Abstract
Moxifloxacin penetrates well into oromaxillary tissue and covers the causative pathogens that show an increasing resistance to standard antibiotics. Clinical reports suggest that moxifloxacin may be effective for the treatment of odontogenic infections that can lead to serious complications. The objective of this prospective, randomized, double-blind, multicenter study was to compare the efficacies and safeties of moxifloxacin and clindamycin for the medical treatment of patients with gingival inflammatory infiltrates and as an adjuvant therapy for patients with odontogenic abscesses requiring surgical treatment. Patients received either 400 mg moxifloxacin per os once daily or 300 mg clindamycin per os four times daily for 5 days consecutively. The primary efficacy endpoint was the percent reduction in patients' perceived pain on a visual analogue scale at days 2 to 3 from baseline. Primary analysis included 21 moxifloxacin- and 19 clindamycin-treated patients with infiltrates and 15 moxifloxacin- and 16 clindamycin-treated patients with abscesses. The mean pain reductions were 61.0% (standard deviation [SD], 46.9%) with moxifloxacin versus 23.4% (SD, 32.1%) with clindamycin (P = 0.006) for patients with infiltrates and 55.8% (SD, 24.8%) with moxifloxacin versus 42.7% (SD, 48.5%) with clindamycin (P = 0.358) for patients with abscesses. A global efficacy assessment at days 2 to 3 and 5 to 7 showed faster clinical responses with moxifloxacin in both abscess and infiltrate patients. Rates of adverse events were lower in moxifloxacin- than in clindamycin-treated patients. In patients with inflammatory infiltrates, moxifloxacin was significantly more effective in reducing pain at days 2 to 3 of therapy than clindamycin. No significant differences between groups were found for patients with odontogenic abscesses.
Odontogenic infections arise from either periapical (necrotic pulp) or periodontal infections and penetrate through the subcutaneous tissue, causing cellulitis or an abscess in the soft and bony oromaxillofacial tissues. They can then spread through the bone and periosteum toward nearby or more distant anatomical structures such as the jaws, the surrounding face, or the neck. While often taking a mild course, odontogenic infections may also lead to potentially life-threatening complications such as cervical necrotizing fasciitis, necrotizing mediastinitis, or orbital and brain abscesses, depending on a patient's immunocompetence and the site of the inflammatory process. Medically compromised patients appear to be more susceptible to systemic rather than local infection complications, with a need for significantly longer hospital stays and with an increased risk of fatal complications (19). In Finland, Seppänen et al. recently observed an increased incidence of odontogenic infections requiring hospital care and at the same time an increased need for intensive care (20).
Antibiotics are an important component in the conservative treatment strategy and are considered to be an essential adjunct even for severe odontogenic infections with primarily surgical management (18, 25). Antibiotic therapy is indicated when there are clear signs of systemic involvement such as pyrexia, lymphadenopathy, difficulty in swallowing, and lockjaw (6, 15).
Odontogenic infections are always polymicrobial, consisting of aerobic pathogens, facultative anaerobes, and strict anaerobes (6, 24, 25). In odontogenic abscesses typically 3 to 6 different bacterial species are present (2). The microbiota involved in endodontic abscesses are dominated by anaerobic bacteria (21) that are considered clinically relevant (24). Therefore, therapy for orofacial odontogenic infections should be effective against both streptococci and anaerobes (14).
For the commonly recommended antibiotics penicillin (PEN) and clindamycin (CLI), increasing resistance rates have been reported (2). Of 87 strains isolated from patients with odontogenic abscesses in a monocentric study, only 69% were susceptible to penicillin and 75% were susceptible to CLI (22). For severe odontogenic infections there was a higher rate of episodes with PEN-resistant bacteria than for local abscesses (1). Therefore, additional antibiotic options are desirable.
The fluoroquinolone moxifloxacin (MXF) exhibits good tissue penetration and high oral bioavailability. It has shown good bactericidal activity against Gram-positive and Gram-negative aerobic and anaerobic pathogens, including those that cause odontogenic infections (8, 17, 22). The activity of MXF is comparable to that of amoxicillin-clavulanate (AMX-CLA) but better than those of CLI, metronidazole (MET), and PEN (17). In vitro, MXF was found to be more efficient than CLI, doxycycline (DOX), and MET in the eradication of strains of periodontopathogenic bacteria from biofilms (9). The penetration of MXF into the mandibular bone and soft tissue of rats is at least as good as that of CLI (6). For humans, a good penetration of MXF into bone, tonsillar tissue, and subcutaneous adipose tissue was shown (10, 12, 13).
