Abstract
The pharmacokinetics, safety, and tolerability of a single 1-hour, 500-mg intravenous infusion of doripenem were assessed in dialysis-dependent subjects with stage 5 chronic kidney disease undergoing continuous renal replacement therapy (CRRT) via 12-hour continuous venovenous hemofiltration (CVVH) (n = 6) or continuous venovenous hemodiafiltration (CVVHDF) (n = 5). Healthy volunteers were also assessed (n = 12). Concentrations of doripenem and the primary metabolite doripenem-M-1 were measured in plasma and ultrafiltrate or ultrafiltrate/dialysate by a validated liquid chromatography-tandem mass spectrometry method. In dialysis-dependent subjects, levels of systemic exposure to doripenem and doripenem-M-1 were approximately 3- and 5-fold greater, respectively, than those in healthy subjects: for doripenem, 98 μg·h/ml for CVVH and 77 μg·h/ml for CVVHDF versus 32 μg·h/ml for healthy subjects, and for doripenem-M-1, 24 μg·h/ml for CVVH and 22 μg·h/ml for CVVHDF versus 4.7 μg·h/ml for healthy subjects. The mean sieving coefficients and saturation coefficients were >0.67 for both doripenem and doripenem-M-1. During CVVH and CVVHDF, respectively, the percentages of administered doripenem dose removed were 38% and 29%, and clearances of doripenem were 22 and 25 ml/min. Both CVVH and CVVHDF efficiently removed doripenem and doripenem-M-1. Despite significant removal of drug by CVVH and CVVHDF, a single 1-hour, 500-mg doripenem infusion produced significantly higher plasma concentrations of doripenem, higher systemic exposure (area under the plasma concentration-time curve from time zero to 12 h after the start of infusion [AUC0-12]), and longer half-life (t1/2) in subjects receiving CVVH or CVVHDF than in healthy volunteers. The recovery of drug in ultrafiltrate and ultrafiltrate/dialysate and the enhanced rate of reduction of plasma concentrations indicate that CVVH and CVVHDF significantly augmented residual total body clearance of doripenem in subjects receiving CRRT. Doripenem dosage regimens for patients receiving CRRT thus need to be adjusted.
Doripenem is a synthetic, parenteral carbapenem with a broad spectrum of microbiologic activity that has been approved in over 60 countries for the treatment of adults with complicated intraabdominal infection, complicated urinary tract infection, including pyelonephritis, and nosocomial pneumonia, including ventilator-associated pneumonia (24). Since use of doripenem in critically ill patients in the intensive care unit is anticipated, investigations have been conducted to ascertain the pharmacokinetics of doripenem in patients with various degrees of renal function, including those with end-stage renal disease (ESRD) requiring dialysis (6). A critical step in the development process was characterization of the relationship between the pharmacodynamic response to doripenem and the pharmacokinetic characteristics of the drug. These studies revealed that, like other carbapenems, the pharmacokinetic-pharmacodynamic index of doripenem that was most closely associated with efficacy (90 to 99% reduction in bacterial burden) against Gram-negative bacilli was the maintenance of plasma concentrations above the MICs (%TMIC) (23).
Continuous renal replacement therapies (CRRTs), such as continuous venovenous hemofiltration (CVVH) and continuous venovenous hemodiafiltration (CVVHDF), are frequently utilized to manage hemodynamically unstable patients, those who are volume overloaded, and those who have acute kidney insufficiency or acute kidney injury (5, 14, 19). Both of these methodologies have been noted to significantly augment the removal of extracellular fluid and waste products, such as urea and creatinine, in those with impaired as well as normal renal function. The total body clearance (CL) of many medications has also been reported to be enhanced by CVVH and CVVHDF (1, 12, 16, 21). The extent of the clinical impact is primarily dependent on the ultrafiltration rate (QCRRT) and the sieving coefficient (Sc) for patients receiving CVVH and the combined ultrafiltration and dialysate flow rate (QUFD) and the saturation coefficient (Sa) for those receiving CVVHDF (12, 21).
The CRRT clearance during CVVH or CVVHDF (CLCRRT) has been investigated for other carbapenems (imipenem, meropenem, ertapenem, and panipenem), and the clearances range from 3.6 to 49.4 ml/min (4, 9-11, 13, 15, 17, 21, 22). In some cases, the stability of the patients' organ function status and consistency of the delivery of the CRRT were poorly characterized (18). These observations coupled with the broad range of the reported values provided limited confidence for the extrapolation of these findings to doripenem. This study was therefore designed to evaluate the clearance of doripenem and its primary inactive metabolite, doripenem-M-1, while performing controlled CVVH and CVVHDF in dialysis-dependent subjects (DDS). This study enrolled subjects undergoing a stable hemodialysis regimen, because structured clinical studies requiring the application of stable prescribed therapeutic CVVH or CVVHDF regimens may not be clinically justifiable or feasible in critically ill patients.
MATERIALS AND METHODS
This prospective, open-label, single-dose pharmacokinetic study of doripenem was conducted in dialysis-dependent subjects (DDS) with ESRD and healthy adult volunteers from April to July 2008. The study protocol was reviewed and approved by an independent ethics committee. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and in compliance with good clinical practices and all applicable regulatory requirements. All subjects participating in the study provided written informed consent prior to study entry.
Subjects. (i) DDS with ESRD.
Adult subjects with ESRD who had been maintained on a standard hemodialysis regimen for at least 1 month preceding study enrollment were eligible to participate in the study if they were between the ages of 18 and 80 years, had a body mass index (BMI) between 18 kg/m2 and 38 kg/m2 (inclusive), weighed at least 50 kg, had a stable hematocrit of greater than 30% at screening, and had no recent history of vascular access complications. Exclusion criteria included renal transplantation or renal carcinoma within 1 year of screening, a relevant history of clinically significant drug allergy, hypersensitivity, or intolerance to β-lactam antibiotics, a history of hypersensitivity or intolerance to heparin, prostacyclin, or citrate (trisodium citrate or anticoagulant citrate dextrose solution A [ACD-A]), or a recent febrile illness. All DDS were allowed to continue to receive concomitant prescribed medications to treat all underlying disease states.
