Abstract
Methodologies for preclinical animal model testing of drugs against Mycobacterium tuberculosis vary from laboratory to laboratory; however, it is unknown if these variations result in different outcomes. Thus, a series of head-to-head comparisons of drug regimens in three commonly used mouse models (intravenous, a low-dose aerosol, and a high-dose aerosol infection model) and in two strains of mice are reported here. Treatment with standard tuberculosis (TB) drugs resulted in similar efficacies in two mouse species after a low-dose aerosol infection. When comparing the three different infection models, the efficacies in mice of rifampin and pyrazinamide were similar when administered with either isoniazid or moxifloxacin. Relapse studies revealed that the standard drug regimen showed a significantly higher relapse rate than the moxifloxacin-containing regimen. In fact, 4 months of the moxifloxacin-containing combination regimen showed similar relapse rates as 6 months of the standard regimen. The intravenous model showed slower bactericidal killing kinetics with the combination regimens tested and a higher relapse of infection than either aerosol infection models. All three models showed similar outcomes for in vivo efficacy and relapse of infection for the drug combinations tested, regardless of the mouse infection model used. Efficacy data for the drug combinations used also showed similar results, regardless of the formulation used for rifampin or timing of the drugs administered in combination. In all three infection models, the dual combination of rifampin and pyrazinamide was less sterilizing than the standard three-drug regimen, and therefore the results do not support the previously reported antagonism between standard TB agents.
For the first time in many years, there is a portfolio of promising new compounds at every level of tuberculosis (TB) drug discovery and development (4; www.tballiance.org). However, careful selection of new drug candidates is imperative, and efficient screening models for new drugs, including pertinent animal models, need to be further developed and studied. Preclinical testing in animals of newly discovered agents alone, and in combination with new and old agents, prior to being tested in humans is a crucial but lengthy process. These drug regimens include agents that provide bactericidal activities against rapidly growing bacilli, but they especially aim to include those that possess potent sterilizing activities and hence prevent relapse (8). The most commonly used animal species in TB drug development is the mouse, mainly because of economical and practical reasons, but also because of the limited requirement of compound (26).
Various mouse Mycobacterium tuberculosis infection models are utilized by both industry and academia, and they differ in the route of infection with M. tuberculosis, inoculum and strain of M. tuberculosis, strain of mice, timing of the start of treatment after infection, the length of treatment, etc. A model where therapy is started immediately after infection is ideally suited in order to quickly assess whether a compound has sufficient bioavailability, adequate absorption after oral administration, and metabolism sufficient to result in in vivo efficacy. A more realistic model might be one in which there is time for the disease to establish and produce granulomas, i.e., more consistent with what is seen in human tuberculosis. Models can also vary based on inoculum size, and both high- and low-dose inocula of M. tuberculosis were included in our study. We define these infection models based on inoculum size as follows. In the “low-inoculum” infection mouse model, mice develop an adequate immune response and can survive for months to more than a year after infection (22), whereas in the “high-inoculum” infection model, the immune response gets overwhelmed quickly and the animals succumb to disease within weeks after infection. There are various infection protocols based on the route of infection in mice. In the low-dose aerosol (LDA) infection method, reported studies generally aim at expected implantations in lungs of 30 to 100 CFU per mouse (5, 6, 53), versus 3,000 to 10,000 CFU for high-dose aerosol (HDA) infections (37, 39-41, 45, 47, 51, 52). The LDA method aims for an infection with few bacilli, leading to a chronic infection. The HDA method leads rapidly to progressive disease, with bacterial numbers reflecting those of a human cavity (41), and the animals succumb to disease without drug intervention. The route using an intravenous (i.v.) injection in the tail vein typically delivers a high-dose inoculum of M. tuberculosis (21, 35, 48-50). Other mouse infection models for TB drug evaluations have used the intratracheal (46) or intranasal (7) route of infection, which are used less frequently. The route and site of inoculum may be critical, as the immune response, especially the memory immune response and hence the rate of relapse, may well depend on the route of delivery of the pathogen. Mouse strains most commonly used for TB drug evaluations are Swiss, BALB/c, and C57BL/6 mice (20, 28, 40, 41). Laboratories generally use their in-house-developed mouse model, which is in most cases thoroughly validated before routine preclinical evaluation of novel compounds. However, to our knowledge, an effort to reevaluate side by side the different mouse models used for TB drug development has never taken place. In this comparative study, we interrogated the LDA and HDA and also the i.v. infection models, based on the rationale that these are the most widely used mouse models.
In this study, we administered different combinations of TB drugs to mice and used these drug treatment regimens to elucidate the most important variables within the different tuberculosis mouse infection models. In addition, the goal was to assess whether the outcome for in vivo efficacy and relapse of infection for the drug combinations tested was the same or different depending on the mouse infection model used. The standard drug regimen of isoniazid (INH), rifampin (RIF), and pyrazinamide (PZA) administered for up to 6 months was evaluated. In addition, moxifloxacin (MXF) was also included as a replacement for INH in the standard regimen; moxifloxacin is a fluoroquinolone currently in phase 3 clinical trials for TB treatment (1). The rationale for the inclusion of MXF-containing combination regimens in this study was to evaluate two relevant drug combination regimens for tuberculosis in the three mouse infection models. In addition, the inclusion of MXF-containing regimens in particular was aimed to see whether it showed improved in vivo efficacy versus the standard drug regimen, as seen in an earlier published mouse study (40), and these mouse data formed the basis of a TB Trials Consortium study (11). Our study is the first to directly compare the various mouse models used in TB drug screening within the same laboratory to identify the most critical parameters and details in the methodology that may influence subsequent results.
MATERIALS AND METHODS
Mice.
Female BALB/c or C57BL/6 mice (Charles River Laboratories, Wilmington, MA) between the ages of 6 and 12 weeks were housed at five animals per cage in HEPA-filtered racks (Thoren Caging Systems Inc., Hazleton, PA) in certified animal biosafety level three (ABSL-3) laboratories and were rested for 1 to 3 weeks before infection with M. tuberculosis.
Bacterial strains.
The virulent M. tuberculosis strain Erdman (TMC 107; ATCC 35801) was used as the standard strain for drug testing in all animal studies (53). Drug susceptibility testing of the M. tuberculosis Erdman strain used was recently performed for PZA and moxifloxacin at the Mycobacteriology Laboratory at the National Jewish Health, Denver, CO, by Bactec (17, 18). MIC testing for isoniazid and rifampin was performed by the microdilution plate method (16). The pncA gene was sequenced by the U.S. Centers for Disease Control and Prevention (Atlanta, GA). MICs were ≤100 μg/ml for pyrazinamide, 0.0078 μg/ml for isoniazid, 0.0156 μg/ml for rifampin, and ≤2 μg/ml for moxifloxacin. No mutations in the pncA gene were detected.
Bacteria were originally grown as pellicles to generate seed lots (28). The working stocks were generated by growing cultures to mid-log phase in Proskauer-Beck medium containing 0.05% Tween 80 (Sigma Chemical Co., St. Louis, MO), enumerated by colony counting on 7H11 agar plates, divided into 1.5-ml aliquots, and stored at −70°C until use.
Antimicrobial agents and formulations.
INH, PZA, and RIF were purchased from Sigma Chemical Co. (St. Louis, MO). MXF was a generous gift of Bayer Corporation in collaboration with the Global Alliance for TB Drug Development. MXF was administered at 100 mg/kg of body weight, RIF at 10 mg/kg, PZA at 150 mg/kg, and INH at 25 mg/kg. All antimicrobial compounds were administered by oral gavage 5 days per week (at 0.2 ml per mouse). All drugs were prepared in water except when specified differently.
Two methods of drug preparation were used for RIF, and two dosing schedules of RIF were used in relation to the drugs in combination. The first method consisted of the following conditions: RIF was ground in a clean mortar and pestle to a small particle size prior to adding sterile distilled water (1, 39, 44). INH, PZA, and MXF were dissolved in sterile distilled water as well. RIF was dosed in the morning, and the other drugs were administered as combinations in the afternoon (approximately 4 h apart). Drugs were prepared on the Friday prior to the dosing week and kept at 4°C. A day before or on the day of dosing, the drug combinations were combined. PZA solutions were incubated at 55°C on the day of dosing until particles were dissolved.