Clinically, MXF was shown previously to be effective for the prevention of bacteremia following dental extractions, whereas CLI was not (8). For the adjuvant therapy of patients with severe chronic periodontitis, MXF was more effective than DOX and reduced the bacterial load and all inflammatory parameters (11). In a pilot study of hospitalized patients with severe odontogenic abscesses, MXF showed promising results (2).
The objective of this study was to investigate the efficacies and safeties of MXF and CLI for the medical treatment of patients with gingival inflammatory infiltrates and as adjuvant therapy for patients with odontogenic abscesses requiring surgical treatment. The endpoints were the clinical response at days 2 to 3 and 5 to 7 (end of therapy). CLI was chosen as a comparator, as it is the drug most commonly prescribed for this indication in Germany.
(The results of this study were presented in part at the 18th European Congress of Clinical Microbiology and Infectious Diseases [ECCMID], Barcelona, Spain, 2008 [6a].)
MATERIALS AND METHODS
Patients.
Outpatients with either a diagnosis of an odontogenic abscess (dentoalveolar or periodontal) including pericoronitis requiring surgical intervention with adjunctive antibiotic treatment or a diagnosis of gingival inflammatory infiltrates requiring medical therapy including an antibiotic were eligible to enroll. Infiltrate and abscess diagnoses and their treatment modalities were based on the International Classification of Disease, revision 10 (ICD-10) (20), using codes associated with odontogenic infections (K04 and K05). Excluded from the study were patients who were younger than 18 years of age; were pregnant or breastfeeding; had a hypersensitivity to MXF, other quinolones, CLI, or other lincosamides; had a history of tendinopathy related to quinolones; had diabetes mellitus; had known HIV infection; had severe infections requiring parenteral antibiotics; had bradycardia, had known cardiac arrhythmias, or were taking an arrhythmia-causing medication; had a known or suspected electrolyte dysbalance, especially hypokalemia; or had hepatic or renal (creatinine clearance of <50 ml/min or serum creatinine level of >2 mg/dl) dysfunction.
Patient enrollment was open from September 2005 to January 2007.
Study design.
This investigator-initiated, prospective, randomized, double-blind, double-dummy, multicentric, phase II clinical study was conducted at 3 clinical centers in Germany. Written informed consent was obtained from all patients. The study protocol was approved by the Ethics Committee of the Hamburg Board of Physicians (permission no. 2141). Monitoring and randomization were done in collaboration with the Clinical Trial Center North, University Medical Center Hamburg-Eppendorf.
At study entry before the start of treatment, baseline data such as medical history and demographic information were collected, blood samples were drawn to measure basic laboratory parameters, and clinical signs and symptoms were assessed (see Table 1 for baseline values). Diagnostic tests for dental and periodontal assessments, e.g., cold bite sticks, percussion, and radiographic findings, were performed in accordance with methods described previously by Brennan et al. (5). Thereafter, surgical treatment and/or antibiotic therapy was started.
TABLE 1.
Patient demographics and baseline values (before start of treatment) for the intent-to-treat population
| Parameter | Value for stratum |
|||
|---|---|---|---|---|
| Infiltrate patients taking: |
Abscess patients taking: |
|||
| MXF | CLI | MXF | CLI | |
| No. of patients | 21 | 19 | 15 | 16 |
| Mean age (yr) (range) | 43.6 (24-65) | 45.4 (26-85) | 45.0 (27-72) | 38.4 (20-74) |
| % men | 57.1 | 68.4 | 60 | 68.8 |
| Median CRP level (mg/liter) (range) | 5.0 (0.41-23) | 5.0 (0.89-121) | 11.0 (0.85-58) | 8.84 (0.63-104) |
| Median no. of leukocytes (109/liter) | 6.3 | 7.55 | 10.2 | 9.95 |
| Median oral temp (°C) | 36.4 | 36.5 | 36.8 | 36.6 |
| Median pain intensity (VAS) (range) | 25 (10-100) | 25 (7-73) | 65 (30-95) | 36 (10-90) |
| Median incisal edge distance (mm) (range) | 38.0 (10-42) | 38.0 (30-40) | 35.0 (14-45) | 36.5 (20-45) |
| % of patients with lymphadenitis | 19.0 | 26.3 | 46.7 | 56.3 |
| % of patients with painful lymphadenitis | 4.8 | 5.3 | 26.7 | 18.8 |
| % of patients with swelling or erythema | 95.2 | 94.7 | 100 | 100 |
| % of patients with extraoral swelling | 14.3 | 15.8 | 60.0 | 68.8 |
Clinical signs and symptoms (e.g., pain intensity, oral temperature, incisal edge distance, extent of lymphadenitis, swelling, erythema, putrid secretions, and sensory status of the trigeminal nerve and facial nerve) were evaluated again during therapy at the visit on days 2 to 3 (48 to 72 h after the start of therapy) and at the end-of-treatment (EOT) visit on days 5 to 7. Laboratory parameters were assessed a second time at the EOT visit.