(ii) Healthy volunteers.
Each healthy subject had normal renal function (estimated creatinine clearance between 70 and 150 ml/min) as calculated by the Cockcroft-Gault formula (8) and was comparable to the DDS, for which they served as a control, with respect to individual age (±20 years) and weight (±30%). These subjects were all judged to be in good health based on a prestudy medical history, physical examination, vital signs, electrocardiogram, and clinical laboratory test results. They were not allowed to use any medication, except acetaminophen (maximum of 3 doses of 500 mg per day, but no more than 3 g per week) and hormonal contraceptive therapy, starting 21 days before and continuing during the study.
Study design.
Subjects who met eligibility criteria were admitted to one of the two clinical research units, Virginia Commonwealth University and Orlando Clinical Research Center, on the evening prior to doripenem administration to confirm their eligibility and verify baseline observations and laboratory measurements which were determined during the screening period. All subjects received doripenem as a single 500-mg, 1-hour intravenous infusion into a forearm vein on the morning of day 1 after an overnight fast. The DDS received doripenem approximately 1 h after the start of their CRRT procedure. Doripenem was infused in the contralateral arm from the dialysis access site or proximal to the CRRT access site when the opposite arm was not possible.
CRRT technique.
DDS were randomly assigned, using computer-based randomization, to receive either CVVH or CVVHDF (6 subjects in each treatment group). The assigned CRRT study procedure was administered on a day the subject was not scheduled to receive the standard hemodialysis treatment. Thus, each 13-h CRRT procedure was performed in addition to the patient's regularly scheduled hemodialysis treatments. Venous access was obtained by cannulation of the patient's hemodialysis arteriovenous fistula or polytetrafluoroethylene graft. The inlet and outlet ports of the filter were connected to the patient and the CRRT apparatus (Prisma; Fresenius Medical Care, New York, NY). This device continuously monitored dialysate, blood, ultrafiltration, and replacement fluid flow rates, as well as arterial and venous pressure, heparin infusion rate, and total net volume removal rate. The same model/brand of hemofilter/dialyzer (polyacrylonitrile AN69 0.9-m2 Prisma M100 dialyzer; Fresenius Medical Care, New York, NY) was used for all CRRT procedures. The CRRT procedure was initiated at a blood flow rate of 125 ml/min. Heparin was infused at a rate of 1,000 units per hour to maintain access and hemofilter patency. A bicarbonate-based dialysate/replacement fluid was utilized in all patients (Prismanate BGK 2/0 dialysate at 5,000 ml plus 12.5 meq calcium chloride plus 7.5 meq potassium chloride). In the CVVH procedure, the ultrafiltration removal rate and replacement solution infusion rates were matched at a target of 2 liters per hour to maintain the patient's fluid status. For CVVHDF, the target ultrafiltrate (UF) removal and dialysate flow rates were each 1 liter per hour. Ultrafiltrate losses were replaced with replacement fluid at a rate of 1 liter per hour.
Pharmacokinetic assessments. (i) DDS with ESRD receiving CRRT.
Serial prefilter and postfilter blood samples (3 ml) were collected in heparinized polypropylene or glass collection tubes by direct venipuncture or via an indwelling catheter from a vein of the opposite arm from which doripenem was administered. No tubes with separation gel were used, and all samples were stored on crushed ice immediately after collection. Samples for the determination of plasma concentrations (CP) of doripenem and doripenem-M-1 were collected during the CRRT treatment at the following time points relative to the start of doripenem infusion: predose and at 0.25, 0.75, 1, 1.25, 1.5, 2, 4, 6, 8, and 12 h. Venous blood samples were collected at 2, 6, 10, and 12 h after the end of the CVVH or CVVHDF procedure. Samples were centrifuged for 10 min at 2,500 to 3,000 rpm to yield at least 1 ml of plasma. The plasma samples were frozen at −70°C or lower until analyzed for doripenem and doripenem-M-1 concentrations.
Serial ultrafiltrate (UF) and ultrafiltrate/dialysate (UFD) samples for the determination of doripenem and doripenem-M-1 concentrations were collected at the following times relative to the start of the doripenem infusion: predose and at 0.25, 0.75, 1, 1.25, 1.5, 2, 4, 6, 8, and 12 h. In addition, the total ultrafiltrate and ultrafiltrate/dialysate fluid was collected during the following intervals: 0 to 4, 4 to 8, and 8 to 12 h. The total volume of fluid collected at each interval was measured, and an aliquot was retained for the determination of doripenem and doripenem-M-1 concentrations. The ultrafiltrate and ultrafiltrate/dialysate samples were frozen at −70°C or lower, where they remained until they were transferred to the bioanalytical facility.
(ii) Healthy volunteers.
All healthy subjects had venous blood samples collected and processed for the determination of plasma concentrations of doripenem and doripenem-M-1, as described above, immediately prior to the start of the doripenem infusion and at the following times after the start of the infusion: 0.25, 0.75, 1, 1.25, 1.5, 2, 4, 6, 8, and 12 h.
Safety assessment.
Safety was assessed throughout the study by evaluating the incidence, severity, onset, resolution, and type of adverse events and their relationship to study drug, as well as changes in clinical laboratory test results, physical examination findings, vital sign measurements, and need for medication or other therapy.
Analytical procedures.
Doripenem and doripenem-M-1 concentrations in plasma (CP), UF (CUF), and UFD (CUFD) were quantified by validated assay procedures using reversed-phase chromatography and detection by tandem mass spectrometry (liquid chromatography-triple quadruple mass spectrometry [LC-MS-MS]).
Plasma.
