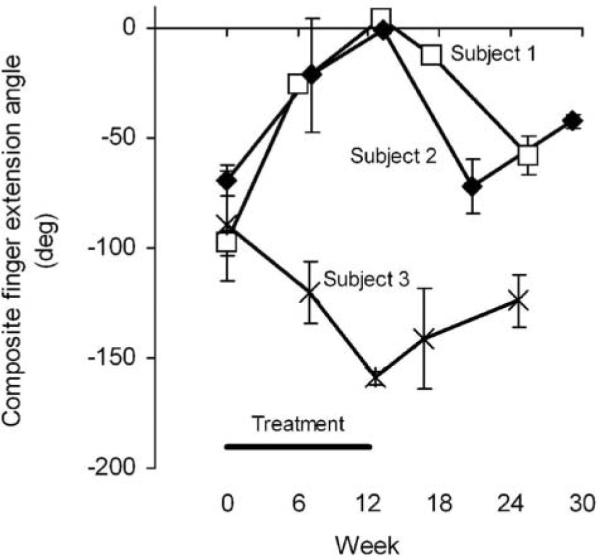
Note: Zero degrees corresponds to a fully extended finger; negative values are flexion. Values are the mean ± standard deviation of 3 repeated measurements.
Finger movement tracking error (Figure 3) decreased by 54% and 87% by end of treatment for subjects 1 and 2, respectively. Most of the improvement in finger movement control occurred by mid-treatment, with slight additional improvements occurring between 6 and 12 weeks. In contrast, the tracking error for subject 3 increased by 26% at mid-treatment and by another 35% at end of treatment. Subjects 1 and 2 maintained their tracking performance during the 3-month follow-up period.
Figure 3. Finger Movement Tracking Error.
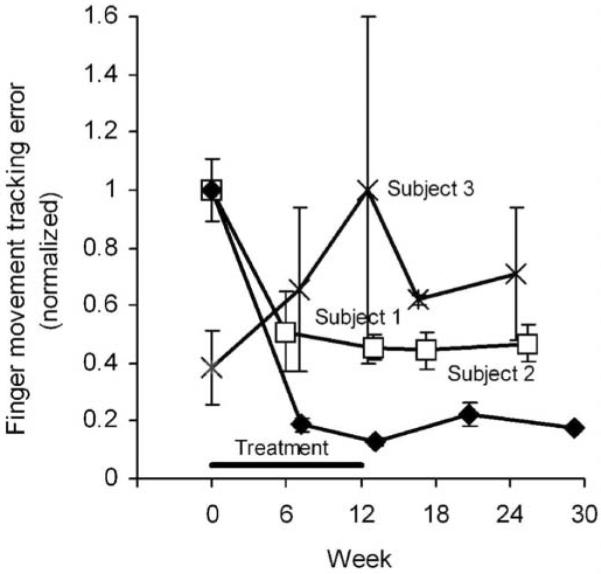
Note: Error was the accumulated distance the cursor was from the track over the 30-second period. Values are the mean ± standard deviation of 3 repeated measurements normalized by the maximum error for each subject.
Maximum voluntary isometric finger extension moment (Figure 4) progressively increased through the treatment period for subjects 1 and 2, increasing by 18 and 16 N cm by mid-treatment, respectively, and by another 21 and 10 N cm by end of treatment. The gains in finger extension moment experienced by subjects 1 and 2 did not persist at follow-up. For subject 3, finger extension moment remained near zero throughout the study, sometimes registering negative (indicating a net flexion moment).
Figure 4. Maximum Isometric Finger Extension Moment.