For abscess patients, swabs from abscesses were taken at all three visits, if possible.
Study interventions.
Patients were randomly assigned to receive either 400 mg MXF (Avalox/Avelox; Bayer Vital GmbH, Germany) per os once daily plus a placebo CLI dummy four times daily or 300 mg CLI (Clindastad; STADApharm GmbH, Germany) per os four times daily plus a placebo MXF dummy once daily, each for 5 days consecutively.
Concomitantly with the study drugs, patients could receive additional medical therapy, except for other systemic antimicrobials, and/or surgical interventions in accordance with the guidelines of the German Society for Oral and Maxillofacial Surgery (DGMKG) (18), if deemed necessary by the patient's physician. These treatments ranged from standardized analgesia with 3 doses of 1 g paracetamol per os and local interventions, such as lavage or trepanation, to surgical incisions, drainages, removal of the involved tooth, debridement, and puncture. All surgical interventions were performed before the start of antibiotic therapy.
Study endpoints.
The primary efficacy endpoint was the percent reduction in patients' perceived pain on a visual analogue scale (VAS) at days 2 to 3 from baseline in the intent-to-treat (ITT) population.
A VAS is a one-dimensional measurement instrument used for assessing a characteristic or a response that ranges across a continuum of values, such as pain or quality of life. A VAS usually consists of a horizontal line (on paper or cardboard), 100 mm in length, with word descriptors at each end and a scale between them. A VAS for pain assessment will use 0 for “no pain” and 100 for “worst pain” as the anchors. In oral medicine, the VAS is an established tool for the assessment of pain intensity (4, 5).
Secondary endpoints were, e.g., a global assessment of the clinical efficacy at days 2 to 3 and 5 to 7 and the safety of the study medication.
Clinical efficacy assessment.
According to the plan of study a clinical efficacy rating of cure required the complete resolution of all signs of inflammation, a decrease of the body temperature to ≤37.5°C in cases with fever, a subjectively unobstructed opening of the mouth and an incisal edge distance of at least 35 mm, a negative palpation finding for lymphadenopathy, no further excretion of pus, no need for further therapies after the study treatment, and avoidance of sensory disturbances of the trigeminal nerve or facial nerve.
The clinical outcome was rated as improvement if the signs of inflammation were decreased at least 50%, if the body temperature was decreased to ≤38.0°C, if the excretion of pus was reduced, if palpation for lymphadenopathy was only slightly positive (soft and palpable), and if the opening of the mouth was slightly obstructed and the incisal edge distance was 35 mm or lower.
If an initial fever did not decrease, the excretion of pus was unchanged, palpation for lymphadenopathy was positive and painful (hard and well palpable), or there were persistent or new sensory disturbances of the trigeminal nerve or facial nerve, this was rated as no response at days 2 to 3 (during treatment) and as failure at days 5 to 7 (EOT).
Laboratory measures.
The following laboratory parameters were analyzed with tests and reagents certified to comply with the In Vitro Diagnostic Directive according to standard protocols provided by the manufacturers: hemoglobin, leukocytes, platelets, aspartate aminotransferase, alanine aminotransferase, gamma-glutamyltransferase, alkaline phosphatase, potassium, C-reactive protein (CRP), and creatinine (Roche/Hitachi modular systems; Roche, Penzberg, Germany).
All swabs (BBL CultureSwab; Becton Dickinson GmbH, Heidelberg, Germany) were cultured for pathogen identification and susceptibility testing by an Etest (AB BioDisk, Solna, Sweden) according to EUCAST (European Committee on Antimicrobial Susceptibility Testing) guidelines.