Doripenem, doripenem-M-1, and the internal standard samples were extracted from plasma (containing sodium heparin) by protein precipitation. The peak areas were quantified using a Perkin-Elmer series 200 liquid chromatograph and an API 5000 series LC-MS-MS instrument (PE Sciex, Foster City, CA) equipped with a Turbo ion spray in the positive ion mode. Calibration curves were obtained by performing a linear regression (weighted 1/x2) on the calibration standards, using Watson 7.0. Peak identification and integration were done using Analyst v.1.4.1. The linear standard curve range was 0.1 to 50 μg/ml, with a lower limit of quantitation of 0.1 μg/ml for both doripenem and doripenem-M-1. The interassay precision (coefficient of variation [CV]) for doripenem and doripenem-M-1 in plasma ranged from 4.2% to 16% and 3.7% to 8.1%, respectively, for quality control samples and 1.5% to 9.1% and 1.3% to 5%, respectively, for calibration standards.
UF and UFD.
Doripenem, doripenem-M-1, and internal standard samples were diluted with acetonitrile. The samples were vortex mixed and then analyzed by reversed-phase high-performance liquid chromatography (HPLC) using an Atlantis C18 column (Waters, Milford, MA) maintained at 30°C for doripenem and 45°C for doripenem-M-1. The peak areas were quantified using a Perkin-Elmer series 200 liquid chromatograph and an API 5000 series LC-MS-MS instrument (PE Sciex, Foster City, CA) equipped with a Turbo ion spray in the positive ion mode. The linear standard curve range was 0.1 to 50 μg/ml, with a lower limit of quantitation of 0.1 μg/ml for both doripenem and doripenem-M-1. The interassay precision (CV) for doripenem and doripenem-M-1 in dialysate ranged from 1.3% to 2.3% and 1.5% to 5.2%, respectively, for quality control samples and 0.7% to 4.6% and 0.8% to 4.2%, respectively, for calibration standards.
Pharmacokinetic analysis. (i) All subjects.
Pharmacokinetic parameters for doripenem and doripenem-M-1 were estimated from the observed individual plasma (prefilter values were used for the subjects receiving CRRT) concentration-time data up to 12 hours after the start of the doripenem infusion, via noncompartmental analysis with validated WinNonlin software version 5.2 (Pharsight Corporation). Plasma concentration values that were below the lower limit of quantification were assigned a value of zero for all pharmacokinetic assessments. The maximum observed plasma concentration (Cmax) and the time the maximum plasma concentration was achieved (Tmax) were determined by inspection of the plasma concentration-time profiles using WinNonlin. The terminal elimination rate constant (λz) was determined by linear least-squares regression analysis of the log-linear concentration-time profile from 1.5 to 12 h for the subjects receiving CRRT or 1.5 to 8 h for the healthy volunteers. The terminal elimination half-life (t1/2) for each subject was then calculated as 0.693/λz. The area under the plasma concentration-time curve from time zero to 12 h after the start of doripenem infusion (AUC0-12) was calculated by linear trapezoidal approximation, and that from time zero to infinity (AUC0-∞) was calculated for the healthy volunteers as the sum of AUC0-12 and Clast/λz, were Clast is the last observed quantifiable concentration. The total body clearance (CL) of doripenem was calculated as dose/AUC0-∞, while the apparent volume of distribution at steady state (Vss) was calculated as MRT × CL, where MRT is mean residence time. The renal clearance (CLR) of doripenem and doripenem-M-1 was calculated as Ae0-12 (the amount of doripenem or doripenem-M-1 excreted into urine over the interval of 0 to 12 h) divided by AUC0-12. The nonrenal clearance (CLNR) of doripenem was calculated as CL − (CLR + CLCRRT) for subjects receiving CRRT and as CL − CLR for the healthy volunteers.
(ii) DDS receiving CRRT.
The sieving coefficient (Sc) during CVVH and the saturation coefficient (Sa) during CVVHDF of doripenem and doripenem-M-1 were calculated as Sc = CUF/CP and Sa = CUFD/CP, where ultrafiltrate concentration (CUF), ultrafiltrate/dialysate concentrations (CUFD), and plasma concentration (CP) were determined from simultaneously collected specimens at 1.25, 1.5, 2, 4, 6, 8, and 12 h after the start of the doripenem infusion. The cumulative amount of doripenem and doripenem-M-1 excreted into UF or UFD (AeCRRT) was calculated as the sum of the UF or UFD volume multiplied by the UF or UFD concentration from each respective collection interval. The CVVH and CVVHDF clearances (CLCRRT) of doripenem and doripenem-M-1 were calculated by multiplying the UF flow rate (QCVVH) or UFD flow rate (QCVVHDF) by the average Sc or Sa, respectively, observed during each interval. QCVVH or QCVVHDF was determined by dividing the volume of UF or UFD, respectively, collected during each timed collection interval by the duration of the collection interval (in hours). The contribution of CLCRRT to the CL of doripenem (%CLCRRT) was calculated as CLCRRT divided by CL. Finally, urea clearance (CLurea) was calculated as QCRRT × [(CUF/UFD)/CPmid], where CUF/UFD is the urea concentration in UF or UFD during the 4- to 8-h collection interval, and CPmid is the urea plasma concentration at the midpoint of the interval.
Statistics.
Based on previous data in healthy subjects, the intersubject coefficient of variation (CV) for AUC and Cmax after a single 500-mg dose of doripenem was estimated to be 30%. Assuming an intersubject CV of 35% for AUC and Cmax of doripenem for dialysis-dependent subjects receiving CRRT, a sample size of 12 subjects was determined to be sufficient to estimate the mean AUC and Cmax of doripenem for that group to fall within 80% and 125% of their true values with 95% confidence.
Plasma concentrations and pharmacokinetic parameters of doripenem and its metabolite doripenem-M-1 were summarized using descriptive statistics and are expressed as means ± standard deviations (SD) unless otherwise indicated.