The second method of RIF formulation and administration was the following: RIF was dissolved in 100% dimethyl sulfoxide (DMSO) and slowly (dropwise) diluted to a final concentration of 5% DMSO in water (28). INH, PZA, and MXF were dissolved in autoclaved tap or deionized water. RIF was dosed at least 1 h after other drugs (but not more than 2 h). Concentrations of drugs were adjusted monthly based on body weights. For combinations of INH and PZA, concentrated stocks of individual drugs were prepared up to 7 days in advance, combined the day before or the day of dosing, and left at room temperature (RIF was kept at 4°C). PZA solutions, if particulate, were sonicated. In both methods, all drugs were visibly completely dissolved and free of obvious particles at the time of drug administration to the animal.
Infection models.
Six- to 12-week-old BALB/c or C57BL/6 mice were exposed to an LDA, HDA, or an i.v. infection with the virulent M. tuberculosis Erdman in ABSL-3 laboratories. All studies were approved after institutional review by the Animal Care and Use Committee and the Biosafety Committee at Colorado State University. All aerosol infections utilized an inhalation exposure system (Glas-Col, Inc., Terre Haute, IN), as described before (27). In the LDA infection experiment, 8- to 10-week-old female BALB/c and C57BL/6 mice were exposed to M. tuberculosis Erdman, derived from a frozen bacterial stock, with a titer of 4.87 × 107 CFU per ml (27). A 5-ml inoculum containing 2 × 106 CFU per ml in autoclaved deionized water was placed in the Glas-Col nebulizer with settings of 10 compressed air and 50 main (negative air) standard cubic feet per hour (SCFH).
For the HDA model (40, 41), 8- to 10-week-old female BALB/c mice were infected with a freshly grown culture of M. tuberculosis. Bacteria were grown in 7H9 medium (Difco Inc., Lawrence, KS) supplemented with 10% oleic acid-albumin-dextrose-catalase and Tween 80 and propagated to late log phase with an optical density at 600 nm (OD600) of 0.8 to 1.0. Ten milliliters of the freshly grown M. tuberculosis culture was placed in the Glas-Col nebulizer with settings of 13 to 17 SCFH compressed air and 80 SCFH main (negative air). The procedures included a 15-min preheat cycle, a nebulizing cycle of 30 to 40 min, a cloud decay cycle of 15 to 30 min, with decontamination for 15 min (Glas-Col, Inc.).
For i.v. infections, 8- to 12-week-old mice were injected with M. tuberculosis Erdman with 6 to 7 log10 CFU delivered in 0.1 ml of sterile phosphate-buffered saline via injection of the lateral tail vein. Bacteria were suspended repetitively through a SurGuard safety hypodermic needle (26 gauge; VWR, Wilmington, DE) in order to obtain a single-cell bacterial suspension. Enumeration of the M. tuberculosis inoculum from all infection routes was determined by CFU counts on 7H11 agar plates (as described below). The actual bacterial load delivered to the animals was determined from three mice per group the day after the infection in the lungs from all aerogenically challenged animals and in lungs and spleens from five mice in the i.v.-infected group. At the start of treatment (defined by convention as day zero), the bacterial load was determined in lungs and spleens. The timing of the drug treatment varied depending on the infection model being evaluated and was based on published and unpublished data. Treatment regimens ranged from 1 to 6 months with 5 to 8 mice per treatment group for each sacrifice point during treatment and 10 to 22 mice per group for assessment of relapse of infection. To assess relapse, animals were observed without drug intervention for 3 months.
Enumeration of bacteria from tissues.
After completion of therapy, mice were sacrificed by CO2 inhalation. After euthanasia, lungs, spleens, and/or livers were aseptically removed and homogenized in Pyrex tubes containing 1 ml (after more than 1 month of treatment) or 4.5 ml (when less than a month had elapsed since start of therapy) of sterile saline. The number of viable organisms was determined by serial dilution in sterile physiologic saline of the homogenates plated on nutrient 7H11 agar plates containing glycerol, oleic acid, dextrose, catalase, albumin, and cycloheximide (GIBCO-BRL, Gaithersburg, MD). Plates were incubated at 37°C in ambient air for 4 to 8 weeks prior to counting CFU. For determination of the emergence of RIF-resistant colonies, tissue homogenates were plated on 7H11 plates containing 4 μg/ml of RIF. RIF resistance plating was performed when indicated on residual frozen organ homogenates plated directly on RIF-containing plates.
Pharmacokinetic analysis.
Uninfected 8-week-old BALB/c mice were administered a single oral dose by gavage of different RIF formulations (see “Antimicrobial agents and formulations,” above). For both formulation methods, whole blood was obtained from mice by cardiocentesis immediately after CO2 euthanasia at 0, 0.5, 1, 2, 4, 8, and 14 h from three mice at each time point (times are in reference to rifampin administration); whole blood was collected in heparinized syringes containing 4.3 USP units of heparin per syringe (Sigma Chemical Co., St. Louis, MO), transferred into plasma separator tubes (BD microtainer; BD Diagnostics, VWR Wilmington, DE), and centrifuged at 4°C (5,410 rpm for 15 min). Plasma samples were snap-frozen in cryovials in liquid nitrogen and stored at −70°C prior to analysis at the Infectious Disease Pharmacokinetics Laboratory (University of Florida, Gainesville, FL), where the samples were analyzed by validated high-pressure liquid chromatography (RIF and INH) or gas chromatography/mass spectrometry (PZA) assays (1). The data on concentrations in plasma were analyzed by using WinNonlin (version 5.2.1, 2008; Pharsight, Mountain View, CA) with standard noncompartmental techniques in order to determine the maximum concentration (Cmax), time of Cmax (Tmax), area under the concentration-time curve (AUC0-∞), half-life, volume of distribution, and clearance.
Statistical analysis.
The CFU counts were converted to log values (log10 CFU), which were then evaluated by analysis of variance (ANOVA) when log10 CFU values appeared approximately normally distributed and by the Wilcoxon rank sum test when the log counts were predominantly <1 log and contained a large proportion of 0 values. BALB/c and C57BL/6 aerosol and BALB/c i.v. routes were compared using one-way ANOVA. When the ANOVA F-test was significant (P < 0.05), pairs of groups were compared using two-sided contrasts of means. Similarly, INH- and MXF-containing combination therapies were compared using one-way ANOVA separately for each infection model (HDA, LDA, and i.v.) and each time point, followed by pairwise contrasts of treatment means.
In the experiment that compared two formulations of four dosing schedule treatments, log10 CFU results were analyzed using a two-way (formulation by treatment) ANOVA. If the interaction was significant (P < 0.05), it was concluded that the differences between treatment means depend on formulation, and treatments were compared by pairwise contrasts, separately for each formulation. If the interaction was not significant, the treatments were compared averaging over formulation, and the formulations were compared averaging over treatment. For comparison of relapse rates, Fisher's exact test was used. Treatments were compared within models, and nonsignificant treatments were combined for comparisons of models.
Analysis was done separately for relapse rates in lungs and spleens for the HDA and i.v. routes for the three regimens by using Fisher's exact test. In addition, the relapse rates for the different drug regimens were analyzed via pairwise two-sided comparisons for every infection model individually, again with Fisher's exact test to identify the regimens with similar and improved relapse rates.
RESULTS
BALB/c and C57BL/6 mice after aerosol infection respond similarly to treatment with standard drugs, whereas mice infected via the i.v. route show a delayed treatment effect.