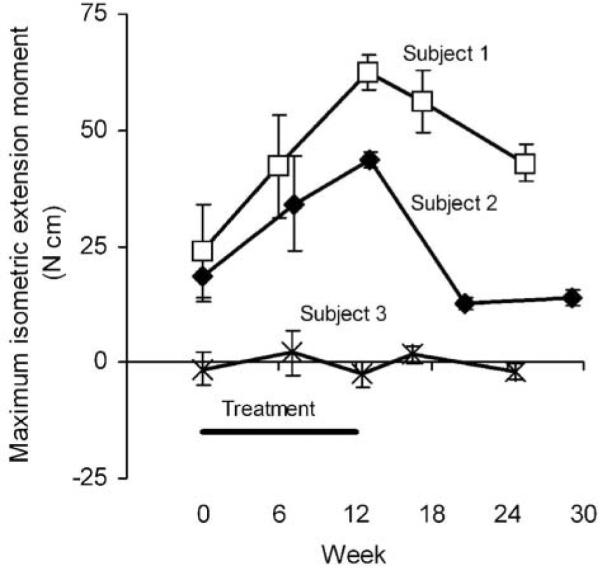
Note: Values are the mean ± standard deviation of 3 repeated measurements.
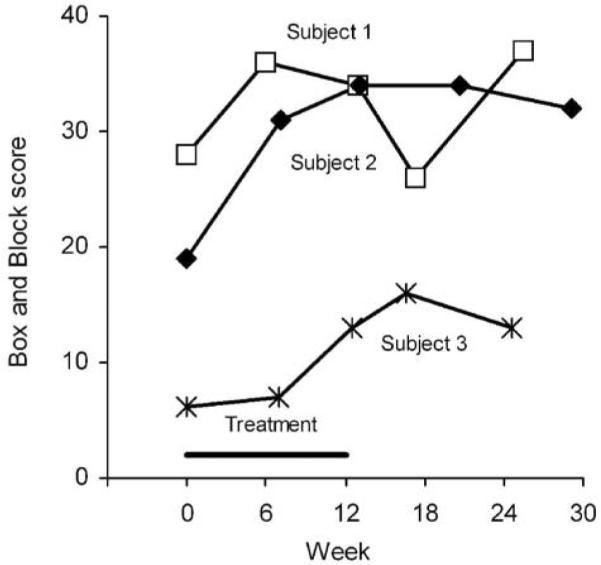
Box and Block scores (Figure 5) increased for all 3 subjects, with increases of 6, 15, and 7 blocks by end of treatment for subjects 1, 2, and 3, respectively. Subject 1 experienced some loss at 1-month follow-up and then rebounded by the 3-month follow-up, whereas subjects 2 and 3 maintained their end of treatment scores throughout the follow-up period.
Figure 5. Box and Block Score, the Number of Blocks Transferred in 60 seconds.
Fugl-Meyer scores (Figure 6) progressively increased for all 3 subjects throughout the treatment period. The scores increased by 11, 15, and 8 points by end of treatment for subjects 1, 2, and 3, respectively. During follow-up, the score for subject 1 decreased at 1 month and then rebounded at 3 months, the score for subjects 2 diminished only slightly, and the score for subject 3 returned to baseline level by 3 months posttreatment.
Figure 6. Upper-Extremity Portion of the Fugl-Meyer Motor Assessment, With a Maximum Score of 66.
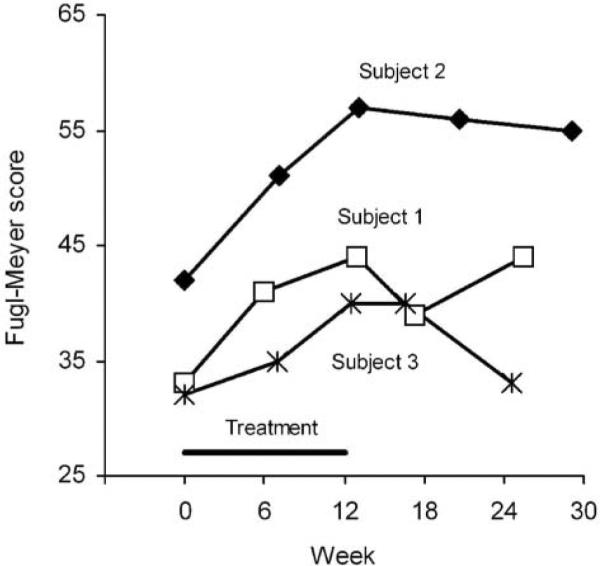
Note: Values are the total score.
