Abstract
Environmental Aeromonas sp. isolates resistant to ceftazidime were recovered during an environmental survey performed with water samples from the Seine River, in Paris, France, in November 2009. Selected isolates were identified by sequencing of the 16S rRNA and rpoB genes. PCR and cloning experiments were used to identify broad-spectrum-β-lactamase-encoding genes and their genetic context. Clavulanic acid-inhibited extended-spectrum-β-lactamase (ESBL) genes were identified in 71% of the Aeromonas sp. isolates. A variety of ESBL genes were detected, including blaVEB-1a, blaSHV-12, blaPER-1, blaPER-6, blaTLA-2, and blaGES-7, suggesting an aquatic reservoir of those ESBL genes. Moreover, the repeated elements and different insertion sequences were identified in association with the blaPER-6 and the blaVEB-1a genes, respectively, indicating a wide diversity of mobilization events, making Aeromonas spp. a vehicle for ESBL dissemination.
Bacteria with intrinsic or acquired resistance to antibiotics are commonly found in aquatic environments, where Pseudomonas, Serratia, and Aeromonas are commonly identified (2, 17). Recent studies reported that the blaTEM-1 gene was detected in most of the Gram-negative isolates resistant to ampicillin recovered from lakes in Brazil or from wastewater treatment plants in China (24, 40). Furthermore, several studies identified extended-spectrum β-lactamases (ESBLs) from water samples. For instance, the clinically relevant ESBL PER-1 was identified from Aeromonas media in a Swiss lake (32), and CTX-M-14 and TEM-52 were detected in Escherichia coli recovered from a South Korean river (21). Besides, nonclinically relevant Ambler class A β-lactamases TLA-2 and PER-6 were identified from uncultured bacteria (43, 12) and Aeromonas allosaccharophila isolates, respectively (15).
In addition, other studies reported on the identification of carbapenem-hydrolyzing β-lactamases in the environment, such as the Ambler class A β-lactamases IMI-2 and BIC-1 from Enterobacter asburiae (1, 5) and Pseudomonas fluorescens (14), respectively; the Ambler class B β-lactamase VIM-2 from Pseudomonas pseudoalcaligenes (41); and the class D β-lactamase OXA-23 from Acinetobacter baumannii (13).
The present study was designed to evaluate the diversity of broad-spectrum β-lactam resistance determinants in Aeromonas species in water samples from the Seine River, Paris, France. The working hypothesis was that Aeromonas spp. could be an important reservoir for ESBLs.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Three water samples (100 ml) were collected from approximately 0.2 m below the water surface in sterile bottles in November 2009 and filtered through nitrocellulose membranes (pore size, 0.45 μm; Millipore), and the filters were resuspended in 3 ml of sterile water. Aliquots (0.1 ml) were plated on MacConkey agar plates supplemented with ceftazidime (2 μg/ml), and 10-fold dilutions were plated (0.1 ml) in parallel on antibiotic-free MacConkey agar plates for bacterial counts. Samples were processed on the day of collection. E. coli DH10B (Invitrogen, Life Technologies, Cergy-Pontoise, France) was used as the host for cloning and transformation experiments as previously described (42). Azide-resistant E. coli J53 was used as the recipient in mating-out assays (32). The kanamycin-resistant plasmid pBKCMV (Invitrogen) was used as a cloning vector. Bacterial cultures were grown in Trypticase soy (TS) broth at 37°C for 18 h.
Antibiotic susceptibility testing.
The antibiotic susceptibility profiles of 80 ceftazidime- or imipenem-resistant isolates were determined by the agar dilution method, and the results were interpreted according to the CLSI guidelines (7). AmpC overproducers were identified by testing susceptibility to ceftazidime on Mueller-Hinton agar plates supplemented with cloxacillin (250 μg/ml). Detection of ESBL production was carried out by the double-disk synergy test (DDST) (19).
Identification of ceftazidime-resistant isolates and genotyping.