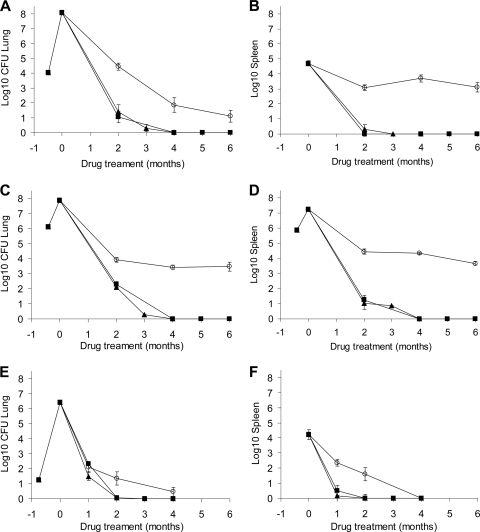
The purpose of this experiment was to evaluate the treatment efficacy of the standard drug regimen (INH, RIF, and PZA) given over 4 months in two mouse strains (BALB/c and C57BL/6) infected by LDA in one single experiment. In addition, parallel groups were also infected by i.v. injection in BALB/c mice only (Table 1). After 1 month of treatment, the bacterial loads were significantly different between the three different infection models (P < 0.0001, ANOVA F-test) (Fig. 1A), with the bacterial burden in lungs of the i.v.-infected BALB/c mice higher than in the aerosol-infected BALB/c and C57BL/6 mice (P < 0.0001 for both mouse strain). The bacterial loads in the lungs of both mouse strains infected by aerosol were similar after 1 month of treatment (P = 0.25) (Fig. 1A). After 2 months of treatment the lung bacterial loads showed similar results, with the i.v.-infected mice showing higher bacterial loads again than the aerosol-infected BALB/c and C57BL/6 mice (P = 0.020 and 0.019, respectively). The treatment efficacies in the two mouse strains infected by aerosol were again statistically similar (P = 0.9) (Fig. 1A). After 4 months, results in the lungs showed bacterial numbers were reduced to only a few bacilli, and this finding was in a single mouse. These results could not be compared statistically.
TABLE 1.
Bacterial loads in lungs and spleens on the day of infection and at the start of treatmenta
| Expt no. (route) | Log10 CFU 1 day after infectionb | Log10 CFU at treatment start |
|
|---|---|---|---|
| Lungs | Spleens | ||
| 1c (LDA; C57BL/6) | 54 | 5.8 | 4.6 |
| 1c (LDA; BALB/c) | 54 | 5.0 | 4.09 |
| 1 (i.v.) | 3.32 | 5.78 | 5.7 |
| 2 (i.v.) | 6.13 | 7.8 | 5.88 |
| 2 (HDA) | 4.04 | 8.09 | 4.71 |
| 2 (LDA) | 1.27 | 6.41 | 4.22 |
| 3 (HDA) | 4.3 | 8.31 | 5.39 |
Data are shown for the different experiments performed and illustrated in the figures, as follows: experiment 1 (Fig. 1), experiment 2 (Fig. 2), and experiment 3 (Fig. 3).
Data are log10 CFU counts (or for CFU counts in experiment 1 via LDA route) in lungs.
In experiment 1, both BALB/c and C57BL/6 mice were infected via LDA. Based on the comparable results and because most laboratories use BALB/c mice for TB drug evaluations, all further experiments were conducted with the BALB/c mouse strain.
FIG. 1.
The i.v. and LDA infection models with M. tuberculosis, using BALB/c and C57BL/6 mice. Data shown are bacterial numbers in lungs (A) or spleens (B) of BALB/c (▪) or C57BL/6 (▴) mice after an LDA infection with M. tuberculosis or i.v. infection of BALB/c (□) after 4 months of treatment with INH, PZA, and RIF. Error bars are standard errors of the means.
In spleens, bacterial loads at the start of treatment were not statistically significantly different for the three models (P = 0.060, ANOVA F-test); this was due to the larger standard deviations in the spleens of the i.v.-infected group. After 1 month of treatment, the bacterial loads in the spleens of i.v.-infected mice were significantly higher than in either group of mice infected by the aerosol route (P < 0.0001, ANOVA F-test) (Fig. 1B). In addition, the LDA-infected BALB/c mice showed a lower bacterial count than the C57BL/6 mice (P = 0.018). After 2 months of treatment (Fig. 1B), drug efficacy in the spleens was not statistically significantly different for the three mouse groups evaluated (P = 0.05, ANOVA F-test). The spleens of i.v.-infected mice indicated only three mice with positive cultures (mean, 0.42 log10 CFU), whereas the aerosol-infected mice had sterile cultures. After 4 months of treatment, mice in all treatment groups failed to show any culturable bacteria in the spleens.
INH- and MXF-containing combination therapies have similar efficacies in the i.v. and aerosol infection models in BALB/c mice.
The purpose of this comparative study was to evaluate three mouse infection models using BALB/c mice in one single experiment (after LDA, HDA, or i.v. infection) and to evaluate three drug regimens in each model. Based on preliminary experiments (data not shown), a set of parameters was established (such as starting inoculum and the time between infection and start of treatment) to ensure similar starting bacillary burden typical for the HDA and the i.v. models (7 to 8 log10 CFU) (38). Treatment included the standard drug regimen of INH, PZA, and RIF (HRZ), a MXF-containing regimen where MXF was substituted for INH in the standard regimen (MRZ), and the combination of RIF and PZA (RZ). PZA was discontinued in all groups after 2 months of therapy, except for the dual-drug RIF and PZA groups. Treatment start for the i.v. animals was at 11 to 12 days, at 14 days for the HDA group, and at day 21 after low-dose infection. Results are shown in Tables 1 and 2 and Fig. 2.
TABLE 2.
Viable M. tuberculosis in lungs and spleens of BALB/c mice 3 months after cessation of treatment with three-drug regimens via the HDA, LDA, and i.v. infection modelsa
| Infection route | Regimen | Lungs |
Spleens |
Relapse rate (%) | ||
|---|---|---|---|---|---|---|
| Log10 CFU ± SEM | n/N (%) | Log10 CFU ± SEM | n/N (%) | |||
| HDA | 2MRZ/2MR | 0.48 ± 0.27 | 3/19 (16) | 0.16 ± 0.16 | 1/19 (5) | 3/19 (16) |
| HDA | 2HRZ/3HR | 0.75 ± 0.27 | 6/20 (30) | 0.13 ± 0.13 | 1/20 (5) | 6/20 (30) |
| HDA | 2HRZ/4HR | 0.13 ± 0.13 | 1/22 (5) | 0.08 ± 0.08 | 1/22 (5) | 1/22 (5) |
| i.v. | 2MRZ/2MR | 1.27 ± 0.30 | 10/19 (53) | 1.00 ± 0.30 | 9/19 (47) | 12/19 (63) |
| i.v. | 2HRZ/3HR | 3.14 ± 0.13 | 20/20 (100) | 1.70 ± 0.31 | 13/20 (65) | 20/20 (100) |
| i.v. | 2HRZ/4HR | 1.62 ± 0.35 | 11/20 (55) | 0.64 ± 0.26 | 5/20 (25) | 11/20 (55) |
| LDA | 2MRZ/1MR | 0 ± 0 | 0/21 (0) | 0 ± 0 | 0/19 (0) | 0/21 (0) |
| LDA | 2MRZ/2MR | 0 ± 0 | 0/21 (0) | 0.15 ± 0.15 | 1/21 (5) | 1/21 (5) |
| LDA | 2HRZ/1HR | 0.53 ± 0.25 | 4/21 (19) | 0.22 ± 0.15 | 2/21 (10) | 5/21 (24) |
| LDA | 2HRZ/2HR | 0.36 ± 0.25 | 2/21 (10) | 0.10 ± 0.10 | 1/21 (5) | 2/21 (10) |
Bacterial numbers are presented as the log10 CFU with standard errors of the means. Drug treatments included 2 months of MXF, RIF, and PZA followed by 2 months of MXF and RIF (2MRZ/2MR), 2 months of INH, RIF, and PZA followed by 2 to 4 months of INH and RIF (2HRZ/2HR), etc. Relapse rates include all lung and spleens positive for bacilli. N, total number of mice/group; n, number of mice with detectable CFU.
FIG. 2.
Comparison of HDA and LDA infection models with i.v. infection in BALB/c mice treated with different drug combination regimens. (A, C, and E) Bacterial numbers in lungs of BALB/c mice after HDA (A), i.v. (C), or LDA (E) infection with M. tuberculosis and 1, 2, 3, 4, 5, or 6 months of drug treatment. (B, D, and F) Bacterial numbers in spleens after HDA (B), i.v. (D), or LDA (F) infection and 1, 2, 3, 4, 5, or 6 months of drug treatment. Drug treatment regimens included either 2 months of INH, PZA, and RIF followed by INH and RIF (▪); 2 months of MXF, PZA, and RIF followed by MXF and RIF (▴); or 6 months of PZA and RIF (○).