Responses to a questionnaire given at the end of treatment indicated that all 3 subjects had a positive attitude toward the treatment. All 3 subjects agreed to the statement, “I wish this intervention was part of my original therapy.” In response to the statement, “The exercises and therapy associated with this study improved my hand function,” subjects 1 and 2 agreed, whereas subject 3 was unsure. Likewise, subjects 1 and 2 agreed with “I can open and use my hand better now than when I started the study,” whereas subject 3 was unsure. Even though subject 3 had the least improvement, she seemed to be the most enthusiastic about the treatment as indicated by a perfect compliance record and her expressed desire to show the CCFES system to her primary care physician and former coworkers. According to the usage data downloaded from the stimulator, compliance with the home exercise portion of the intervention was 78%, 0%, and 100% for subjects 1, 2, and 3, respectively.
Discussion
This study extends our pilot work on CCFES.33 CCFES is an intervention that incorporates several rehabilitation principles considered to be important in promoting neuroplastic changes leading to motor recovery. Such principles include active, repetitive, goal-oriented movement,3–5,32,49 bilateral symmetric movement,9–11,35, 50,51 neuromuscular electrical stimulation,21–31,36–41 and synchronous activation of upper and lower motor neurons and afferent fibers.52–54 CCFES may be more widely applicable than existing treatments because it does not require the patient to retain any residual hand movement and may require less therapist time because it can be self-administered, in part, at home.
In this study, hand impairment progressively improved over the 12-week CCFES treatment period, especially for subjects 1 and 2. Improvements were made both during the interval between baseline and mid-treatment and from mid-treatment (6 weeks) to end of treatment (12 weeks), suggesting that lengthening the treatment duration beyond 6 weeks produced greater gains. The active range of finger extension, extensor strength, and finger-movement control (tracking) for subject 3 were exceptions to the general finding of progressive improvement over the treatment period; this probably was due to muscle fatigue caused by self-administration of her home CCFES exercises on the day of her mid-treatment and end of treatment assessments, despite instructions to refrain from exercising those days. Her tendency to have greater upper extremity flexor hypertonia, as indicated by baseline modified Ashworth score55 on elbow flexors (Table 1), may also account for her poorer outcomes. Nevertheless, the Box and Block and Fugl-Meyer scores for subject 3 increased during the treatment period.
Increasing the treatment period to 12 weeks allowed more time for greater gains to be achieved, but the optimum treatment duration (ie, time at which the gains plateau) and dosage remain unknown. Treatment durations ranging from 2 to 12 weeks can be found in the research literature on poststroke upper-extremity rehabilitation treatments (eg, constraint induced therapy, robot-assisted therapy, neuromuscular electrical stimulation), but more research is needed to determine the optimum duration of any of these treatments.56,57 For subjects 1 and 2 in our study, the greatest improvements occurred in the interval between baseline and 6 weeks, with additional but less improvement occurring in the subsequent 6 weeks. For subject 3, who had greater impairment at baseline, the opposite was true; she had more improvement in Box and Block and Fugl-Meyer scores during the last 6 weeks of the treatment than during the first 6 weeks. The duration of treatment required for maximum benefit may depend on the baseline severity of paresis.
Subjective responses from research participants regarding treatment duration may help determine the duration of treatment that patients will tolerate. Two of the 3 subjects in the original CCFES pilot study with the 6-week treatment period wanted to continue using the CCFES system after the treatment period was over; they reported that they thought they could have experienced greater gains if they had been allowed to use the system longer than 6 weeks.33 When the subjects in this study were asked at the end of the treatment to respond to the statement, “The 12-week treatment was too long,” all 3 subjects disagreed. Subject 3 added that she thought she could have benefited from using the device longer than 12 weeks, but subject 1 was unsure, and subject 2 said she did not believe she would gain any additional benefit from a longer treatment period. Thus, a period of 12 weeks was tolerable for our subjects, but whether a longer duration would be acceptable depends on the gains patients perceive to be possible by continued use. In response to questions about the intensity of the treatment (ie, 2 hours/day daily and 1½-hour task practice sessions twice a week), all 3 subjects indicated that it was not too much. However, 1 subject indicated that a greater intensity would be too much, and another subject suggested that the 2 hours daily home exercise be broken into 3 equal segments throughout the day instead of 2. A future dose–response study will compare the effects of different durations of CCFES treatment in stroke survivors with different degrees of baseline impairment to optimize the treatment duration for a particular level of baseline impairment.