Safety assessments.
All patients were monitored externally for adverse events daily during study treatment and at days 5 to 7. Adverse events had to be documented independently of their suspected relation to the study drugs and were graded by their seriousness, severity, and relatedness to the study medication.
Randomization and blinding.
Patients with abscesses and patients with infiltrates were randomized separately and were analyzed as two strata. A blocked randomization with equal block sizes was used. Randomization was carried out in collaboration with the Department of Medical Biometry and Epidemiology of the University Medical Center Hamburg-Eppendorf.
MXF verum and placebo were capsuled by Bayer Schering Pharma, Germany, and CLI verum and placebo were capsuled by the pharmacy of the University Hospital Hamburg-Eppendorf, using Capsugel hard gelatin capsules provided by Bayer Schering Pharma.
Populations for analysis.
The safety population included all randomized patients who took at least 1 dose of the study drug and had at least one postrandomization assessment. The ITT population included all patients who took at least 1 dose of the study drug and had a postrandomization primary efficacy measurement. The available case analysis of the clinical efficacy population included those patients within the ITT population who also had secondary efficacy measurements.
Statistical analysis.
The study was designed to test for the following null hypothesis: the pain reduction [(VASday 2/3 − VASday 1)/VASday 1] is identical in both treatment groups (MXF and CLI groups), versus the pain reduction is different in both treatment groups (MXF and CLI groups).
The evaluation was carried out by using a t test for unpaired samples and an error correction by the Bonferroni method to ensure a total type I error margin of an α of 0.05. Therefore, each of both t tests was performed up to a level of an α of 0.025. The sample size required to achieve a statistical power of 80% under these conditions was calculated to be 21 patients for each of the 4 treatment groups.
The secondary endpoints were analyzed exploratively. For the global clinical efficacy an exact Mann-Whitney rank sum test was performed at each point of time.
All statistical analyses were performed separately for the two strata, patients with abscesses and patients with infiltrates.
RESULTS
Overall, 71 patients were evaluable for the primary endpoint analysis of the ITT population, 40 patients with infiltrates and 31 with abscesses. The treatment groups were well matched with regard to age, whereas the proportion of male patients was somewhat larger in the CLI groups. Within the infiltrate and abscess strata there were no statistically significant differences between the MXF and CLI groups for clinical signs and symptoms at baseline (Table 1).
Before the unblinding of data, a plausibility check identified three patients with implausible VAS baseline values of 0 (no pain) in one center. The true VAS values could not be determined at the time of analysis. Due to doubts regarding the validity of the VAS values reported by this center, the trial manager removed all patients included by this center from the primary analysis, well aware of the resulting reduction in the statistical power of the study. However, these patients remained in the safety population. After the exclusion of this center, 4 patients in the infiltrate group had to be removed from the primary analysis, which corresponded to a reduction of the post hoc power from 86% to 83%. For the abscess group, the sample size dropped from 46 to 31, which corresponded to a reduction of the post hoc power from 21% to 15%. However, this loss in power did not change the findings.
A further 5 patients were rated as dropouts, 1 due to an adverse event and 4 due to missing data. According to the study protocol, data for these 5 patients remained in the primary analysis of the ITT population, with their VAS baseline value carried forward to the end of treatment, resulting in a pain reduction of zero.
Pain reduction.
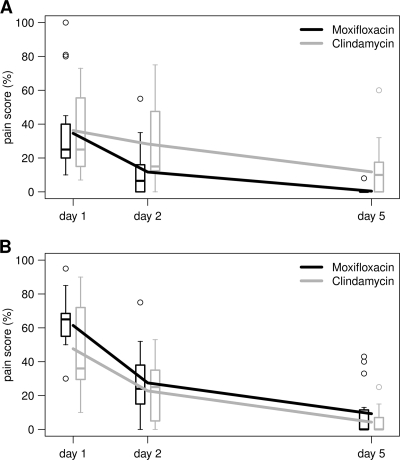
For patients with infiltrates, the mean pain reduction on days 2 to 3 from baseline as measured by the VAS (Table 2) was significantly higher in the MXF-treated than in the CLI-treated group. This treatment effect difference was largely maintained at the end-of-treatment (EOT) analysis on days 5 to 7, as shown in Fig. 1.
TABLE 2.