After 2 months of treatment in lungs, similar reductions in bacterial loads of the three drug regimens were observed for all three infection models in the BALB/c mice (Fig. 2A, C, and E). For all three models, the RZ treatment was significantly less effective than MRZ or HRZ (P < 0.001), and the efficacies for both the MRZ and HRZ drug regimens were not significantly different (HDA, P = 0.48; i.v., P = 0.51; LDA, P = 1.0). Only in the LDA group did the MRZ treatment group approach statistical significance for improved activity over the standard drug regimen at 1 month, but this was statistically not different from the standard regimen (P = 0.06). Resistance to RIF of the remaining colonies was tested after 4 months of RZ treatment in lungs, and all remaining colonies were found to be susceptible to 4 μg/ml RIF. Of note, we need to mention here that the organ homogenates were frozen, and upon thawing for resistance plating a certain loss of bacterial viability was observed that might have affected our ability to grow all resistant colonies. After 4 months of treatment, mice treated with either MRZ or HRZ showed no detectable CFU in lungs. In contrast, mice treated with RZ showed significantly higher bacterial loads in lungs (P < 0.001) and after 4 months of treatment there were still culturable bacteria in lungs. Based on a Wilcoxon rank sum comparison, the number of mice carrying bacteria was significantly higher for the HDA (P = 0.003) and i.v.-infected animals (P < 0.001), but not in the LDA animals (P = 0.3). Even after 6 months of therapy with RZ in the HDA group and the i.v.-infected group, bacteria could be detected.
In spleens a similar trend was seen, although it was less pronounced than in lungs. After 2 months of treatment, in the HDA and the i.v. infection models the RZ treatment was again significantly less effective than the MRZ and HRZ treatment regimens (P < 0.0001), and both MRZ and HRZ were as effective in spleens (P = 0.36 for HDA and P = 0.60 for i.v.) (Fig. 2B, D, and F). In the LDA infection model, after 2 months of treatment, for the HRZ and MRZ treatments the mice were all culture negative, and six out of seven mice of the RZ group showed bacterial growth in the spleens. Due to some culture negativity, the treatment groups were compared using the Wilcoxon rank sum test (P < 0.0004). After 4 months of treatment, the mice from the MRZ and HRZ groups did not show any detectable bacterial growth in spleens, and no additional activity of RZ was seen between 4 and 6 months. At 4 months, mice treated with RZ still had detectable CFU in spleens in mice of the HDA and i.v. infection model groups, but no bacterial growth was found in the spleens of mice in the LDA infection group (P < 0.0001). After 6 months of therapy with RZ, the bacterial loads in the spleens from the HDA and i.v. infection groups remained the same as after 2 or 4 months of RZ treatment (3.11 and 3.67 log10 CFU, respectively). From the RZ group frozen residual homogenates were plated for RIF resistance determinance, and only one mouse was found to have 30 RIF-resistant colonies in the spleen in the i.v.-infected group. Since the organ homogenates were frozen and thawed, it is likely that the number of resistant colonies might have been higher, but we were not able to grow resistant colonies on agar plates.
Relapse rates were studied for all treatment groups 3 months after cessation of treatment (Table 2). When the relapse rates were compared for the two drug treatments in every infection model tested, there was no significant difference within each model. Relapse rates for 2 months of INH, RIF, and PZA followed by 4 months of INH and RIF (2HRZ/4HR) were similar to the 2MRZ/2MR groups (HDA, P = 0.32; i.v., P = 0.75), and for LDA the relapse rate of 2HRZ/2HR was similar to that of 2MRZ/1MR (P = 1.0). When the relapse rates were compared between the tested infection models for corresponding drug treatment regimens, the i.v. model showed a significantly higher relapse rate for both the MRZ and HRZ regimens than for either aerosol model (P < 0.0001) (Table 2). Comparison of relapse rates by using Fisher's exact test for data from the lungs and spleens revealed that in lungs of i.v.-infected animals, there were significantly more relapses for 2HRZ/3HR than in the 2MRZ/2MR group (P = 0.00042). In the spleens of the i.v.-infected animals, there was again a significantly greater relapse seen for 2HRZ/4HR than for 2MRZ/2MR (P = 0.04). For the HDA and LDA infection groups, the 2HRZ/3HR treatment groups showed a statistically similar relapse rate as 2MRZ/2MR for both lungs and spleens (P > 0.05).
A different dosing schedule of rifampin in combination with other drugs does not affect treatment efficacy in BALB/c mice infected with M. tuberculosis by HDA infection.
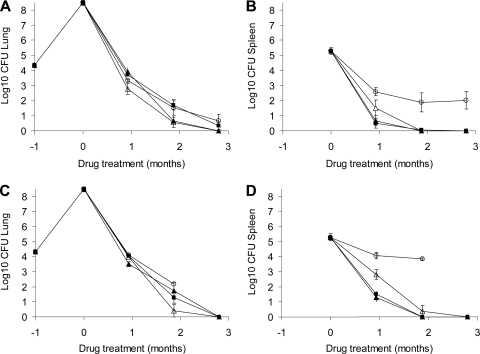
The goal of the HDA experiment was to determine the effects of RIF formulation and dosing schedules of drugs in combination treatment regimens on outcomes of TB infection in BALB/c mice. Two different RIF formulations with an alternative schedule of drug delivery were used. In the first method RIF was dissolved in water and dosed 4 h before other drugs, and in the second method RIF was administered in 5% DMSO 1 h after combinations of INH or MXF with PZA. PZA was discontinued in all groups after 2 months of therapy, except for the RZ group. Infection was initiated in two runs, and mice were randomly allocated. There were six animals assigned to each treatment group, and treatment began 14 days post-HDA infection. Results are shown in Table 1 and Fig. 3. Due to the high mortality in the RZ arm when we used the second formulation, there were no mice available for the last time point (3 months after treatment). We believe the mortality was caused by TB infection, based on clinical observations. No other treatment groups had any mortality.
FIG. 3.
HDA infection in BALB/c mice treated with different drug combination regimens, using two different RIF formulations and two dosing schedules of the drug combinations. (A and B) Bacterial numbers in lungs (A) or spleens (B) of BALB/c mice infected via HDA and treated with RIF dissolved in water and delivered 4 h before other drugs. (C and D) Bacterial numbers in lungs (C) or spleens (D) of BALB/c mice infected via HDA and treated with RIF dissolved in 5% DMSO and delivered 1 h after other drugs. The following drug treatments were administered: 2 months of MXF, RIF, and PZA followed by 1 month of MXF and RIF (▵); 2 months of INH, RIF, MXF, and PZA followed by 1 month of INH, RIF, and MXF (▴); 2 months of INH, RIF, and PZA followed by 1 month of RIF and INH (▪); 3 months of RIF and PZA (○).
After the first month of treatment in which we compared the two formulation methods to each other, the bacterial loads in lungs of mice treated with drugs using method one showed significantly lower numbers for all treatment regimens compared to mice treated with the method two (across all comparisons, P = 0.001 [two-way ANOVA]) (Fig. 3A and C). The lung bacterial numbers in treatment groups using formulation method one were significantly different from each other (Fig. 3A), except for HRZ being similar to RZ (P = 0.13) and HRZ being similar to HMRZ as well (P = 0.48). Lung bacterial numbers in all treatment groups using formulation method two were mostly not significantly different from each other (P ≥ 0.05) (Fig. 3C), except HMRZ was superior to HRZ and RZ. RIF resistance in frozen homogenates was not detected in RZ groups by either formulation. After 2 months of treatment, bacterial numbers in the lungs of all treatment groups were not significantly different (P = 0.59, two-way ANOVA). By 3 months, the CFU burdens in the lungs of mice treated with formulation one were low and statistically similar (P > 0.05). At 3 months after treatment using formulation two, there were no mice left in the parallel RZ treatment group for enumeration of bacilli, and the other treatment arms had reached culture negativity.