How long the effects of CCFES persist is unclear; some outcomes were maintained after 3 months whereas some had declined. Maximum voluntary finger extension and finger extension strength declined during the follow-up period, but the finger movement control (tracking), Box and Block score, and Fugl-Meyer scores generally persisted for 3 months. This pattern is consistent with the results of our first pilot study. We hypothesized that lengthening the treatment duration would lead to greater improvement, and that greater improvement would in turn lead to better longevity of the gains. Subjects 1 and 2 did in fact have greater gains in finger extension and strength than the subjects in our first pilot study; nevertheless, those greater gains did not translate into greater permanence of the gains over time.
One possible explanation for some outcomes persisting during follow-up and others not is that the neuromuscular stimulation strengthened the extensor muscles during the treatment period, and once the treatment ended, the muscles became weak again causing losses in active finger strength, and therefore, extension. Loss of strength, however, would not necessarily cause a decrease in Box and Block score, Fugl-Meyer score, or finger-movement control because those outcomes also depend on motor coordination, perhaps more than on strength. So it is possible that strength diminished during the follow-up period but motor coordination was maintained. If that is the case, then a standard electrical stimulator could be used periodically to re-strengthen the muscles if needed. Another possible explanation for the finger extension and strength gains not persisting over time is that the subjects may be regressing back to a state of “learned nonuse” once the treatment period is over. Thus, if they do not continue to use their affected arm and hand they may lose the gains they had made, losing not only strength gains but also gains in motor coordination as more time elapses. The problem of nonenduring improvements could possibly be addressed by redosing with CCFES after certain periods of time, a practice that is commonly done in medicine (eg, corticosteroid injections for shoulder pain) and should be considered for motor relearning as well.
Although this study did not include any measures of activity limitation (ie, activities of daily living), subjects 1 and 2 reported at end of treatment that their hand function had improved. When asked what daily tasks they thought they were able to perform better, the following responses were given: opening and closing doors, carrying plates, turning on/off light switches, putting on/taking off jewelry, and cutting vegetables. Future studies will include assessments of activity limitation.
In spite of remarkably positive results for subject 2, the compliance data downloaded from the stimulator's data logger indicated that she did not complete any of the home exercise sessions. This was in contrast to her diary, which indicated that she completed 75% of the self-administered home exercise sessions. The stimulator accurately logged every session in which it was used in the lab, providing evidence that it was functioning properly. Thus it appears that her positive outcomes may be attributed only to the therapist-guided CCFES functional task practice that took place in the lab. This raises questions regarding the relative importance of the home exercises and the functional task practice, and what results would have been obtained if she had also complied with the CCFES home exercise portion. Subject 2 gave no information that would have explained why she did not comply with the home regimen.
In summary, the results of this and the previous pilot study suggest that there is an association between CCFES treatment and improvements in upper extremity impairment. The small sample sizes of these studies do not allow a conclusion to be made regarding the strength of association or the probability of causation. Nevertheless, the data suggest a positive effect of the intervention, and further investigation of CCFES is underway.
Future studies include randomized controlled trials of CCFES in stroke survivors with acute and chronic hemiplegia to delineate the specific effects of CCFES and studies aimed at optimizing the treatment duration and dose for patients with various levels of baseline impairment. Other studies will seek to apply the CCFES concept to other paretic muscle groups, such as the ankle dorsiflexors.
Acknowledgments
This work was supported in part by the State of Ohio Biomedical Research and Technology Transfer Trust (grant no. BRTT 03–10), the National Institutes of Health National Center for Research Resources Multi disciplinary Clinical Research Career Development Programs (grant no. 8K12RR023264), and the National Institute of Child Health and Human Development (grant no. K24HD 054600). We thank Jeff Weisgarber, BS, Tina Vrabec, MS, and Stephen Trier, MS, at the Technical Development Laboratory of the Cleveland FES Center for their development of the stimulator used in this study.