Isolates were identified by the API 32GN system (bioMérieux, Marcy l'Etoile, France) and/or sequencing of the 16S RNA and/or rpoB or gyrB genes by using previously described primers (for 16S, primers 27F and 1492R [16]; for gyrB, primers gyrB3F and gyrB14R [17]; for rpoB, primers rpoBLAPS and LAPS27 [26]). Amplicons were sequenced and their sequences were compared to those in the nucleotide data library using leBIBI software (http://umr5558-sud-str1.univ-lyon1.fr/lebibi/lebibi.cgi) in order to determine their closest phylogenetic relatives. The genetic diversity of the ESBL-producing Aeromonas sp. isolates was assessed by pulsed-field gel electrophoresis (PFGE) using XbaI, as previously described (21). The chromosomal fingerprints were compared by eye and assigned to PFGE types and subtypes (44).
PCR amplification for detection of ESBL genes, analysis of genetic environment, and sequencing.
Specific primers were used under standard PCR conditions to detect ESBL-encoding genes, namely, blaTEM (9), blaSHV (30), blaCTX-M (30), blaGES (33), blaPER-1/-2 (31, 35), blaPER-6 (15), blaVEB (39), and blaBEL (34). The genetic environments of the blaPER-1, blaTLA-2, and blaSHV genes were determined by PCR mapping using previously published specific primers together with primers designed to anneal at the 5′ and the 3′ conserved segments (CSs) of class 1 integrons (35, 12). The genetic environments of blaPER-6, blaVEB-1a, and blaGES-7 were precisely determined by cloning those genes into plasmid pBKCMV. For that purpose, BamHI-, SacI-, ScaI-, or SalI-restricted DNAs were ligated into restricted plasmid pBKCMV and introduced into E. coli DH10B by electroporation as described previously (42). Recombinant plasmids were selected on TS agar plates containing ceftazidime (2 μg/ml) and kanamycin (30 μg/ml). All enzymes for DNA manipulations were used according to the recommendations of the supplier (GE Healthcare, Orsay, France). Recombinant plasmids were sequenced by using combinations of universal T3 and T7 primers and specific gene primers (Table 1), designed on the basis of sequences obtained on an Applied Biosystems sequencer (ABI 3130).
TABLE 1.
Oligonucleotides used for PCR amplification and sequencing
| Primer | Sequence (5′ to 3′) | Reference or source |
|---|---|---|
| VEB-inv1 | GTCAGTTTGAGCATTTGAATAC | 37 |
| VEB-inv2 | AAGCGTATTTGTTGCAGAGTC | 37 |
| IS26out | CCCACAGAATGATGTCACGC | This study |
| aadB-B | CGCATATCGCGACCTGAAAGC | This study |
| Av9_1 A | AAGTGCCTAGAGATTGTTTCC | This study |
| TnpF-A | AACTACTGCTGGAGTATATGG | This study |
| IS4B | TAAATCATCAGGCTACAGGTC | This study |
| IS6100-A | ATTTCTACCTGTCGCCGACC | This study |
Genetic support of β-lactamase-encoding genes.
Plasmid extraction was performed by the Kieser technique (20). E. coli NCTC 50192, harboring four plasmids of 154, 66, 38, and 7 kb, was used as a size marker for plasmids. Transformation assays were performed by electroporation with plasmid extracts from the identified positive isolates, and E. coli DH10B was used as the recipient strain. Selection was performed on agar plates supplemented with ceftazidime (2 μg/ml). Conjugation experiments were carried out as described previously (32). The I-CeuI restriction enzyme (Ozyme; New England Biolabs, Saint-Quentin-en-Yvelines, France), which digests a 26-bp sequence in the rrn genes for the 23S large-subunit rRNA, was used to determine whether the β-lactamase gene had a chromosomal location, and the fragments were separated by pulsed-field gel electrophoresis (36). DNA-DNA hybridization of plasmid extracts was performed after a Southern transfer onto a Hybond N+ nylon membrane (GE Healthcare), as previously described (42). Labeling of the probe and signal detection were carried out using an enhanced chemiluminescence labeling and detection kit, according to the manufacturer's instructions (GE Healthcare). PCR-based replicon-typing (PBRT) analysis was performed as described by Carattoli et al. (6). The 17 primer pairs targeting the FIA, FIB, FIC, HI1, HI2, I1-I, L/M, N, P, W, T, A/C, K, B/O, X, Y, and FII replicons were used in separate PCRs.