Comparisons of the effect of CVVH versus that of CVVHDF on doripenem and its metabolite were performed using Student's t test. Analysis of variance (ANOVA) was performed using SAS version 9.2 (SAS Institute, Cary, NC) on all systemic pharmacokinetic parameters for all subjects, and for AUC0-12 and Cmax, the values were natural log transformed before analysis was conducted. Each ANOVA included calculations of the least-squares mean (LSM) for each group and the standard errors associated with the differences between the CVVH, CVVHDF, and healthy-volunteer groups. A P value of 0.05 or less was considered to indicate statistical significance.
RESULTS
A total of 25 subjects were enrolled and completed the study, with 23 subjects included in the pharmacokinetic analyses (11 subjects with ESRD receiving CRRT and 12 healthy volunteers). Detailed information regarding patient demographics is given in Table 1. There were no statistically significant differences in gender, age, weight, or BMI score among the dialysis-dependent subjects who received CVVH versus those who received CVVHDF.
TABLE 1.
Baseline demographic characteristicsa
| Parameter | Result for CRRT subjects |
Result for healthy subjects (n = 12) | |
|---|---|---|---|
| CVVH subjects (n = 6) | CVVHDF subjects (n = 5) | ||
| Age (yr) | 43.5 (6.89) | 43.6 (9.45) | 46.3 (5.14) |
| Weight (kg) | 87.1 (29.5) | 103 (17.4) | 87.6 (9.82) |
| Height (cm) | 174 (11.9) | 178 (4.75) | 174 (8.42) |
| BMI (kg/m2) | 28.3 (6.14) | 33.7 (3.69) | 32.6 (3.64) |
| Gender | |||
| Male | 3 (50) | 5 (100) | 9 (75) |
| Female | 3 (50) | 0 (0) | 3 (25) |
Data are presented as means (standard deviations), except for gender data, which indicate numbers (percentages) of patients.
Doripenem pharmacokinetics.
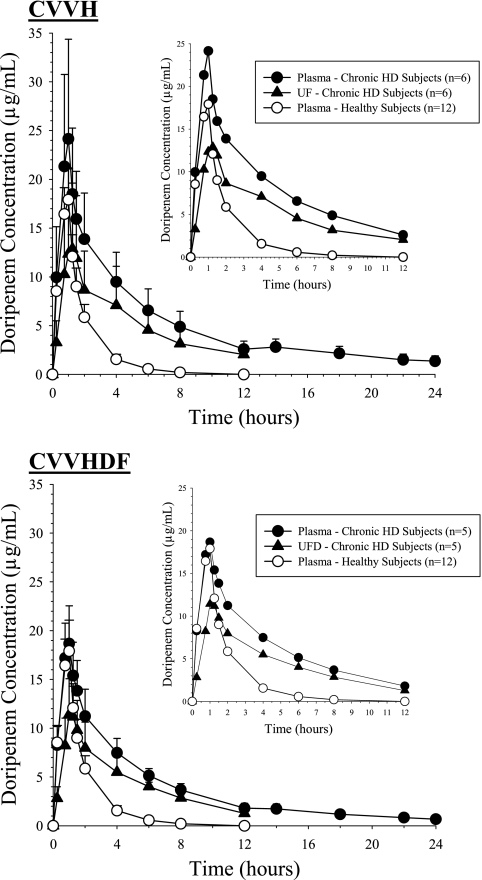
The plasma concentration-time profiles of doripenem in the subjects receiving CRRT (Fig. 1), after a single 500-mg doripenem infusion, were markedly elevated compared to those in the healthy volunteers. Doripenem AUC0-12 was approximately 3-fold or 2.5-fold greater for those receiving CVVH (97.6 μg·h/ml) or CVVHDF (77.2 μg·h/ml), respectively, than for the healthy subjects (32.1 μg·h/ml) (P < 0.0001). The observed doripenem plasma concentrations in the healthy subjects were similar to data reported for other healthy subjects receiving doripenem at 500 mg (7). The half-life of doripenem was approximately 4-fold longer for subjects receiving CVVH (mean [SD] of 4.24 [0.56] hours) and CVVHDF (3.87 [0.62] hours) than for healthy subjects (1.29 [0.24] hours). Consistent with these differences in exposure between the populations, the total body clearance of doripenem was significantly reduced in subjects receiving CVVH (4.94 [2.13] liters/h) and CVVHDF (5.89 [1.18] liters/h) compared to that in healthy subjects (15.95 [3.13] liters/h). However, the total and body weight-adjusted Vss values were not significantly different among the three groups of subjects (Table 2).
FIG. 1.
Mean (SD) concentration-time profiles of doripenem. The inset presents the concentration-time profile truncated to 12 h after the start of doripenem infusion.
TABLE 2.