References
- 1.Kamper DG, Harvey RL, Suresh S, Rymer WZ. Relative contributions of neural mechanisms versus muscle mechanics in promoting finger extension deficits following stroke. Muscle Nerve. 2003;28:309–318. doi: 10.1002/mus.10443. [DOI] [PubMed] [Google Scholar]
- 2.Lai SM, Studenski S, Duncan PW, Perera S. Persisting consequences of stroke measured by the Stroke Impact Scale. Stroke. 2002;33:1840–1844. doi: 10.1161/01.str.0000019289.15440.f2. [DOI] [PubMed] [Google Scholar]
- 3.Boake C, Noser E, Ro T, et al. Constraint-induced movement therapy during early stroke rehabilitation. Neurorehabil Neural Repair. 2007;21:14–24. doi: 10.1177/1545968306291858. [DOI] [PubMed] [Google Scholar]
- 4.Taub E, Uswatte G, King DK, Morris D, Crago JE, Chatterjee A. A placebo-controlled trial of constraint-induced movement therapy for upper extremity after stroke. Stroke. 2006;37:1045–1049. doi: 10.1161/01.STR.0000206463.66461.97. [DOI] [PubMed] [Google Scholar]
- 5.Wolf SL, Winstein CJ, Miller JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 6.Lum PS, Burgar CG, Shor PC, Majmundar M, Van der Loos M. Robot-assisted movement training compared with conventional therapy techniques for the rehabilitation of upper-limb motor function after stroke. Arch Phys Med Rehabil. 2002;83:952–959. doi: 10.1053/apmr.2001.33101. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi CD, Der-Yeghiaian L, Le V, Motiwala RR, Cramer SC. Robot-based hand motor therapy after stroke. Brain. 2008;131:425–437. doi: 10.1093/brain/awm311. [DOI] [PubMed] [Google Scholar]
- 8.Kwakkel G, Kollen BJ, Krebs HI. Effects of robot-assisted therapy on upper limb recovery after stroke: a systematic review. Neurorehabil Neural Repair. 2008;22:111–121. doi: 10.1177/1545968307305457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luft AR, McCombe-Waller S, Whitall J, et al. Repetitive bilateral arm training and motor cortex activation in chronic stroke: a randomized controlled trial. JAMA. 2004;292:1853–1861. doi: 10.1001/jama.292.15.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waller SM, Harris-Love M, Liu W, Whitall J. Temporal coordination of the arms during bilateral simultaneous and sequential movements in patients with chronic hemiparesis. Exp Brain Res. 2006;168:450–454. doi: 10.1007/s00221-005-0235-3. [DOI] [PubMed] [Google Scholar]
- 11.Whitall J, McCombe-Waller S, Silver KH, Macko RF. Repetitive bilateral arm training with rhythmic auditory cueing improves motor function in chronic hemiparetic stroke. Stroke. 2000;31:2390–2395. doi: 10.1161/01.str.31.10.2390. [DOI] [PubMed] [Google Scholar]
- 12.Richards LG, Senesac C, Davis S, Woodbury M, Nadeau S. Bilateral arm training with rhythmic auditory cueing in chronic stroke: not always efficacious. Neurorehabil Neural Repair. 2008;22:180–184. doi: 10.1177/1545968307305355. [DOI] [PubMed] [Google Scholar]
- 13.Dijkerman HC, Letswaart M, Johnston M, MacWalter RS. Does motor imagery training improve hand function in chronic stroke patients? A pilot study. Clin Rehabil. 2004;18:538–549. doi: 10.1191/0269215504cr769oa. [DOI] [PubMed] [Google Scholar]
- 14.Page SJ, Levine P, Leonard A. Mental practice in chronic stroke: results of a randomized, placebo-controlled trial. Stroke. 2007;38:1293–1297. doi: 10.1161/01.STR.0000260205.67348.2b. [DOI] [PubMed] [Google Scholar]