Nucleotide sequence accession numbers.
The nucleotide sequence data reported in this paper will appear in the GenBank nucleotide database under accession numbers HM453325, HM370390 to HM370393, HM453326, HM453327, and HM626463.
RESULTS
Bacterial counts and susceptibility testing.
The total bacterial count on MacConkey agar was 1.5 × 105 CFU/liter of Seine River water, of which 2.6% grew on ceftazidime-agar plates (3.6 × 103 CFU/liter). A total of 73 isolates were selected from ceftazidime-containing plates and subjected to identification. Fifty-six percent (n = 41) of the isolates that were resistant or of reduced susceptibility to ceftazidime were identified as Aeromonas spp., and the others were mostly Pseudomonas spp. and Stenotrophomonas maltophilia. Among those 41 ceftazidime-resistant Aeromonas spp., 29 (71%) displayed an ESBL phenotype, as evidenced by DDST. These isolates were identified as A. punctata, A. allosaccharophila, A. veronii, and A. media. Our study then focused on those 29 ESBL-producing Aeromonas isolates. The MICs of β-lactams, the patterns of resistance to other antimicrobial agents, and the molecular typing were used to evaluate that collection, and the findings are presented in Table 2. The 29 ceftazidime-resistant isolates were resistant to ticarcillin and had reduced susceptibility to cefotaxime and cefepime, but none of them were resistant to imipenem (Table 2). Addition of clavulanic acid significantly decreased the MICs of ceftazidime and aztreonam (Table 2). More than 86% of these isolates were resistant to sulfonamides and to nalidixic acid, although Aeromonas spp. are naturally fully susceptible to quinolones (Table 2) (22).
TABLE 2.
Resistance pattern of ESBL-producing Aeromonas sp. isolates
| Isolate | Aeromonas species | Clone | ESBL identified | MIC (μg/ml)b |
Non-β-lactam(s) to which isolate was resistant | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTX | FEP | CAZ | CAZ + CLAV | TIC | TIC + CLAV | ATM | ATM + CLAV | IPM | |||||
| 1 | A. punctata | A1a | VEB-1a | 8 | 2 | 32 | 0.25 | 128 | 16 | 8 | <0.06 | 0.12 | KAN, TOB, GEN, AMK, TET, NAL, CIP, FOF, CHL, SXT |
| 2 | A. punctata | A2 | VEB-1a | 8 | 4 | 32 | 0.25 | 128 | 1 | 8 | <0.06 | 0.12 | KAN, TOB, GEN, AMK, TET, NAL, CIP, FOF, CHL, SXT |
| 3 | A. punctata | A3 | VEB-1a | 4 | 1 | 32 | 0.06 | 128 | 0.5 | 2 | <0.06 | <0.06 | KAN, TOB, AMK, TET, NAL, CIP, SXT |
| 4 | A. punctata | A3 | VEB-1a | 8 | 2 | 32 | 0.12 | 64 | 2 | 4 | <0.06 | <0.06 | KAN, TOB, TET, NAL, CIP, SXT |
| 5 | A. punctata | A4 | VEB-1a | 4 | 2 | 16 | 0.25 | 128 | 128 | 2 | <0.06 | 0.12 | SXT |