Systemic pharmacokinetic parameters of doripenem and doripenem-M-1a
| Drug and parameter | Result for CRRT subjects |
Result for healthy subjects (n = 12) | P valued | |
|---|---|---|---|---|
| CVVH subjects (n = 6) | CVVHDF subjects (n = 5) | |||
| Doripenem | ||||
| Tmax (h)b | 1.00 (0.97-1.03) | 1.00 (0.75-1.00) | 1.00 (1.00-1.00) | |
| Cmax (μg/ml) | 24.1 (10.2) | 18.9 (3.88) | 17.9 (3.17) | 0.125 |
| AUC0-12 (μg·h/ml) | 97.6 (33.4) | 77.2 (14.3) | 32.1 (6.08)c | <0.0001 |
| AUC0-∞ (μg·h/ml) | 114 (37.4) | 87.5 (16.3) | 32.5 (6.07) | <0.0001 |
| t1/2 (h) | 4.24 (0.562) | 3.87 (0.622) | 1.29 (0.233) | <0.0001 |
| Vss (liter) | 28.2 (14.1) | 29.6 (6.36) | 20.4 (3.77) | 0.0657 |
| Vss (liter/kg) | 0.343 (0.199) | 0.297 (0.0923) | 0.233 (0.0294) | 0.1441 |
| CL (ml/min) | 82.4 (35.5) | 98.2 (19.8) | 265 (52.1) | <0.0001 |
| CL (liter/h) | 4.94 (2.13) | 5.98 (1.19) | 15.9 (3.13) | <0.0001 |
| CLR (liter/h) | NAe | NA | 9.20 (1.52) | |
| CLNR (liter/h) | 3.58 (2.33) | 4.22 (0.818) | 6.73 (2.02) | 0.0097 |
| Doripenem-M-1 | ||||
| Tmax (h)b | 1.00 (0.97-1.03) | 1.00 (0.75-1.00) | 1.00 (1.00-1.00) | |
| Cmax (μg/ml) | 3.02 (0.818) | 2.87 (1.33) | 1.80 (0.366) | 0.0670 |
| AUC0-12 (μg·h/ml) | 24.4 (4.58) | 21.8 (6.63) | 4.73 (0.955)c | <0.0001 |
| AUC0-∞ (μg·h/ml) | NA | NA | 5.22 (1.00) | |
| t1/2 (h) | NA | NA | 2.15 (0.282) | |
| CLNR (liter/h) | NA | NA | 14.2 (4.64) | |
Data are presented as means (SD) unless otherwise noted.
Median (range).
Values represent the last quantifiable AUC0-12 (AUClast); median time of last quantifiable concentration (tlast) value, 8.00 h (range, 6.00 to 12.00).
ANOVA (SAS 9.2) with the SHEFFE option was used, and P values represent comparisons between the healthy subjects and the two CRRT groups.
NA, not assessable.
After termination of CRRT, mean doripenem plasma concentrations in the subjects receiving CRRT showed a slight rise followed by a gradual decline until the end of the sampling period (24 h), at which point low but quantifiable concentrations were still detectable, 1.36 μg/ml for CVVH subjects and 0.67 μg/ml for CVVHDF subjects.
Doripenem CRRT pharmacokinetic parameters.
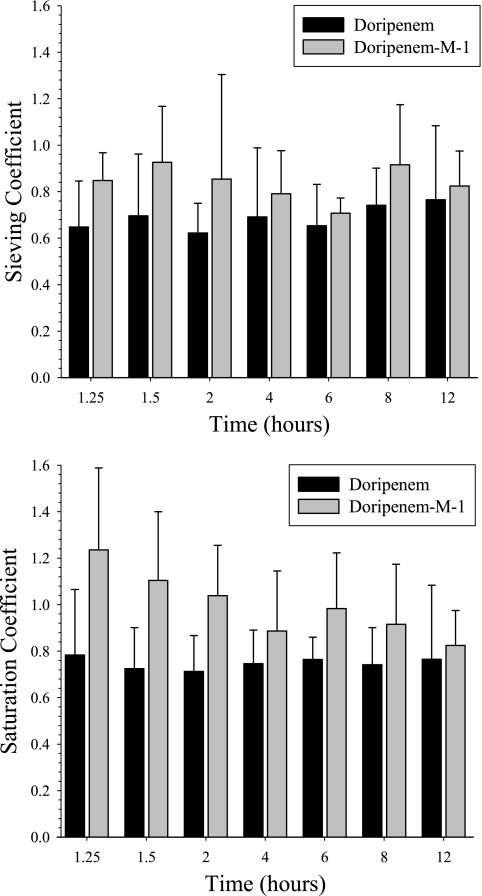
Doripenem plasma, UF, and UFD concentrations in the subjects who received CVVH were similar over the entire sampling period to concentrations in subjects who received CVVHDF (Fig. 1). The mean (SD) Sc and Sa for doripenem during CVVH and CVVHDF were 0.67 (0.149) and 0.75 (0.163), respectively, indicating that doripenem is extensively removed by both procedures. These calculated values were consistent throughout the sampling periods among all subjects, and no statistically significant trends were evident (Fig. 3). The mean (SD) AeCRRT (% per dose) for doripenem was slightly higher during CVVH (27.5% [12.46%]) than during CVVHDF (20.5% [3.99%]). The clearances of doripenem by CVVH and CVVHDF were similar, at 22.2 (4.99) ml/min and 24.5 (5.03) ml/min, respectively. The urea clearances by CVVH and CVVHDF were similar, and these values did not significantly differ from the CLCRRT of doripenem (Table 3).
TABLE 3.
Clearance of doripenem and doripenem-M-1 by CVVH and CVVHDFa
| Drug/product and parameter | Result for CVVH subjects (n = 6) | Result for CVVHDF subjects (n = 5) | P value |
|---|---|---|---|
| Doripenem | |||
| QCRRT (liter/h) | 1.99 (0.0272) | 1.96 (0.0487) | 0.2967 |
| Sc or Sa | 0.67 (0.15) | 0.76 (0.16) | 0.4119 |
| AUC0-12 (μg·h/ml) | 63.5 (30.7) | 53.6 (11.4) | 0.5143 |
| AeCRRT (% per dose) | 27.5 (12.5) | 20.5 (3.99)b | 0.3213 |
| CLCRRT (ml/min) | 22.2 (4.99) | 24.5 (5.03) | 0.6254 |
| %CLCRRT | 32.0 (15.2) | 25.4 (5.06) | 0.2795 |
| Doripenem-M-1 | |||
| Sc or Sa | 0.82 (0.11) | 0.93 (0.17) | 0.4119 |
| AUC0-12 (μg·h/ml) | 18.7 (4.10) | 18.9 (4.90) | 0.2094 |
| AeCRRT (% per dose) | 10.1 (3.82) | 8.44 (1.65)b | 0.9571 |
| CLCRRT (ml/min) | 27.1 (3.59) | 30.3 (5.00) | 0.2515 |
| Urea | |||
| CLurea (ml/min) | 26.8 (6.22) | 26.7 (8.33) | NAc |
Data are presented as means (SD) unless otherwise noted.
n = 4.