- 15.Page SJ, Levine P, Leonard AC. Effects of mental practice on affected limb use and function in chronic stroke. Arch Phys Med Rehabil. 2005;86:399–402. doi: 10.1016/j.apmr.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Stevens JA, Stoykov ME. Simulation of bilateral movement training through mirror reflection: a case report demonstrating an occupational therapy technique for hemiparesis. Top Stroke Rehabil. 2004;11:59–66. doi: 10.1310/GCFE-QA7A-2D24-KHRU. [DOI] [PubMed] [Google Scholar]
- 17.Altschuler EL, Wisdom SB, Stone L, et al. Rehabilitation of hemiparesis after stroke with a mirror. Lancet. 1999;353:2035–2036. doi: 10.1016/s0140-6736(99)00920-4. [DOI] [PubMed] [Google Scholar]
- 18.Gaggioli A, Meneghini A, Morganti F, Alcaniz M, Riva G. A strategy for computer-assisted mental practice in stroke rehabilitation. Neurorehabil Neural Repair. 2006;20:503–507. doi: 10.1177/1545968306290224. [DOI] [PubMed] [Google Scholar]
- 19.Sisto SA, Forrest GF, Glendinning D. Virtual reality applications for motor rehabilitation after stroke. Top Stroke Rehabil. 2002;8:11–23. doi: 10.1310/YABD-14KA-159P-MN6F. [DOI] [PubMed] [Google Scholar]
- 20.Deutsch JE, Merians AS, Adamovich S, Poizner H, Burdea GC. Development and application of virtual reality technology to improve hand use and gait of individuals post-stroke. Restor Neurol Neurosci. 2004;22:371–386. [PubMed] [Google Scholar]
- 21.Kimberley TJ, Lewis SM, Auerbach EJ, Dorsey LL, Lojovich JM, Carey JR. Electrical stimulation driving functional improvements and cortical changes in subjects with stroke. Exp Brain Res. 2004;154:450–460. doi: 10.1007/s00221-003-1695-y. [DOI] [PubMed] [Google Scholar]
- 22.Kraft GH, Fitts SS, Hammond MC. Techniques to improve function of the arm and hand in chronic hemiplegia. Arch Phys Med Rehabil. 1992;73:220–227. [PubMed] [Google Scholar]
- 23.Fields RW. Electromyographically triggered electric muscle stimulation for chronic hemiplegia. Arch Phys Med Rehabil. 1987;68:407–414. [PubMed] [Google Scholar]
- 24.Francisco G, Chae J, Chawla H, et al. Electromyogram-triggered neuromuscular stimulation for improving the arm function of acute stroke survivors: a randomized pilot study. Arch Phys Med Rehabil. 1998;79:570–575. doi: 10.1016/s0003-9993(98)90074-0. [DOI] [PubMed] [Google Scholar]
- 25.Chae J, Fang ZP, Walker M, Pourmehdi S. Intramuscular electromyographically controlled neuromuscular electrical stimulation for upper limb recovery in chronic hemiplegia. Am J Phys Med Rehabil. 2001;80:935–941. doi: 10.1097/00002060-200112000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Cauraugh J, Light K, Kim S, Thigpen M, Behrman A. Chronic motor dysfunction after stroke: recovering wrist and finger extension by electromyography-triggered neuromuscular stimulation. Stroke. 2000;31:1360–1364. doi: 10.1161/01.str.31.6.1360. [DOI] [PubMed] [Google Scholar]
- 27.Popovic DB, Popovic MB, Sinkjaer T, Stefanovic A, Schwirtlich L. Therapy of paretic arm in hemiplegic subjects augmented with a neural prosthesis: a cross-over study. Can J Physiol Pharmacol. 2004;82:749–756. doi: 10.1139/y04-057. [DOI] [PubMed] [Google Scholar]
- 28.Popovic MB, Popovic DB, Sinkjaer T, Stefanovic A, Schwirtlich L. Clinical evaluation of functional electrical therapy in acute hemiplegic subjects. J Rehabil Res Dev. 2003;40:443–453. doi: 10.1682/jrrd.2003.09.0443. [DOI] [PubMed] [Google Scholar]