After the first month of treatment, the bacterial numbers in spleens in all treatment groups in the formulation method one group were similar to those of the method two group for all treatment regimens (P = 0.55, two-way ANOVA) (Fig. 3B and D). For formulation two, MRZ was significantly less effective than HMRZ (P = 0.002) or HRZ (P = 0.007). The bacterial loads were higher for the RZ group than for any of the other drug combination groups. After 2 months of treatment, a similar result was observed. HRZ, HMRZ, and MRZ were all significantly more effective than RZ (P < 0.001) but were statistically similar to one another (P > 0.3). After 3 months of treatment, for all drug regimens except RZ, the CFU burden was very low and not significantly different between treatment groups (P = 1). The RZ arm with formulation method one revealed a significantly inferior performance than with any other arms (P < 0.001). Due to early mortality, there were no mice left in the parallel RZ treatment group (formulation method two) at 3 months for enumeration of bacilli. There was no RIF resistance detected after plating the lungs and spleens on RIF-containing plates to account for poor bactericidal activity of the RZ regimen; however, drug-free plates at the time of rifampin plating were not used in this study.
No significant difference was found between pharmacokinetic parameters when we used different RIF dosing schedules in BALB/c mice.
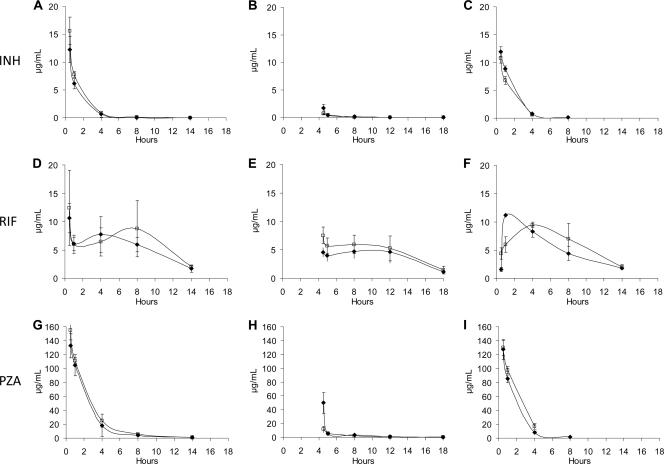
Analysis of drug concentrations was performed on plasma samples of mice to assess pharmacokinetics after single-dose drug exposures to INH, RIF, and PZA, using the same formulation methods as described above for the efficacy studies. Pharmacokinetic parameters of INH, PZA, and RIF were measured after a single dose of RIF was given either 4 h before (in water) or 1 h after (in DMSO) the two companion drugs, INH and PZA. The results showed that, overall, the concentrations (in μg/ml) of all three drugs, INH, RIF, and PZA, over time were largely similar for both RIF formulation methods (Fig. 4).
FIG. 4.
Single-dose pharmacokinetic plasma drug levels of standard TB drugs in BALB/c mice when the drugs were administered as drug combinations. (A) INH drug levels over time when administered with PZA 4 h after RIF in DMSO (⧫) or water (□). (B) INH concentrations when administered with PZA 4 h before RIF was given in DMSO (⧫) or water (□). The data in the graphs are shown from the time of RIF administration. (C) INH concentrations when INH was given alone (⧫) or in combination with PZA (□) without RIF. (D) RIF concentrations after PZA and INH, RIF in DMSO (⧫), or water (□). (E) RIF concentrations when RIF in DMSO (⧫) or water (□) was given 4 h before PZA and INH. (F) RIF plasma levels when given alone in water (□) or DMSO (⧫). (G) PZA concentrations when RIF was administered 4 h before PZA and INH, with RIF dissolved in DMSO (⧫) or water (□). (H) PZA concentrations when PZA and INH were given 4 h prior to RIF dissolved in DMSO (⧫) or water (□). (I) PZA plasma concentrations when PZA was given alone (⧫) or in combination with INH (□). Doses: INH, 25 mg/kg; RIF, 10 mg/kg; PZA, 150 mg/kg (as a single dose by oral gavage). All time points refer to the time after RIF administration.
INH concentrations over time were similar when administered 4 h after RIF delivered in water or DMSO (Fig. 4A). When INH was administered 4 h before RIF, the INH levels were, as expected, very low at the 4-h time point, due to the short half-life of INH (Fig. 4B). The low INH drug levels did not allow us to make direct statistical comparisons between the two formulations. INH drug concentrations were similar whether the drug was administered as a single agent or combined with PZA in water (Fig. 4C). PZA groups demonstrated similar drug concentrations over time when administered after RIF (Fig. 4G) when RIF was administered in either water or DMSO. When PZA was administered 4 h before RIF, the plasma levels of PZA were, as expected, very low at the 4-h time point, due to the short half-life of PZA (Fig. 4H). The low PZA drug levels did not allow us to make direct statistical comparisons between the two formulations. PZA levels did not differ over time when administered alone or combined with INH (Fig. 4I).
The median AUC0-∞ values for single drugs were determined in order to compare the drug combinations when administered in water versus DMSO. The AUC0-∞ for RIF was slightly higher in water versus DMSO, 97.46 and 86.02 μg·h/ml, respectively (+13%). After a single dose of INH administered with RIF in water compared to RIF in DMSO, the AUC0-∞ of INH was 24.41 and 19.34 μg·h/ml, respectively (+26%). Lastly, the AUC of PZA was 433.95 and 419.38 μg·h/ml when PZA was given with RIF in water versus DMSO, respectively (+3%). When administered as a single agent, RIF demonstrated a median Cmax_D (the concentration maximum, normalized to dose) of 47.83 and 60.83 μg/ml in water versus DMSO, respectively (−21%). When RIF was administered in combination with INH and PZA, the Cmax_D of RIF was 39.14 and 32.93 μg/ml in water versus DMSO, respectively (+19%).
DISCUSSION
In preclinical testing of experimental compounds against tuberculosis, various mouse models have been used by different investigators in the field. This study is the first, to our knowledge, to evaluate the most widely used mouse infection models side by side in one laboratory. The rationale of this work was to understand the most crucial parameters of the mouse infection models that may change the outcomes of drug efficacy trials in TB drug development.
Different drug combination treatment regimens were evaluated in three different mouse infection models: the LDA, HDA, and i.v. infection mouse models, using BALB/c mice. An initial experiment indicated that the treatment efficacy of the standard drug regimen, HRZ administered over 4 months, was equivalent for BALB/c and C57BL/6 mice infected via LDA. Since most laboratories use BALB/c mice for TB drug evaluations, we opted for this mouse strain for all further comparative studies. The experiments aimed to achieve the same bacterial loads in the lungs at the start of treatment and mimic the protocols used by other investigators in the field employing HDA or i.v. infection models. The results of the mouse studies showed that the killing kinetics of the drug regimens in lungs were significantly slower for the i.v.-infected versus aerosol-infected animals in lungs as well as in spleens. The reason for this is not entirely clear but might be explained by the state of the bacilli (the proportion of intra- versus extracellular bacilli) as well as the growth rates of the bacilli being possibly slower in the i.v. model. In addition, the i.v. route of infection induces an altered state of lung immunity, compared to the direct deposition of the bacteria in the lungs by the other routes (19), which might alter the responsiveness of the bacteria to treatment.
The efficacy results of the three infection models showed that the MXF-containing regimen (MRZ/MR) reduced the bacterial load in a statistically similar way as the standard regimen (HRZ/HR), until no detectable bacilli could be determined in lungs and in spleens. Therefore, all three models showed a similar outcome based on drug combination comparisons regardless of the mouse model used. In all three models, the RZ combination was far less efficacious than any of the three-drug combinations. Drug resistance was likely not an issue, as no RIF-resistant colonies were obtained after plating on 4 μg/ml of RIF, although isolates having a lower level of resistance could have been missed. The killing kinetics for RZ also differed between lungs and spleens. In the lungs a steady reduction in bacterial numbers was generally seen in the aerosol infection models, while in spleens after 1 month of treatment the RZ activity was significantly reduced in the HDA model. Whether this was caused by a difference in replication rates of the organism in the different organs, the distribution of the bacteria in the host, or lower drug penetration into the different organs is not clear at this time.