NA, not assessable.
Doripenem-M-1 pharmacokinetics.
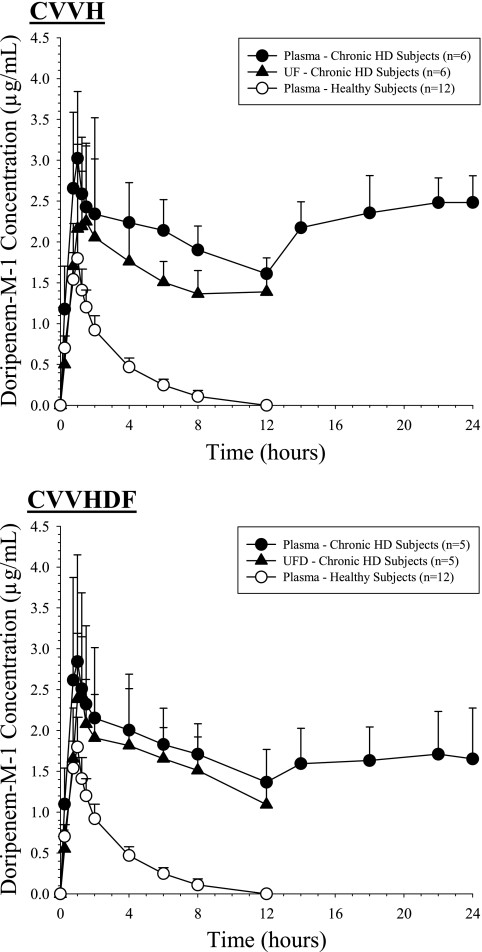
The maximum doripenem-M-1 concentrations were similar, but the AUC of doripenem-M-1 was significantly greater in subjects receiving CRRT than in healthy subjects (Table 2). Plasma concentrations in the elimination phase for subjects receiving CRRT decreased at a lower rate than those for healthy subjects and did not return to baseline by the end of the CRRT procedure (mean concentrations, 1.61 μg/ml [CVVH] and 1.29 μg/ml [CVVHDF]). After termination of CRRT, doripenem-M-1 plasma concentrations rebounded and remained above those observed at the end of CRRT for the subsequent 12-hour sampling period for subjects receiving CVVH or CVVHDF. The terminal elimination half-life thus could not be reliably estimated for doripenem-M-1 (Table 2).
Doripenem-M-1 CRRT pharmacokinetic parameters.
Doripenem-M-1 exposure in plasma during the CRRT procedure, AUC0-12, was approximately 5-fold greater for the DDS (24.4 [4.58] μg·h/ml during CVVH and 21.8 [6.63] μg·h/ml during CVVHDF) than for the healthy subjects (4.73 [0.96] μg·h/ml) (Fig. 2). The mean (SD) Sc and Sa for doripenem-M-1 during CVVH and CVVHDF, respectively, were 0.817 (0.109) and 0.928 (0.174), indicating that doripenem-M-1 is extensively removed by both procedures. These calculated values were consistent throughout the sampling periods among all subjects, and no statistically significant trends were evident (Fig. 3). The mean (SD) AeCRRT (% per dose) for doripenem-M-1 was slightly higher during CVVH (10.1% [3.82%])than during CVVHDF (8.44% [1.65%]). The clearances of doripenem-M-1 by CVVH and CVVHDF were similar, i.e., 27.1 (3.59) versus 30.3 (4.97) ml/min, respectively (Table 3).
FIG. 2.
Mean (SD) concentration-time profiles of doripenem-M-1.
FIG. 3.
Mean (SD) sieving coefficients and saturation coefficients versus time.
Safety and tolerability.
Treatment-emergent adverse events were reported in 6 subjects: 3 healthy volunteers, 2 subjects receiving CVVH, and 1 subject receiving CVVHDF. All of these events were either mild or moderate in severity, and all resolved by the end of the study. Three events, acute pancreatitis, acute abdominal pain, and hyperkalemia, were assessed by the investigator as related to study drug. The event of acute pancreatitis was reported on day 1 in a healthy volunteer, resolved within 2 days, and was confounded by predisposing conditions, including the possibility of a preexisting biliary stone in a middle-aged woman with a BMI of 30.5 kg/m2 and a history of hypercholesterolemia. The abdominal pain, described as stomach cramps, occurred in a healthy male volunteer on day 1 and resolved spontaneously after 2 h. The event of hyperkalemia was reported the day after study drug administration in a subject with ESRD with a medical history of hyperkalemia. There were no deaths or serious adverse events reported in the study.
DISCUSSION
The disposition of several carbapenems during CRRT has been reported in single case reports or as a series of clinical cases. Unfortunately, it is difficult, if not impossible, to control the variables that may affect the clearance of carbapenems in acutely ill patients. In this study, we prospectively measured the systemic pharmacokinetics of doripenem and its primary metabolite, doripenem-M-1, in DDS with ESRD undergoing a session of CRRT. The mean CL and CLNR in these subjects were similar to those in previous reports of subjects with ESRD (6) and significantly lower than values observed in healthy subjects (7). No significant differences in Vss were noted. The impacts of two CRRT procedures, CVVH and CVVHDF, on the disposition of doripenem and doripenem-M-1 were also rigorously assessed. The mean Sc and Sa for doripenem were comparable with those from previous reports of imipenem and meropenem, in which the same hemofilter was used (10, 13, 21). The CLCRRT of doripenem accounted for 25 to 32% of the observed CL, and the mean values ranged from 82 to 92% of simultaneous CLurea values, clearly indicating that either mode of CRRT has a marked effect on the disposition of doripenem, which needs to be accounted for with an increase in the daily doripenem dosage.