- 29.Alon G, Levitt AF, McCarthy PA. Functional electrical stimulation enhancement of upper extremity functional recovery during stroke rehabilitation: a pilot study. Neurorehabil Neural Repair. 2007;21:207–215. doi: 10.1177/1545968306297871. [DOI] [PubMed] [Google Scholar]
- 30.Alon G, Sunnerhagen KS, Geurts AC, Ohry A. A home-based, self-administered stimulation program to improve selected hand functions of chronic stroke. NeuroRehabilitation. 2003;18:215–225. [PubMed] [Google Scholar]
- 31.Kowalczewski J, Gritsenko V, Ashworth N, Ellaway P, Prochazka A. Upper-extremity functional electric stimulation-assisted exercises on a workstation in the subacute phase of stroke recovery. Arch Phys Med Rehabil. 2007;88:833–839. doi: 10.1016/j.apmr.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 32.Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996;272:1791–1794. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- 33.Knutson JS, Harley MY, Hisel TZ, Chae J. Improving hand function in stroke survivors: a pilot study of contralaterally controlled functional electric stimulation in chronic hemiplegia. Arch Phys Med Rehabil. 2007;88:513–520. doi: 10.1016/j.apmr.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong Y, Winstein CJ, Albistegui-DuBois R, Dobkin BH. Evolution of fMRI activation in perilesional primary motor cortex and cerebellum with rehabilitation training-related motor gains after stroke. Neurorehabil Neural Repair. 2007;21:412–428. doi: 10.1177/1545968306298598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stinear JW, Byblow WD. Rhythmic bilateral movement training modulates corticomotor excitability and enhances upper limb motricity poststroke: a pilot study. J Clin Neurophysiol. 2004;21:124–131. doi: 10.1097/00004691-200403000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Powell J, Pandyan AD, Granat M, Cameron M, Stott DJ. Electrical stimulation of wrist extensors in poststroke hemiplegia. Stroke. 1999;30:1384–1389. doi: 10.1161/01.str.30.7.1384. [DOI] [PubMed] [Google Scholar]
- 37.de Kroon JR, Ijzerman MJ, Chae J, Lankhorst GJ, Zilvold G. Relation between stimulation characteristics and clinical outcome in studies using electrical stimulation to improve motor control of the upper extremity in stroke. J Rehabil Med. 2005;37:65–74. doi: 10.1080/16501970410024190. [DOI] [PubMed] [Google Scholar]
- 38.Chae J, Bethoux F, Bohine T, Dobos L, Davis T, Friedl A. Neuromuscular stimulation for upper extremity motor and functional recovery in acute hemiplegia. Stroke. 1998;29:975–979. doi: 10.1161/01.str.29.5.975. [DOI] [PubMed] [Google Scholar]
- 39.Ring H, Rosenthal N. Controlled study of neuroprosthetic functional electrical stimulation in sub-acute post-stroke rehabilitation. J Rehabil Med. 2005;37:32–36. doi: 10.1080/16501970410035387. [DOI] [PubMed] [Google Scholar]
- 40.de Kroon JR, IJzerman MJ, Lankhorst GJ, Zilvold G. Electrical stimulation of the upper limb in stroke: stimulation of the extensors of the hand vs. alternate stimulation of flexors and extensors. Am J Phys Med Rehabil. 2004;83(8):592–600. doi: 10.1097/01.phm.0000133435.61610.55. [DOI] [PubMed] [Google Scholar]
- 41.Hendricks HT, de Kroon JR, Groen FA, Zilvold G. Functional electrical stimulation by means of the `Ness Handmaster Orthosis' in chronic stroke patients: an exploratory study. Clin Rehabil. 2001;15:217–220. doi: 10.1191/026921501672937235. [DOI] [PubMed] [Google Scholar]