Primary efficacy endpoints
| Stratum | No. of patients | Median pain reduction on days 2-3 (%) ± SD | % difference between MXF and CLI treatment (95% CI)a | P value |
|---|---|---|---|---|
| Infiltrate patients taking: | ||||
| MXF | 21 | 61.0 ± 10.2 | 37.6 (11.6, 63.8) | 0.006 |
| CLI | 19 | 23.4 ± 7.4 | ||
| Abscess patients taking: | ||||
| MXF | 15 | 55.8 ± 6.4 | 13.1 (−15.5, 41.7) | 0.358 |
| CLI | 16 | 42.7 ± 12.1 |
CI, confidence interval.
FIG. 1.
Lines representing the course of pain intensity measured on a visual analogue scale for the infiltrate (A) and abscess (B) strata of the ITT population. Box plots illustrate the distribution of the pain score at three time points.
For patients with abscesses, the mean pain reduction on days 2 to 3 was higher for MXF-treated than for CLI-treated patients, but the difference did not reach statistical significance. The difference in the treatment effect was maintained in the EOT analysis on days 5 to 7.
Global clinical efficacy.
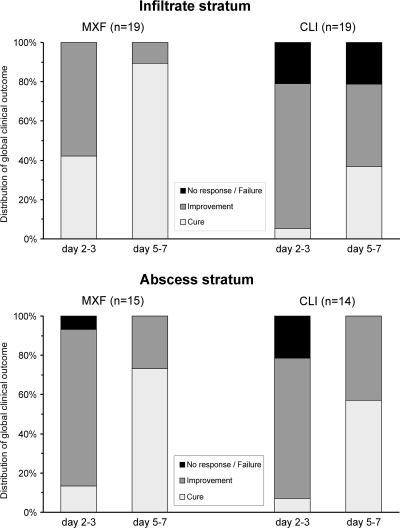
In the global clinical efficacy assessment of both strata, a higher proportion of CLI-treated patients was rated as having no response to treatment at days 2 to 3, and a higher proportion of MXF-treated patients was rated as being cured at days 5 to 7. Of patients with infiltrates treated with CLI, 21% were treatment failures at days 5 to 7, whereas there were no failures in the MXF group. In the infiltrate stratum, the difference between treatment groups for this secondary endpoint was statistically significantly in favor of MXF both at days 2 to 3 (P = 0.003) and at days 5 to 7 (P = 0.001) (Fig. 2). For patients with abscesses the difference between treatment groups did not reach statistical significance.
FIG. 2.
Course of clinical efficacy for the infiltrate and abscess strata of available cases.
Safety.
The most frequent adverse events were diarrhea and nausea. The total rate of adverse events as well as the rates of nausea and diarrhea were markedly higher for CLI- than for MXF-treated patients in both strata (Table 3). CLI-treated patients, especially those in the infiltrate stratum, showed high rates of diarrhea and nausea. There were no reports of nausea for MXF-treated patients. Of the 22 adverse events in total, 18 were judged to be at least possibly related to the study medication. None of the adverse events were serious, and only two were severe, one each of diarrhea and aggression. Treatment was discontinued due to adverse events in one case, a CLI-treated abscess patient who showed severe aggression. This patient behaved violently toward his wife after having taken a single dose of CLI, as reported by his father. Although the patient himself denied alcohol abuse, we suppose that this aggression was due to the consumption of alcohol and had no relation to the medication, especially as there is no such adverse effect described for CLI in the literature.
TABLE 3.
Rates of adverse events in the safety population
| Parameter | Value for stratum |
|||
|---|---|---|---|---|
| Infiltrate patientsa taking: |
Abscess patientsb taking: |
|||
| MXF | CLI | MXF | CLI | |
| No. of patients | 23 | 21 | 22 | 24 |
| No. of patients with adverse event/ % of patients with adverse event (95% confidence interval) | 3/13.0 (2.8-33.6) | 7/33.3 (14.6-57.0) | 2/9.1 (1.1-29.2) | 5/20.8 (7.1-42.2) |
| No. of adverse events | 4 | 9 | 2 | 7 |
| Diarrhea | 1 | 6 | 1 | 3 |
| Nausea | 0 | 2 | 0 | 1 |
| Other | 3 | 1 | 1 | 3 |
The P value was 0.11 for MXF versus CLI.
The P value was 0.27 for MXF versus CLI.