| 6 | A. punctata | A4 | VEB-1a | 8 | 4 | 32 | 0.25 | 256 | 128 | 8 | <0.06 | 0.12 | SXT |
| 7 | A. allosaccharophila | B1 | VEB-1a | 4 | 2 | 16 | 0.06 | 32 | 2 | 2 | <0.06 | 0.25 | KAN, TOB, TET, NAL, SXT |
| 8 | A. allosaccharophila | B2 | VEB-1a | 8 | 2 | 16 | 0.06 | 128 | 16 | 4 | <0.06 | 0.12 | KAN, TOB, TET, NAL, CIP, SXT |
| 9 | A. veronii | C1 | VEB-1a | 0.25 | 0.12 | 2 | 0.06 | 16 | 4 | 0.5 | <0.06 | 0.25 | SXT |
| 10 | A. veronii | C2 | VEB-1a | 1 | 0.25 | 4 | 0.06 | 32 | 4 | 0.25 | <0.06 | 0.5 | |
| 11 | A. media | D1 | VEB-1a | 32 | 4 | 32 | 0.5 | 512 | 512 | 16 | <0.06 | <0.06 | NAL |
| 12 | A. allosaccharophila | B3 | SHV-12 | 0.25 | 0.25 | 2 | 0.06 | 128 | 1 | 1 | <0.06 | 0.25 | NAL |
| 13 | A. allosaccharophila | B3 | SHV-12 | 1 | 1 | 4 | 0.06 | 512 | 2 | 2 | <0.06 | 0.25 | NAL |
| 14 | A. allosaccharophila | B4 | SHV-12 | 4 | 0.25 | 4 | 0.06 | 256 | 2 | 2 | <0.06 | 0.25 | NAL |
| 15 | A. allosaccharophila | B4 | SHV-12 | 1 | 0.5 | 4 | 0.12 | 512 | 2 | 2 | <0.06 | 0.25 | NAL |
| 16 | A. allosaccharophila | B4 | SHV-12 | 1 | 0.25 | 4 | 0.06 | 256 | 2 | 2 | <0.06 | 0.25 | NAL |
| 17 | A. allosaccharophila | B4 | SHV-12 | 1 | 0.25 | 4 | 0.06 | 256 | 2 | 1 | <0.06 | 0.25 | NAL |
| 18 | A. veronii | C3 | SHV-12 | 4 | 1 | 4 | 0.06 | 512 | 16 | 4 | <0.06 | 0.25 | NAL |
| 19 | A. veronii | C3 | SHV-12 | 1 | 1 | 4 | 0.06 | 512 | 8 | 4 | <0.06 | 0.25 | NAL |
| 20 | A. veronii | C3 | SHV-12 | 4 | 2 | 8 | 0.06 | 128 | 8 | 2 | <0.06 | 0.25 | NAL |
| 21 | A. media | D2 | SHV-12 | 8 | 2 | 32 | 0.5 | 512 | 256 | 16 | <0.06 | <0.06 | NAL, RIF, SXT |
| 22 | A. punctata | A5 | PER-1 | 8 | 4 | 32 | 0.25 | 256 | 64 | 16 | <0.06 | 0.12 | KAN, NAL, SXT |
| 23 | A. punctata | A6 | PER-1 | 8 | 4 | 32 | 0.25 | 128 | 128 | 16 | <0.06 | 0.12 | NAL, SXT |
| 24 | A. media | D3 | PER-1 | 16 | 4 | 64 | 0.25 | 128 | 16 | 64 | <0.06 | 0.12 | KAN, NAL |
| 25 | A. veronii | C4 | PER-6 | 4 | 2 | 64 | 0.25 | 128 | 128 | 32 | <0.06 | 0.12 | TET, NAL, SXT |
| 26 | A. allosaccharophila | B5 | PER-6 | 4 | 0.5 | 2 | 0.06 | 512 | 64 | 4 | <0.06 | 0.5 | TET, NAL, SXT |
| 27 | A. allosaccharophila | B5 | PER-6 | 4 | 0.5 | 8 | 2 | 512 | 512 | 8 | 0.25 | 0.5 | TET, NAL |
| 28 | A. veronii | C4 | GES-7 | 2 | 0.5 | 16 | 2 | 64 | 32 | 0.25 | <0.06 | 0.25 | KAN, TOB, NAL |
| 29 | A. allosaccharophila | B6 | TLA-2 | 2 | 1 | 8 | 0.12 | 128 | 64 | 1 | <0.06 | 0.12 | NAL, RIF |
The letter A was arbitrarily attributed to A. punctata, B to A. allosaccharophila, C to A. veronii, and D to A. media. Different numbers correspond to different clones.