- 42.Carey J, Durfee W, Bhatt E, et al. Comparison of finger tracking versus simple movement training via telerehabilitation to alter hand function and cortical reorganization after stroke. Neurorehabil Neural Repair. 2007;21:216–232. doi: 10.1177/1545968306292381. [DOI] [PubMed] [Google Scholar]
- 43.Halaney ME, Carey JR. Tracking ability of hemiparetic and healthy subjects. Phys Ther. 1989;69:342–348. doi: 10.1093/ptj/69.5.342. [DOI] [PubMed] [Google Scholar]
- 44.Knutson JS, Kilgore KL, Mansour JM, Crago PE. Intrinsic and extrinsic contributions to the passive moment at the metacarpophalangeal joint. J Biomech. 2000;33:1675–1681. doi: 10.1016/s0021-9290(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 45.Desrosiers J, Bravo G, Hebert R, Dutil E, Mercier L. Validation of the Box and Block Test as a measure of dexterity of elderly people: reliability, validity, and norms studies. Arch Phys Med Rehabil. 1994;75:751–755. [PubMed] [Google Scholar]
- 46.Mathiowetz V, Volland G, Kashman N, Weber K. Adult norms for the Box and Block Test of manual dexterity. Am J Occup Ther. 1985;39:386–391. doi: 10.5014/ajot.39.6.386. [DOI] [PubMed] [Google Scholar]
- 47.Duncan PW, Propst M, Nelson SG. Reliability of the Fugl-Meyer assessment of sensorimotor recovery following cerebrovascular accident. Phys Ther. 1983;63:1606–1610. doi: 10.1093/ptj/63.10.1606. [DOI] [PubMed] [Google Scholar]
- 48.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 49.Butefisch C, Hummelsheim H, Denzler P, Mauritz KH. Repetitive training of isolated movements improves the outcome of motor rehabilitation of the centrally paretic hand. J Neurol Sci. 1995;130:59–68. doi: 10.1016/0022-510x(95)00003-k. [DOI] [PubMed] [Google Scholar]
- 50.McCombe Waller S, Harris-Love M, Liu W, Whitall J. Temporal coordination of the arms during bilateral simultaneous and sequential movements in patients with chronic hemiparesis. Exp Brain Res. 2006;168:450–454. doi: 10.1007/s00221-005-0235-3. [DOI] [PubMed] [Google Scholar]
- 51.Stewart KC, Cauraugh JH, Summers JJ. Bilateral movement training and stroke rehabilitation: a systematic review and meta-analysis. J Neurol Sci. 2006;244:89–95. doi: 10.1016/j.jns.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 52.Conforto AB, Kaelin-Lang A, Cohen LG. Increase in hand muscle strength of stroke patients after somatosensory stimulation. Ann Neurol. 2002;51:122–125. doi: 10.1002/ana.10070. [DOI] [PubMed] [Google Scholar]
- 53.Jackson A, Mavoori J, Fetz EE. Long-term motor cortex plasticity induced by an electronic neural implant. Nature. 2006;444:56–60. doi: 10.1038/nature05226. [DOI] [PubMed] [Google Scholar]
- 54.Rushton DN. Functional electrical stimulation and rehabilitation—an hypothesis. Med Eng Phys. 2003;25:75–78. doi: 10.1016/s1350-4533(02)00040-1. [DOI] [PubMed] [Google Scholar]
- 55.Gregson JM, Leathley M, Moore AP, Sharma AK, Smith TL, Watkins CL. Reliability of the Tone Assessment Scale and the modified Ashworth scale as clinical tools for assessing poststroke spasticity. Arch Phys Med Rehabil. 1999;80:1013–1016. doi: 10.1016/s0003-9993(99)90053-9. [DOI] [PubMed] [Google Scholar]
- 56.Dobkin B. Rehabilitation and functional neuroimaging dose-response trajectories for clinical trials. Neurorehabil Neural Repair. 2005;19:276–282. doi: 10.1177/1545968305281892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sterr A, Saunders A. CI therapy distribution: theory, evidence and practice. NeuroRehabilitation. 2006;21:97–105. [PubMed] [Google Scholar]