Many investigations of the pharmacokinetics of carbapenems in critically ill individuals have been published in the last 10 to 15 years. The results of these investigations suggest that there is marked variability between the agents within the class and that critical illness and/or acute impairment of kidney function is associated with a reduction in renal, as well as nonrenal, clearance. The reduction in renal clearance of imipenem and meropenem is correlated with the patient's degree of residual renal function and independent of the duration of the renal injury, i.e., whether the patient has acute or chronic kidney disease. The reduction in nonrenal clearance of these agents in subjects with acute impairment of kidney function is significant relative to clearance in those with normal renal function, but clearance is not as low as the values which have been observed in subjects with ESRD who are dialysis dependent. The renal and nonrenal clearance of doripenem in stable chronic kidney disease patients, and those who are dialysis dependent, has been noted to be lower than that observed in healthy subjects; the results of these investigations can be utilized to estimate the clearance of doripenem (6, 7).
Continuous replacement renal therapies have emerged as the foundational renal replacement therapies for the critically ill patient (5, 19). CVVH, as the preferred therapy for the management of fluid-overloaded patients and at ultrafiltration rates greater than 1 liter/h, has also proven to be an efficient means of removing accumulated waste products. CVVHDF augments convection with diffusion and as a result is considered by some to be the most efficient method of CRRT. In clinical practice, these therapies are tailored to the individual patient's needs by modification of the blood flow and ultrafiltration, dialysate, and replacement fluid flow rates, as well as the hemofilter or dialyzer. Results of recent investigations suggest that although the achieved clearances of urea, a marker of renal insufficiency and CRRT efficiency, may vary 2- to 3-fold, the delivery of an increased therapeutic regimen was not associated with an improvement in clinical outcomes (2, 20).
The influences of CRRT on the removal of drugs have also been noted to be markedly variable, although there is a clear trend in the last 10 to 15 years toward higher clearances with all agents that have been evaluated throughout this time period (3, 9, 12, 21). The clearances of imipenem by CVVH ranged from 6.5 to 13.3 ml/min in the 1990s and have increased to 22.9 to 36.0 ml/min during the last decade (9, 10, 21). This increase is in part due to the utilization of larger-surface-area hemofilters and higher ultrafiltration rates. The CVVHDF clearance of imipenem has generally been greater than that observed with CVVH; values have ranged from 18.7 to 57 ml/min, with higher values being observed in patients who had greater QUFD rates (9, 10, 21).
The disposition of meropenem has been evaluated extensively in critically ill patients with creatinine clearances ranging from 0 to 118 ml/min (13, 15, 17, 21). The total body clearances reported by the nine investigative teams which have assessed the influence of CVVH and/or CVVHDF vary widely (52 to 1,064 ml/min) and thus are relatively noninformative in terms of application of the data to prospective patient care situations. Focusing on those studies in patients with CLCR less than 70 ml/min dramatically reduces the variability in CL, to 52 to 143 ml/min (13, 17, 21). The mean nonrenal clearances from a subgroup of 5 investigations of 38 patients ranged from 35 to 59 ml/min and are comparable to the values observed in individuals with normal renal function (Table 2). The CRRT procedure clearances are higher with CVVHDF than with CVVH, and when the same hemofilter was utilized (AN69 with a surface area of 0.9 m2), the values ranged from 27.0 to 38.9 ml/min and 17.2 to 27.0 ml/min, respectively. The mean (SD) saturation and sieving coefficients derived from these investigations were less variable, 0.92 (0.05) and 0.74 (0.13), respectively, and thus these parameters may be the most useful measure of CRRT efficiency for prospective utilization.
Since the dialysis-dependent subjects evaluated in this study had chronic renal disease and were not acutely or critically ill but were anuric (n = 9) or had minimal residual renal function (urine output less than 300 ml/day) (n = 3), the systemic pharmacokinetics of doripenem were expected to be, and were, similar to previous observations in ESRD patients (Fig. 3). The ability to maintain constant CVVH and CVVHDF procedures throughout the observation period facilitated the rigorous characterization of the clearance, Sc, and Sa of doripenem by these two CRRTs. The doripenem Sc and Sa were stable over the 12-h observation period and were consistent with previous reports from other evaluation of carbapenems using the same hemofilter. CLCVVH and CLCVVHDF were in the range of values reported for imipenem and meropenem when a similar CRRT prescription was delivered. This information provides clinicians with a sound foundation upon which to plan the optimal dosage regimen for doripenem in critically ill patients receiving CRRT.
The primary challenge to the clinician's determination of the optimal individualized dosage regimen is thus the broad range of residual renal and other organ functions that one may encounter in the critically ill patient population. Indeed, the composite organ function of one critically ill population may bear little resemblance to that of another population. The clinician is thus faced with the need to evaluate and quantify the functionality of each relevant organ system, as well as the influence of therapeutic interventions, such as CRRT, to arrive at the optimal dosage regimen for the patient. This investigation provides the foundational knowledge of the influence of CVVH and CVVHDF on the disposition of doripenem, which can be used to guide the initiation of therapy and to perform additional analysis to establish an optimal dosage regimen for patients treated with doripenem and undergoing these modalities of CRRT.
Acknowledgments
The work was supported by Johnson & Johnson Pharmaceutical Research & Development, L.L.C.
We thank Daksha Desai-Kreiger for her support in the validations of bioanalytical assays and drug concentration analysis, Christopher Curtin for assistance in protocol development, and Bradford Challis for editorial support for the manuscript (employees of Johnson & Johnson Pharmaceutical Research & Development). Daniel E. Carl from Virginia Commonwealth University (Richmond, VA) and Thomas C. Marbury from the Orlando Clinical Research Center (Orlando, FL) are acknowledged for their contribution in the evaluation and care of study subjects.
Footnotes
Published ahead of print on 3 January 2011.