Abbreviations: AMK, amikacin; ATM, aztreonam; CHL, chloramphenicol; CAZ, ceftazidime; CIP, ciprofloxacin; CLA, clavulanic acid; CTX, cefotaxime; FEP, cefepime; FOF, fosfomycin; GEN, gentamicin; IPM, imipenem; KAN, kanamycin; NAL; nalidixic acid; RIF, rifampin; SXT, trimethoprim-sulfamethoxazole; SSS, sulfonamide; TET, tetracycline; TIC, ticarcillin; TOB, tobramycin.
Identification of acquired β-lactamase genes.
PCRs and sequencing performed on the 29 Aeromonas sp. isolates exhibiting an ESBL phenotype, using primers specific for β-lactamase genes, identified the blaVEB-1a (n = 11), blaSHV-12 (n = 10), blaPER-1 (n = 3), blaPER-6 (n = 3), blaTLA-2 (n = 1), and blaGES-7 (n = 1) genes (Table 2).
Clonal relationship.
PFGE analysis performed on the 29 Aeromonas sp. isolates showed 19 distinct genotypes, including 4 clonally related A. allosaccharophila isolates harboring an identical blaSHV-12 gene (Table 2; data not shown). The ESBL genes were therefore identified in a large diversity of Aeromonas species (Table 2).
Genetic support of blaSHV-12 gene.
PCR mapping and sequencing revealed that the blaSHV-12 gene, identified in 10 Aeromonas sp. isolates, was preceded by insertion sequence (IS) IS26, as previously reported (10). Southern blot hybridization of plasmid DNA extracted from the blaSHV-12-positive Aeromonas sp. isolates using the corresponding probe did not give any signal, suggesting a chromosomal location of that ESBL gene (data not shown).
Genetic support of blaTLA-2 gene.
PCR mapping to determine the genetic environment of the blaTLA-2 gene in the single A. allosaccharophila isolate was performed with combinations of primers located in the 5′ CS, blaTLA-2, and 3′ CS sequences. The blaTLA-2 gene was not part of a gene cassette but was located in a class 1 integron, located on a ca. 50-kb plasmid. Mating-out transfer of the plasmid harboring the blaTLA-2 gene to E. coli remained unsuccessful, suggesting that this plasmid has a narrow host range or was nonconjugative (43).
Genetic support of blaPER-1 gene.
The blaPER-1 gene identified in three Aeromonas sp. isolates was preceded by insertion sequence ISPa12 and was followed by ISPa13, thus being part of a composite transposon, Tn1213, as previously reported (35). Southern blot hybridization of plasmid DNA extracts recovered from the blaPER-1-positive Aeromonas sp. isolates using the corresponding probes did not give any signal, suggesting a chromosomal location of the blaPER-1 gene (data not shown).
Genetic support of blaPER-6 gene.