Global microbiological results.
In total, 205 bacteria were isolated from 71 patients (77 viridans streptococci, 56 Prevotella spp., 19 Neisseria spp., 17 Streptococcus anginosus group and hemolytic streptococci, 15 other anaerobes, and 21 other bacteria). The overall susceptibility rates for MXF and CLI were 98% and 60%, respectively (23). Detailed data will be reported in a separate paper.
DISCUSSION
To our knowledge, this is the first study of MXF for the treatment of gingival inflammatory infiltrates and the first prospective, randomized, double-blind clinical study of MXF for the treatment of odontogenic abscesses. According to previously reported studies and case reports, MXF has been shown to be successful for the treatment of abscesses of other origins, e.g., skin (26) and abdominal cavity (16).
Our study covers two distinct but etiologically related disease entities, odontogenic abscesses and gingival inflammatory infiltrates, which also differ in the roles of antibiotic therapy. For odontogenic abscesses the antibiotic was an adjuvant to surgical therapy, whereas for inflammatory infiltrates the antibiotic was the main component of the medical therapy. Therefore, both patient strata were analyzed separately.
For both strata the percent pain reduction from baseline was higher for MXF-treated than for CLI-treated patients, although the difference between treatment groups did not reach statistical significance for abscess patients. MXF treatment resulted in a faster clinical improvement, as shown by the rates of pain reduction and cure on days 2 to 3 (Fig. 2). This was true for both strata, although the effect was more pronounced for patients with infiltrates.
This study has several limitations. The number of actually evaluable patients was below the statistically calculated sample size for all but one treatment group (MXF-treated infiltrate group). Patients requiring parenteral antibiotics, thereby suggesting a more severe course, were excluded. There was no control for concomitant interventions, such as surgery, which are frequently required for patients with abscesses. However, the latter might be compensated for by the double-blind/double-dummy design of the study.
The use of pain medications during the first 3 days of study therapy is a potential confounder of the primary endpoint. We therefore analyzed the reported use of pain drugs during the assessment period for the primary endpoint in both strata of our study. Additional pain medications were reported by 4 out of 40 infiltrate patients, 2 of whom were in the CLI group and 2 of whom were in the MXF group. The use of pain medications was also reported by 5 out of 31 abscess patients, all of whom were treated with CLI. While the even distribution of pain drug use in the infiltrate stratum makes a confounding effect unlikely, the imbalance of pain medication use in abscess patients likely decreased the effect size of the difference between CLI and MXF.
Factors contributing to the somewhat different clinical responses of patients with infiltrates versus patients with abscesses might be differences in the spectra of causative pathogens among odontogenic infiltrates and abscesses as well as the major impact of surgical interventions on the clinical course of patients with abscesses. In addition, the number of patients in the abscess stratum was below the calculated sample size due to the exclusion of all patients from one study center.
Our results are well in line with the only other clinical trial with patients with odontogenic abscesses by Al-Nawas et al. (2). In their pilot study of adjunctive antibiotic treatment after extraoral incision for 21 hospitalized patients overall with severe odontogenic abscesses, the time to clinical remission was used as the primary efficacy endpoint. MXF at 400 mg once daily showed promising results in comparison to 2.2 g AMX-CLA three times a day (t.i.d.) (2).
In addition to concerns about the increasing resistance of odontogenic pathogens (7), the availability of other antibiotic options is crucial in cases of treatment failure and/or hypersensitivity to current standard antibiotics.
According to the results of this study, MXF seems to offer several potential advantages over CLI for the treatment of odontogenic infections. The faster decrease in pain and the faster overall clinical resolution not only will be a subjective advantage, especially for nonhospitalized patients, but also might decrease the need for additional days of antibiotic therapy and thereby decrease the risk of a development of resistance. The once-daily dosing of MXF may enhance the patient's compliance, in comparison to the four-times-daily dosing with CLI. The lower daily dose of MXF (400 mg) than of CLI (1200 mg) reduces the metabolic load of the patients. The markedly better tolerability of MXF than of CLI, as shown in this study, may lower adverse-event-related comorbidity and costs, enable patients to return to work earlier, and increase the patient's contentedness and compliance with the therapy.