Another measure of treatment efficacy, namely, relapse of infection rate, was evaluated 3 months after cessation of drug treatment in the different mouse infection models. The 2MRZ/2MR-treated group showed statistically similar relapse rates as the 2HRZ/4HR group in the HDA and i.v. infection models. In fact, the relapse studies revealed that for the i.v. infection model, the standard drug regimen (HRZ) showed a significantly higher number of mice relapsing after 5 months of treatment than the MXF-containing regimen (MRZ) after 4 months of treatment. This result shows the superior sterilizing activity of the moxifloxacin-containing regimen over the standard regimen in the i.v. infection model. In the HDA model, a similar trend was observed between the 5-month standard regimen and the 4-month treatment with the moxifloxacin-containing regimen, although this result was not statistically significant. In the LDA model, virtually no relapse was observed after 2MRZ/1MR treatment, while there was a 10% relapse seen after the 2HRZ/2HR treatment. Although the relapse rates for the mice in the LDA group were very low, resulting in an insufficient statistical power to allow a statement of the significance, this was a meaningful result, as it showed a trend. We published previously the statistical limitations of relapse studies, pointing out that small studies can still be useful as “screening” experiments (25). In that earlier study, we calculated the need for obtaining a difference in relapse rates of a >40 to 50% difference between the comparison groups in order to achieve a power of 0.8 when using 20 mice per group (25). In these studies only a difference of 40 to 50% in relapse rates was observed for the i.v. infection group, and therefore a strong statistical statement can be made only for this infection group, whereas for the aerosol infection groups only a trend of relapse rates can be shown. Nevertheless, for all three infection models in BALB/c mice, a similar trend was seen regarding the relapse of infection in the MXF-containing regimen versus the standard drug regimen, regardless of the mouse infection model used.
In contrast to observing similar efficacies of MRZ and HRZ over the initial months of treatment as described above, the relapse rates for the MXF-containing regimen (2MRZ/2MR) were significantly lower for the i.v. infection group than for the standard drug regimen (2HRZ/3HR). And, in the aerosol infection models, a similar trend was observed. These results show the importance of such relapse studies, versus only evaluating the bactericidal efficacies of drugs in mouse models during treatment in order to select the drug regimen that can lead to a cure. That bactericidal activity during treatment does not always provide a good correlation with relapse data has been seen before in a mouse study at John Hopkins University (38). Of importance, a recent paper by Koen Andries et al. demonstrated that bactericidal potencies of new TB drug regimens do not always predict relapse potential (2). In that study, the investigators rank ordered the bactericidal and sterilizing potencies of several regimens and found that drug regimens with very good bactericidal properties did not necessarily have good sterilizing properties. For instance, treatment with a three-drug regimen (TMC207, PZA, and MXF) for 4 weeks resulted in culture conversion, but 5 months of treatment with the same regimen was needed to achieve acceptable relapse rates. In contrast, treatment with the regimen of TMC207, PZA, and rifapentine (RPT) for 4 weeks did not result in complete culture conversion, but only 3 months of treatment was needed to achieve an acceptable relapse rate. In these studies, it was clear that MXF was more bactericidal than RPT but that RPT was far more sterilizing (2). The results presented provided additional credence to the inclusion of relapse studies as part of the preclinical evaluation in order to assess the true sterilizing potential of a new regimen. Ultimately, the clinical trials conducted with these regimens will provide definite answers to the question of the predictive value of bactericidal versus sterilizing activities seen in animal models, and they are required for further validation of the animal model data.
The i.v. infection model showed a significantly greater rate of relapse than either aerosol infection model. After an i.v. infection, bacteria are primarily retained in the liver (90%) and spleen (10%), and only 1% will implant into the lungs (36, 43). We found in our first experiment, when we evaluated the two mouse strains, most bacilli were found in the livers (99%), compared to 0.3% in lungs and 0.4% in the spleens. Therefore, the difference in relapse could be caused by a difference in immune response generated between the i.v. and aerosol infection models from the start of infection. Host immune responses, such as innate, adaptive, and memory immune responses, including T regulatory cell populations, have recently been found to account for differences in outcomes of animal TB infection studies and could be at play in the different models (19, 42).
When comparing our data with earlier published data, our i.v. infection groups showed a higher rate of relapse in the HRZ group than results reported in a recently published study (55% versus 17%, respectively) (21), while for the HDA-infected groups relapse was very similar to studies described in the literature (5% versus 0%, respectively) (41). For the i.v. infection models, our studies also revealed higher relapse rates than in historical trials that were performed in Paris at either Institut Pasteur in the 1950s, 1960s, and 1970s (13-15), in the Pitié-Salpêtrière School of Medicine from the 1980s to the present (11, 24), or those performed at Cornell University (30-33). These important historic studies, which reflect the human clinical relapse rate, often used outbred Swiss mice, which may have resulted in different outcomes and could explain these results. A more likely explanation is that our studies may have resulted in a higher CFU burden at the start of therapy. In general, aerosol infection models are more expensive, due to the necessity to purchase and maintain a specialized aerosol apparatus; however, this model may be more relevant to human infection with TB, which is acquired by direct implantation into the alveoli. The human infectious inoculum is more closely recapitulated by the LDA rather than the HDA model. The LDA model never achieves a bacterial load greater than 6 to 6.5 log10 CFU in immunocompetent animals and therefore has a smaller window in which one can assess bactericidal activity compared to the HDA and the i.v. models, and thus the treatment periods chosen should be carefully assessed and shortened. On the other hand, the HDA model achieves a bacterial burden of 7 to 8 log10 CFU, thereby simulating a human cavitary lesion. However, in humans there are usually only one or a few cavitary lesions, while in a mouse with an equivalently high burden, there will be hundreds of lesions. Due to the high bacterial burden at the start of treatment in the HDA model, it is possible to determine the resistance frequency of a drug or drug combination.
In the studies presented here, the combination of HRZ was in all three infection mouse models significantly more effective than RZ dual therapy. In none of the three infection models was an antagonism of H in the HRZ combination observed. Antagonism between the three standard drugs has been shown by others (10), where it was demonstrated that the dual regimen of RZ after removal of INH performed better than the standard three-drug regimen HRZ. However, this antagonism was not always seen to the same extent by the same investigators, and the antagonism was recently suggested to be dependent on the INH concentration (especially at 25 mg/kg) (1). In the studies described here as well as in studies reported by others, INH is administered at 25 mg/kg, and in our case antagonism was never observed. In one other historic study in mice, PZA actually antagonized the bactericidal effect of INH when treatment with both agents was started within 20 min of infection, and this effect diminished entirely when treatment of an established infection was evaluated (30). Certain conditions appear to influence standard TB drug therapy, such as sequential administration of INH followed by PZA, showing different efficacies than when INH and PZA are simultaneously administered (34). In the studies presented here, we also found some variations between experiments, with HRZ being far more effective than RZ in the initial experiment, while this difference was less pronounced in a second experiment. However, true antagonism between the standard drugs was never observed, and HRZ always showed equivalent or better activity than RZ. Similar to our results, other investigators using models of in vitro (23) and in vivo combination drug efficacy experiments have failed to find antagonism (9). Others have also described better or equivalent activity of HRZ versus RZ (20). In the reports by Ibrahim et al., no antagonism was found; in fact, HRZ was found to decrease the bacterial burden in lungs by 1.5 log10 CFU greater than RZ in their model after 1 month of treatment (20). In addition, results in their laboratory showed the combination of MRZ to be as effective as HRZ and equivalent to RZ at 2 months of treatment. The same authors also found that an MRZ regimen for 4 months resulted in a relapse rate that was not significantly different from that observed after the HRZ regimen for 6 months (21). Their mouse model uses the M. tuberculosis H37Rv strain, Swiss mice, and a high-dose i.v. infection model. Grosset, Nuermberger, and colleagues observed antagonism in HRZ that resulted in less activity than RZ with the M. tuberculosis H37Rv strain, BALB/c mice, a high-dose aerosol infection model, and dosing of RIF at least 1 h ahead of HZ (12, 39). As highlighted by Nuermberger in a recent publication, until the specific conditions of antagonism of standard drugs in certain laboratories are fully delineated, it is recommended that future studies of drug combinations include appropriate control arms in long-term trials (37). We absolutely agree with this statement and strongly urge investigators new to the field that they should, for every study that substitutes a single agent in the standard HRZ regimen, include both an HRZ and an RZ treatment arm in their mouse model.