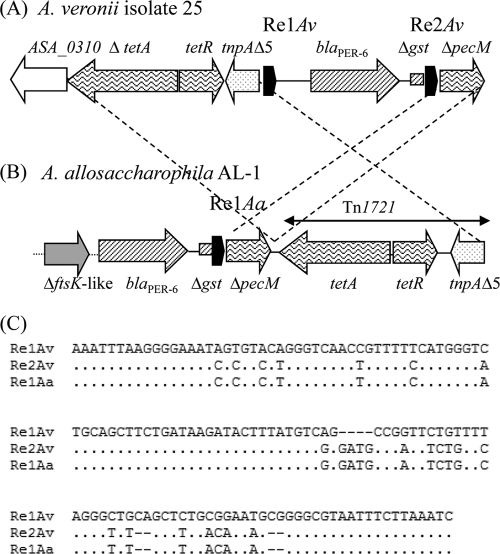
The blaPER-6 gene was identified in an A. veronii isolate and in clonally related A. allosaccharophila (isolates 26 and 27). Southern blot hybridization of plasmid DNA extracted from these isolates, using a blaPER-6-specific probe, did not give any positive signal, suggesting a chromosomal location of this gene (data not shown). Analysis of the sequences surrounding the blaPER-6 gene by PCR mapping showed that they were different from those initially identified in A. allosaccharophila recovered from the Seine River in 2009 (15). Thus, cloning of the blaPER-6 gene from A. veronii isolate 25 was performed, and sequencing revealed that the blaPER-6 gene interrupted the Tn1721 feature, including the tetA, tetR, and tnpAΔ5 genes, as previously identified in the A. allosaccharophila AL-1 isolate (15) (Fig. 1). Interestingly, the blaPER-6 gene was bracketed by 135-bp repeated elements (Res; named Re1Av and Re2Av for A. veronii) sharing 84 and 80% nucleotide sequence identities with the reverse complementary sequence of the previously reported Re1 that bracketed the blaVEB-1a gene (3). Re1-like sequences have been identified in association with blaVEB-like genes in P. aeruginosa (3), Providencia stuartii (4), and Proteus mirabilis (48). In silico analysis of previously reported sequences surrounding the blaPER-6 gene in A. allosaccharophila AL-1 showed a downstream-located Re1-like copy (namely, Re1Aa, for A. allosaccharophila) (Fig. 1). Re1Aa shares 80% nucleotide sequence identity with Re1Av.
FIG. 1.
Schematic representation of the genetic environment of β-lactamase genes. The genetic environment of the blaPER-6 gene in A. veronii isolate 25 and A. allosaccharophila isolates 26 and 27 (A) is compared to the previously identified structure in A. allosaccharophila AL-1 (B) (15). Arrows indicate the direction of transcription of the coding regions. Repeated elements ReAv and ReAa are indicated as short black arrows. The open reading frame named ASA_0310 is a sugar-phosphate isomerase from A. salmonicida; TnpAΔ5 is a truncated relaxase/helicase. (C) Comparison of the sequences of Re1Av, Re2Av, and Re1Aa. Dashes indicate gaps introduced to optimize alignment, and periods indicate nucleotides identical to those in the Re1Av sequence.
Genetic support of blaGES-7 gene.
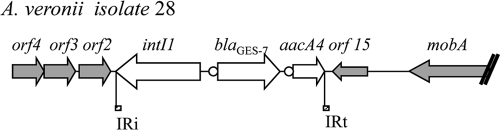
Southern blot hybridization of plasmid DNA extracted from A. veronii isolate 28, using a probe specific for blaGES-like genes, showed that the blaGES-7 gene was located on a ca. 60-kb plasmid. Partial sequencing of this natural plasmid (named Tf28) showed that the blaGES-7 gene was located inside an original structure in which the 3′ extremity of the class 1 integron was absent (Fig. 2). The blaGES-7 gene was in the form of a gene cassette, located at the first position of a class 1 integron and followed by the aacA4 gene. Downstream of the aacA4 gene, there was no 3′ CS sequence identified, but instead, the orf15 and mobA gene arrays were identified. The structure containing orf15, mobA, orf2, orf3, and orf4 was previously identified in plasmid pRSB101 (43). Between the aacA4 and orf15 genes, the terminal inverted repeat (IRt) of Tn402 was identified. The terminal inverted repeat of class 1 integrons (IRi) is present upstream of the gene encoding the IntI1 integrase and has the same boundary with the adjacent sequence as in pRSB101 (43). In order to determine whether the backbone of the Tf28 plasmid was the same as that of pRSB101, PCR amplification was performed with primers located in the transporter module (orf11, encoding a resistance-nodulation-cell division [RND] efflux membrane fusion protein) and in the repA gene. The absence of PCR amplification suggested a different backbone. Mating-out assays between A. veronii isolate 28 and E. coli DH10B, performed at 37°C or at 30°C, remained unsuccessful, suggesting that the Tf28 plasmid was not self-transferable.