Like most classes of antibiotics, fluoroquinolones can select for Clostridium difficile in patients colonized with this anaerobic pathogen, possibly leading to C. difficile-associated diarrhea (CDAD). In a case-control study with 1,142 patients with hospital-acquired CDAD in Northern California, an increased risk of acquiring CDAD was found for treatment with imipenem-cilastin (odds ratio [OR], 2.77), clindamycin (OR, 2.31), moxifloxacin (OR, 1.88), and cephalosporins (e.g., cefuroxime) (OR, 2.16) (3). As with other infections, antibiotics should also be used judiciously for odontogenic infections, and the choice of empirical antibiotic for the individual patient should also take into account recent antibiotic use. As these patients are often treated as outpatients, they may have a lower risk of nosocomial cross-infection with C. difficile. In addition, one could hypothesize that the faster response with MXF may allow a shorter duration of therapy and thereby decrease the risk of CDAD in comparison to standard therapy with CLI.
In summary, 400 mg MXF once daily for 5 days resulted in significantly better pain reduction and overall clinical response than 300 mg CLI four times daily for patients with odontogenic inflammatory infiltrates. As an adjunctive therapy for patients with odontogenic abscesses, MXF was at least as effective as CLI with regard to pain reduction, clinical outcome, and safety. As of the reporting of these results, MXF seems to be an effective and well-tolerated option for the treatment of odontogenic infections. Therefore, a larger clinical trial appears to be justified.
Acknowledgments
This investigator-sponsored study was supported in part by an unrestricted grant by Bayer Vital GmbH, Leverkusen, Germany. G.C., R.H.B., K.W., and I.S. received financial support from Bayer Vital GmbH.
We thank Klaus A. Schmidt, Aachen, Germany, for his assistance in the preparation of the manuscript.
Footnotes
Published ahead of print on 20 December 2010.
REFERENCES
- 1.Al-Nawas, B., and M. Maeurer. 2008. Severe versus local odontogenic bacterial infections: comparison of microbial isolates. Eur. Surg. Res. 40:220-224. [DOI] [PubMed] [Google Scholar]
- 2.Al-Nawas, B., et al. 2009. Clinical and microbiological efficacy of moxifloxacin versus amoxicillin/clavulanic acid in severe odontogenic abscesses: a pilot study. Eur. J. Clin. Microbiol. Infect. Dis. 28:75-82. [DOI] [PubMed] [Google Scholar]
- 3.Baxter, R., G. T. Ray, and B. H. Fireman. 2008. Case-control study of antibiotic use and subsequent Clostridium difficile-associated diarrhea in hospitalized patients. Infect. Control Hosp. Epidemiol. 9:44-50. [DOI] [PubMed] [Google Scholar]
- 4.Breivik, E. K., G. A. Björnsson, and E. Skovlund. 2000. A comparison of pain rating scales by sampling from clinical trial data. Clin. J. Pain 16:22-28. [DOI] [PubMed] [Google Scholar]
- 5.Brennan, M. T., et al. 2006. Odontogenic signs and symptoms as predictors of odontogenic infection. A clinical trial. J. Am. Dent. Assoc. 137:62-66. [DOI] [PubMed] [Google Scholar]
- 6.Cachovan, G., et al. 2009. Penetration of moxifloxacin into rat mandibular bone and soft tissue. Acta Odontol. Scand. 67:182-186. [DOI] [PubMed] [Google Scholar]
- 6a.Cachovan, G., et al. 2008. Efficacy and tolerability of moxifloxacin versus clindamycin in the treatment of odontogenic abscesses and inflammatory infiltrates (MOCLI Study), abstr. O83. Abstr. 18th Eur. Congr. Clin. Microbiol. Infect. Dis., Barcelona, Spain. http://registration.akm.ch/einsicht.php?XNABSTRACT_ID=67338&XNSPRACHE_ID=2&XNKONGRESS_ID=73&XNMASKEN_ID=900.