The significance of the antagonism between the drugs in the HRZ regimen is most apparent when comparing a new drug regimen to the standard drug regimen prior to conducting clinical trials. With an observed antagonism between the three drugs of the standard regimen, one might quickly interpret data for a new drug regimen as being superior to standard therapy. Recent mouse trials with MXF substituted for INH showed significant improvement in efficacy over standard therapy (HRZ), and most of this benefit was attributed to removal of the antagonism between INH with RIF plus PZA (37, 40, 41). The results of our mouse studies showed a superior activity with the substitution of MXF for INH in the standard regimen, based on the relapse studies, while no advantage was seen for the bactericidal activity during treatment. The results of our mouse studies support the findings of human TBTC Study 28, in which no significant difference was seen in bactericidal activity when MXF was substituted for INH in a daily regimen for 8 weeks (11). This leads to the question of whether the 8-week sputum conversion results in patients will be predictive for the sterilizing activity of a regimen measured by relapse after 2 years. Sputum samples contain only one compartment of the bacterial population, whereas the bacteria in the cavitary lesions within the lungs might have a completely different phenotype and environment, which will affect their drug responsiveness. The mouse studies predict that MXF might only show significant benefit in later stages of a clinical trial. The long-time follow-up in the ReMox TB clinical trial might bring the definite answer to these questions, and this trial is under way (www.tballiance.org).
In the past, the dosing schedule of RIF and formulations of drugs in the standard drug regimen for tuberculosis have been described to have a significant effect on the pharmacokinetics in mice (12). In the studies described here, we evaluated the pharmacokinetics of the individual drugs of the standard regimen in different dosing schedules and formulations of RIF when combined with INH and PZA. Besides the pharmacokinetics, we also studied the effects of RIF formulation and dosing schedule on the efficacy of the drug combination by using the HDA model in BALB/c mice. The different formulations and dosing schedule of RIF with other drugs in combination gave similar efficacy results in lungs and spleens. Interestingly, in the mice treated with RZ using formulation method two, a higher mortality was observed than in mice treated with RZ in formulation one. The combination of RZ has been shown to have significant adverse effects in mice (29) and has led to mortality in TB patients (3). The reason for the mortality with only one formulation in our studies reported here is unclear at this time. There were some differences seen in the kinetics of the concentration-time curves of RIF that depended on whether the drug was ground in a mortar in water or prepared as a DMSO solution, but overall drug exposures of RIF were not different. In this study, we did not address the situation when RIF was given simultaneously with INH and PZA, as this was beyond the scope of our studies.
One last variable not studied in this work is the bacterial strain used for the infection of mice. With the results obtained here, the study of different bacterial strains (laboratory and clinical M. tuberculosis strains) is the current focus of our laboratory for extensive in vitro and in vivo drug combination evaluations. We also realize that the studies described here only evaluated the standard drug combination and MXF-containing drug combinations, and therefore more combination regimens should be tested in the future in the different infection models. In summary, our studies demonstrate that the evaluation of TB drug regimens in mouse M. tuberculosis infection models do not differ between the two mouse strains, by the inoculum size or the route of infection used, for drug efficacy as well as for relapse of infection. In addition, the organ CFU counts of the standard drug regimen were not affected by a difference in formulation or dosing schedule of RIF in combination with INH and PZA. Significant differences in results of long-term efficacy mouse studies are, however, observed between various laboratories, such as is seen with the phenomenon of antagonism between the standard drugs INH, RIF, and PZA. Given that many variables are present in animal studies for drug development, we therefore recommend that preclinical animal studies not be standardized but that critical studies be confirmed in a second laboratory using a different strain of M. tuberculosis and a different animal model, and that such studies include the appropriate treatment control groups, prior to moving to expensive human trials.
Acknowledgments
We thank Phillip Chapman (Statistics Department, Colorado State University) for statistical assistance, Jena Valdez, Courtney Hastings, Amanda Marsh, and Jordan Clark for technical assistance, and the Laboratory Animal Resources staff for assistance with care and analysis of the animals. We give a special thanks to the Centers for Disease Control and Prevention Tuberculosis Laboratory for sequencing the pncA gene product and to Leonid Heifets and the staff of the Mycobacteriology Laboratory at the National Jewish Health, Denver, CO, for the MIC testing of our M. tuberculosis strain.
This project was supported by the Bill and Melinda Gates Foundation under Drug Accelerator grant ID number 42589, “Assay Standardization.”
Footnotes
Published ahead of print on 6 December 2010.
REFERENCES
- 1.Almeida, D., et al. 2009. Paradoxical effect of isoniazid on the activity of rifampin-pyrazinamide combination in a mouse model of tuberculosis. Antimicrob. Agents Chemother. 53:4178-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andries, K., T. Gevers, and N. Lounis. 2010. Bactericidal potencies of new regimens are not predictive of their sterilizing potencies in a murine model of tuberculosis. Antimicrob. Agents Chemother. 54:4540-4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anonymous. 2003. Update: adverse event data and revised American Thoracic Society/CDC recommendations against the use of rifampin and pyrazinamide for treatment of latent tuberculosis infection—United States, 2003. MMWR Morb. Mortal. Wkly. Rep. 52:735-739. [PubMed] [Google Scholar]
- 4.Barry, P. J., and T. M. O'Connor. 2007. Novel agents in the management of Mycobacterium tuberculosis disease. Curr. Med. Chem. 14:2000-2008. [DOI] [PubMed] [Google Scholar]
- 5.Bartek, I. L., et al. 2009. The DosR regulon of M. tuberculosis and antibacterial tolerance. Tuberculosis (Edinb.) 89:310-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Botha, T., and B. Ryffel. 2002. Reactivation of latent tuberculosis by an inhibitor of inducible nitric oxide synthase in an aerosol murine model. Immunology 107:350-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cynamon, M. H., and M. Sklaney. 2003. Gatifloxacin and ethionamide as the foundation for therapy of tuberculosis. Antimicrob. Agents Chemother. 47:2442-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies, G. R., and E. L. Nuermberger. 2008. Pharmacokinetics and pharmacodynamics in the development of anti-tuberculosis drugs. Tuberculosis (Edinb.) 88(Suppl. 1):S65-S74. [DOI] [PubMed] [Google Scholar]
- 9.Dhillon, J., J. M. Dickinson, K. Sole, and D. A. Mitchison. 1996. Preventive chemotherapy of tuberculosis in Cornell model mice with combinations of rifampin, isoniazid, and pyrazinamide. Antimicrob. Agents Chemother. 40:552-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dickinson, J. M., V. R. Aber, and D. A. Mitchison. 1977. Bactericidal activity of streptomycin, isoniazid, rifampin, ethambutol, and pyrazinamide alone and in combination against Mycobacterium tuberculosis. Am. Rev. Respir. Dis. 116:627-635. [DOI] [PubMed] [Google Scholar]
- 11.Dorman, S. E., et al. 2009. Substitution of moxifloxacin for isoniazid during intensive phase treatment of pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 180:273-280. [DOI] [PubMed] [Google Scholar]
- 12.Grosset, J., C. Truffot-Pernot, C. Lacroix, and B. Ji. 1992. Antagonism between isoniazid and the combination pyrazinamide-rifampin against tuberculosis infection in mice. Antimicrob. Agents Chemother. 36:548-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grumbach, F. 1969. Experimental “in vivo” studies of new antituberculosis drugs: capreomycin, ethambutol, rifampicin. Tubercle 50(Suppl.):12-21. [PubMed] [Google Scholar]
- 14.Grumbach, F. 1965. Experimental basis for the chemotherapy of tuberculosis. Antimicrob. Agents Chemother. 5:1058-1064. [PubMed] [Google Scholar]
- 15.Grumbach, F., G. Canetti, and J. Grosset. 1964. Further experiments on long-term chemotherapy of advanced murine tuberculosis, with emphasis on intermittent regimes. Tubercle 45:125-135. [DOI] [PubMed] [Google Scholar]