REFERENCES
- 1.Aronoff, G. R., et al. 2007. Drug prescribing in renal failure: dosing guidelines for adults, 5th ed. American College of Physicians, Philadelphia, PA. [DOI] [PubMed]
- 2.Bellomo, R., et al. 2009. Intensity of continuous renal-replacement therapy in critically ill patients. N. Engl. J. Med. 361:1627-1638. [DOI] [PubMed] [Google Scholar]
- 3.Bugge, J. F. 2001. Pharmacokinetics and drug dosing adjustments during continuous venovenous hemofiltration or hemodiafiltration in critically ill patients. Acta Anaesthesiol. Scand. 45:929-934. [DOI] [PubMed] [Google Scholar]
- 4.Burkhardt, O., et al. 2009. Pharmacokinetics of ertapenem in critically ill patients with acute renal failure undergoing extended daily dialysis. Nephrol. Dial. Transplant. 24:267-271. [DOI] [PubMed] [Google Scholar]
- 5.Cerda, J., and C. Ronco. 2009. Modalities of continuous renal replacement therapy: technical and clinical considerations. Semin. Dial. 22:114-122. [DOI] [PubMed] [Google Scholar]
- 6.Cirillo, I., et al. 2008. Pharmacokinetics of doripenem in subjects with varying degrees of renal impairment, abstr. A-1886. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
- 7.Cirillo, I., et al. 2009. Pharmacokinetics, safety, and tolerability of doripenem after 0.5-, 1-, and 4-hour infusions in healthy volunteers. J. Clin. Pharmacol. 49:798-806. [DOI] [PubMed] [Google Scholar]
- 8.Cockcroft, D. W., and M. H. Gault. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31-41. [DOI] [PubMed] [Google Scholar]
- 9.Cotton, A., B. D. Franklin, S. Brett, and A. Holmes. 2005. Using imipenem and cilastatin during continuous renal replacement therapy. Pharm. World Sci. 27:371-375. [DOI] [PubMed] [Google Scholar]
- 10.Fish, D. N., I. Teitelbaum, and E. Abraham. 2005. Pharmacokinetics and pharmacodynamics of imipenem during continuous renal replacement therapy in critically ill patients. Antimicrob. Agents Chemother. 49:2421-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayakawa, M., Y. Ito, I. Fujita, K. Iseki, and S. Gando. 2006. Pharmacokinetics and the most suitable regimen of panipenem/beta mipron in critically ill patients receiving continuous renal replacement therapy: a pilot study. ASAIO J. 52:398-403. [DOI] [PubMed] [Google Scholar]
- 12.Heintz, B. H., G. R. Matzke, and W. E. Dager. 2009. Antimicrobial dosing concepts and recommendations for critically ill adult patients receiving continuous renal replacement therapy or intermittent hemodialysis. Pharmacotherapy 29:562-577. [DOI] [PubMed] [Google Scholar]
- 13.Isla, A., et al. 2005. Meropenem and continuous renal replacement therapy: in vitro permeability of 2 continuous renal replacement therapy membranes and influence of patient renal function on the pharmacokinetics in critically ill patients. J. Clin. Pharmacol. 45:1294-1304. [DOI] [PubMed] [Google Scholar]
- 14.Joy, M. S., G. R. Matzke, D. K. Armstrong, M. A. Marx, and B. J. Zarowitz. 1998. A primer on continuous renal replacement therapy for critically ill patients. Ann. Pharmacother. 32:362-375. [DOI] [PubMed] [Google Scholar]
- 15.Kielstein, J. T., et al. 2006. Pharmacokinetics and total elimination of meropenem and vancomycin in intensive care unit patients undergoing extended daily dialysis. Crit. Care Med. 34:51-56. [DOI] [PubMed] [Google Scholar]
- 16.Kuang, D., A. Verbine, and C. Ronco. 2007. Pharmacokinetics and antimicrobial dosing adjustment in critically ill patients during continuous renal replacement therapy. Clin. Nephrol. 67:267-284. [DOI] [PubMed] [Google Scholar]
- 17.Langgartner, J., A. Vasold, T. Gluck, M. Reng, and F. Kees. 2008. Pharmacokinetics of meropenem during intermittent and continuous intravenous application in patients treated by continuous renal replacement therapy. Intensive Care Med. 34:1091-1096. [DOI] [PubMed] [Google Scholar]
- 18.Li, A. M., et al. 2009. A systematic review of antibiotic dosing regimens for septic patients receiving continuous renal replacement therapy: do current studies supply sufficient data? J. Antimicrob. Chemother. 64:929-937. [DOI] [PubMed] [Google Scholar]
- 19.Palevsky, P. M. 2006. Dialysis modality and dosing strategy in acute renal failure. Semin. Dial. 19:165-170. [DOI] [PubMed] [Google Scholar]
- 20.Palevsky, P. M., et al. 2008. Intensity of renal support in critically ill patients with acute kidney injury. N. Engl. J. Med. 359:7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pea, F., P. Viale, F. Pavan, and M. Furlanut. 2007. Pharmacokinetic considerations for antimicrobial therapy in patients receiving renal replacement therapy. Clin. Pharmacokinet. 46:997-1038. [DOI] [PubMed] [Google Scholar]
- 22.Stevenson, J. M., et al. 2008. Ertapenem clearance during modeled continuous renal replacement therapy. Int. J. Artif. Organs 31:1027-1034. [DOI] [PubMed] [Google Scholar]
- 23.Van Wart, S. A., D. R. Andes, P. G. Ambrose, and S. M. Bhavnani. 2009. Pharmacokinetic-pharmacodynamic modeling to support doripenem dose regimen optimization for critically ill patients. Diagn. Microbiol. Infect. Dis. 63:409-414. [DOI] [PubMed] [Google Scholar]
- 24.Walsh, F. 2007. Doripenem: a new carbapenem antibiotic a review of comparative antimicrobial and bactericidal activities. Ther. Clin. Risk Manag. 3:789-794. [PMC free article] [PubMed] [Google Scholar]