- 7.Chan, Y., and C.-H. Chan. 2003. Antibiotic resistance of pathogenic bacteria from odontogenic infections in Taiwan. J. Microbiol. Immunol. Infect. 36:105-110. [PubMed] [Google Scholar]
- 8.Diz Dios, P., et al. 2006. Comparative efficacies of amoxicillin, clindamycin, and moxifloxacin in prevention of bacteremia following dental extractions. Antimicrob. Agents Chemother. 50:2996-3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eick, S., T. Seltmann, and W. Pfister. 2004. Efficacy of antibiotics to strains of periodontopathogenic bacteria within a single species biofilm—an in vitro study. J. Clin. Periodontol. 31:376-383. [DOI] [PubMed] [Google Scholar]
- 10.Esposito, S., et al. 2006. Concentration of moxifloxacin in plasma and tonsillar tissue after multiple administration in adult patients. J. Antimicrob. Chemother. 57:789-792. [DOI] [PubMed] [Google Scholar]
- 11.Guentsch, A., H. Jentsch, W. Pfister, T. Hoffmann, and S. Eick. 2008. Moxifloxacin as an adjunctive antibiotic in the treatment of severe chronic periodontitis. J. Periodontol. 79:1894-1903. [DOI] [PubMed] [Google Scholar]
- 12.Joukhadar, C., et al. 2003. Penetration of moxifloxacin into healthy and inflamed subcutaneous adipose tissues in humans. Antimicrob. Agents Chemother. 47:3099-3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landersdorfer, C. B., et al. 2009. Penetration of moxifloxacin into bone evaluated by Monte Carlo simulation. Antimicrob. Agents Chemother. 53:2074-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Limeres, J., I. Tomás, M. Álvarez, and P. Diz. 2005. Empirical antimicrobial therapy for odontogenic infections. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 100:263-264. [DOI] [PubMed] [Google Scholar]
- 15.Lin, Y. T., and P. W. Lu. 2006. Retrospective study of pediatric facial cellulitis of odontogenic origin. Pediatr. Infect. Dis. 25:339-342. [DOI] [PubMed] [Google Scholar]
- 16.Malangoni, M. A., J. Song, J. Herrington, S. Choudhri, and P. Pertel. 2006. Randomized controlled trial of moxifloxacin compared with piperacillin-tazobactam and amoxicillin-clavulanate for the treatment of complicated intra-abdominal infections. Ann. Surg. 244:204-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milazzo, I., et al. 2002. Antibacterial activity of moxifloxacin against periodontal anaerobic pathogens involved in systemic infections. Int. J. Antimicrob. Agents 20:451-456. [DOI] [PubMed] [Google Scholar]
- 18.Piesold, J. U., B. Al-Nawas, J. E. Otten, and K. A. Grötz. 13 December 2010, accession date. Guidelines of the DGMKG. Odontogenic infections and abscesses. AWMF science-based guidelines for diagnostics and therapy. AWMF guideline registry no. 007/006. Association of the Scientific Medical Societies in Germany, Düsseldorf, Germany. http://www.awmf.org/leitlinien/detail/ll/007-006.html. (In German.)
- 19.Seppänen, L., A. Lauhio, C. Lindqvist, R. Suuronen, and R. Rautemaa. 2008. Analysis of systemic and local odontogenic infection complications requiring hospital care. J. Infect. 57:116-122. [DOI] [PubMed] [Google Scholar]
- 20.Seppänen, L., R. Rautemaa, C. Lindqvist, and A. Lauhio. 2010. Changing clinical features of odontogenic maxillofacial infections. Clin. Oral Invest. 14:459-465. [DOI] [PubMed] [Google Scholar]
- 21.Siqueira, J. F., Jr., and I. N. Rôças. 2009. The microbiota of acute apical abscesses. J. Dent. Res. 88:61-65. [DOI] [PubMed] [Google Scholar]
- 22.Sobottka, I., et al. 2002. In vitro activity of moxifloxacin against bacteria isolated from odontogenic abscesses. Antimicrob. Agents Chemother. 46:4019-4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sobottka, I., et al. 2009. Mikrobiologische Analyse der MOCLI-Studie. Infection 37(Suppl. IV):60-61.17973076 [Google Scholar]
- 24.Stefanopoulos, P. K., and A. E. Kolokotronis. 2004. The clinical significance of anaerobic bacteria in acute orofacial odontogenic infections. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 98:398-408. [DOI] [PubMed] [Google Scholar]
- 25.Uluibau, I. C., T. Jaunay, and A. N. Goss. 2005. Severe odontogenic infections. Aust. Dent. J. 50(Suppl. 2):S74-S81. [DOI] [PubMed] [Google Scholar]
- 26.Vick-Fragoso, R., et al. 2009. Efficacy and safety of sequential intravenous/oral moxifloxacin versus intravenous/oral amoxicillin/clavulanate for complicated skin and skin structure infections. Infection 37:407-417. [DOI] [PubMed] [Google Scholar]