- 16.Gruppo, V., et al. 2006. Rapid microbiologic and pharmacologic evaluation of experimental compounds against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 50:1245-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heifets, L. 2002. Susceptibility testing of Mycobacterium tuberculosis to pyrazinamide. J. Med. Microbiol. 51:11-12. [DOI] [PubMed] [Google Scholar]
- 18.Heifets, L., M. Iseman, and P. Lindholm-Levy. 1988. Application of rapid methods (BACTEC system) in clinical mycobacteriology and in the search for new drugs. Bull. Int. Union Tuber. Lung Dis. 63:19. [PubMed] [Google Scholar]
- 19.Henao-Tamayo, M. I., et al. 2010. Phenotypic definition of effector and memory T-lymphocyte subsets in mice chronically infected with Mycobacterium tuberculosis. Clin. Vaccine Immunol. 17:618-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibrahim, M., et al. 2007. Synergistic activity of R207910 combined with pyrazinamide against murine tuberculosis. Antimicrob. Agents Chemother. 51:1011-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ibrahim, M., C. Truffot-Pernot, K. Andries, V. Jarlier, and N. Veziris. 2009. Sterilizing activity of R207910 (TMC207)-containing regimens in the murine model of tuberculosis. Am. J. Respir. Crit. Care Med. 180:553-557. [DOI] [PubMed] [Google Scholar]
- 22.Kelly, B. P., S. K. Furney, M. T. Jessen, and I. M. Orme. 1996. Low-dose aerosol infection model for testing drugs for efficacy against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 40:2809-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kubendiran, G., C. N. Paramasivan, S. Sulochana, and D. A. Mitchison. 2006. Moxifloxacin and gatifloxacin in an acid model of persistent Mycobacterium tuberculosis. J. Chemother. 18:617-623. [DOI] [PubMed] [Google Scholar]
- 24.Lecoeur, H. F., P. H. Lagrange, C. Truffot-Pernot, M. Gheorghiu, and J. Grosset. 1989. Relapses after stopping chemotherapy for experimental tuberculosis in genetically resistant and susceptible strains of mice. Clin. Exp. Immunol. 76:458-462. [PMC free article] [PubMed] [Google Scholar]
- 25.Lenaerts, A. J., P. L. Chapman, and I. M. Orme. 2004. Statistical limitations to the Cornell model of latent tuberculosis infection for the study of relapse rates. Tuberculosis (Edinb.) 84:361-364. [DOI] [PubMed] [Google Scholar]
- 26.Lenaerts, A. J., M. A. Degroote, and I. M. Orme. 2008. Preclinical testing of new drugs for tuberculosis: current challenges. Trends Microbiol. 16:48-54. [DOI] [PubMed] [Google Scholar]
- 27.Lenaerts, A. J., V. Gruppo, J. V. Brooks, and I. M. Orme. 2003. Rapid in vivo screening of experimental drugs for tuberculosis using gamma interferon gene-disrupted mice. Antimicrob. Agents Chemother. 47:783-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lenaerts, A. J., et al. 2005. Preclinical testing of the nitroimidazopyran PA-824 for activity against Mycobacterium tuberculosis in a series of in vitro and in vivo models. Antimicrob. Agents Chemother. 49:2294-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lenaerts, A. J., C. M. Johnson, K. S. Marrieta, V. Gruppo, and I. M. Orme. 2005. Significant increases in the levels of liver enzymes in mice treated with anti-tuberculosis drugs. Int. J. Antimicrob. Agents 26:152-158. [DOI] [PubMed] [Google Scholar]
- 30.McCune, R. M., F. M. Feldmann, H. P. Lambert, and W. McDermott. 1966. Microbial persistence. I. The capacity of tubercle bacilli to survive sterilization in mouse tissues. J. Exp. Med. 123:445-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCune, R. M., F. M. Feldmann, and W. McDermott. 1966. Microbial persistence. II. Characteristics of the sterile state of tubercle bacilli. J. Exp. Med. 123:469-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCune, R. M., Jr., W. McDermott, and R. Tompsett. 1956. The fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. II. The conversion of tuberculous infection to the latent state by the administration of pyrazinamide and a companion drug. J. Exp. Med. 104:763-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCune, R. M., Jr., and R. Tompsett. 1956. Fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. I. The persistence of drug-susceptible tubercle bacilli in the tissues despite prolonged antimicrobial therapy. J. Exp. Med. 104:737-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDermott, W., R. M. McCune, Jr., and R. Tompsett. 1956. Dynamics of antituberculous chemotherapy. Am. Rev. Tuberc. 74:100-108. [DOI] [PubMed] [Google Scholar]
- 35.Nikonenko, B. V., R. Samala, L. Einck, and C. A. Nacy. 2004. Rapid, simple in vivo screen for new drugs active against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 48:4550-4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.North, R. J., and A. A. Izzo. 1993. Mycobacterial virulence. Virulent strains of Mycobacteria tuberculosis have faster in vivo doubling times and are better equipped to resist growth-inhibiting functions of macrophages in the presence and absence of specific immunity. J. Exp. Med. 177:1723-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nuermberger, E. 2008. Using animal models to develop new treatments for tuberculosis. Semin. Respir. Crit. Care Med. 29:542-551. [DOI] [PubMed] [Google Scholar]
- 38.Nuermberger, E., et al. 2006. Combination chemotherapy with the nitroimidazopyran PA-824 and first-line drugs in a murine model of tuberculosis. Antimicrob. Agents Chemother. 50:2621-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nuermberger, E., et al. 2008. Powerful bactericidal and sterilizing activity of a regimen containing PA-824, moxifloxacin, and pyrazinamide in a murine model of tuberculosis. Antimicrob. Agents Chemother. 52:1522-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nuermberger, E. L., et al. 2004. Moxifloxacin-containing regimen greatly reduces time to culture conversion in murine tuberculosis. Am. J. Respir. Crit. Care Med. 169:421-426. [DOI] [PubMed] [Google Scholar]
- 41.Nuermberger, E. L., et al. 2004. Moxifloxacin-containing regimens of reduced duration produce a stable cure in murine tuberculosis. Am. J. Respir. Crit. Care Med. 170:1131-1134. [DOI] [PubMed] [Google Scholar]
- 42.Ordway, D., et al. 2007. The hypervirulent Mycobacterium tuberculosis strain HN878 induces a potent TH1 response followed by rapid down-regulation. J. Immunol. 179:522-531. [DOI] [PubMed] [Google Scholar]
- 43.Orme, I. M., and F. M. Collins. 1983. Protection against Mycobacterium tuberculosis infection by adoptive immunotherapy. Requirement for T cell-deficient recipients. J. Exp. Med. 158:74-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenthal, I. M., M. Zhang, D. Almeida, J. H. Grosset, and E. L. Nuermberger. 2008. Isoniazid or moxifloxacin in rifapentine-based regimens for experimental tuberculosis? Am. J. Respir. Crit. Care Med. 178:989-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenthal, I. M., et al. 2007. Daily dosing of rifapentine cures tuberculosis in three months or less in the murine model. PLoS Med. 4:e344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rullas, J., et al. 2010. Fast standardized therapeutic-efficacy assay for drug discovery against tuberculosis. Antimicrob. Agents Chemother. 54:2262-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tyagi, S., et al. 2005. Bactericidal activity of the nitroimidazopyran PA-824 in a murine model of tuberculosis. Antimicrob. Agents Chemother. 49:2289-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Veziris, N., et al. 2009. A once-weekly R207910-containing regimen exceeds activity of the standard daily regimen in murine tuberculosis. Am. J. Respir. Crit. Care Med. 179:75-79. [DOI] [PubMed] [Google Scholar]
- 49.Veziris, N., N. Lounis, A. Chauffour, C. Truffot-Pernot, and V. Jarlier. 2005. Efficient intermittent rifapentine-moxifloxacin-containing short-course regimen for treatment of tuberculosis in mice. Antimicrob. Agents Chemother. 49:4015-4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Veziris, N., C. Truffot-Pernot, A. Aubry, V. Jarlier, and N. Lounis. 2003. Fluoroquinolone-containing third-line regimen against Mycobacterium tuberculosis in vivo. Antimicrob. Agents Chemother. 47:3117-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams, K. N., et al. 2009. Addition of PNU-100480 to first-line drugs shortens the time needed to cure murine tuberculosis. Am. J. Respir. Crit. Care Med. 180:371-376. [DOI] [PubMed] [Google Scholar]
- 52.Williams, K. N., et al. 2009. Promising antituberculosis activity of the oxazolidinone PNU-100480 relative to that of linezolid in a murine model. Antimicrob. Agents Chemother. 53:1314-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woolhiser, L. K., D. R. Hoff, K. S. Marietta, I. M. Orme, and A. J. Lenaerts. 2009. Testing of experimental compounds in a relapse model of tuberculosis using granulocyte-macrophage colony-stimulating factor gene-disrupted mice. Antimicrob. Agents Chemother. 53:306-308. [DOI] [PMC free article] [PubMed] [Google Scholar]