FIG. 2.
Genetic environment of the blaGES-7 gene in A. veronii isolate 28. Arrows indicate the direction of transcription of the coding regions. The orf2, orf3, orf4, orf15, and mobA genes previously identified in plasmid pRSB101 (43) are indicated as gray arrows. IRt of the Tn402-like sequence and IRi are also shown.
Genetic support of blaVEB-1a gene.
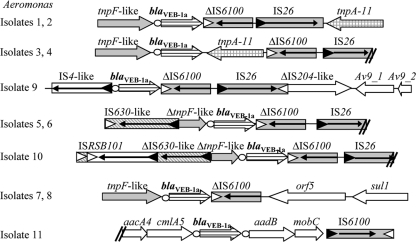
The blaVEB-1a gene was identified in 11 Aeromonas sp. isolates. Plasmid extraction and Southern hybridization showed that the blaVEB-1a gene was chromosomally located in A. punctata isolates 1 and 2, whereas it was plasmid located in all the other Aeromonas sp. isolates. The sizes of the plasmids harboring the blaVEB-1a gene varied from 30 kb in A. media isolate 11 to 50 kb in A. allosaccharophila isolates 7 and 8 and up to 170 kb in all other isolates. Investigation of the genetic context with primers designed from the previously identified structures failed. The identification of blaVEB-1-like genes has been reported from the family Enterobacteriaceae, P. aeruginosa, and A. baumannii recovered worldwide (27, 36, 39). The blaVEB-1 gene is often part of a gene cassette located in class 1 integrons. However, in some cases, the blaVEB-1 and blaVEB-1a genes have been identified in association with Res in P. aeruginosa and the Enterobacteriaceae (3, 28, 38). More recently, the blaVEB-1a gene has been identified in association with two copies of insertion sequence ISCR2 (37). PCR assays revealed that none of these previously described genetic structures was bracketing the blaVEB-1a gene in the Aeromonas isolates recovered in the present study. Sequence analysis of the obtained recombinant or natural plasmids harboring the blaVEB-1a gene revealed some similarities among all genetic environments, as shown in Fig. 3. In particular, partial sequences of a gene encoding a TnpF-like putative integrase from Acinetobacter genomospecies 3 (GenBank accession no. GQ926879) and of insertion sequences IS6100 and IS26 were identified (Fig. 3). Mating-out assays performed with Aeromonas isolates 3 to 11 as donors and E. coli J53 as the recipient, either at 37°C or at 30°C, remained unsuccessful, suggesting that these plasmids were not self-transferable. PBRT analysis showed that the blaVEB-1a-bearing plasmids did not belong to any of the tested Inc groups that correspond to plasmids identified in the Enterobacteriaceae (6).
FIG. 3.
Genetic environment of the blaVEB-1a gene in A. punctata isolates 1 to 6, A. allosaccharophila isolates 7 and 8, A. veronii isolates 9 and 10, and A. media isolate 11. The coding genes are represented by arrows indicating their translation orientation, the left and right inverted repeats of IS elements are shown by filled and empty triangles, respectively, and the core sites are indicated as circles. IS6100, IS26, and tnpF-like genes (gray rectangles) are recovered in most structures. TnpF-like is a putative integrase from Acinetobacter genomospecies 3 (GenBank accession no. GQ926879). TnpA-11 (arrow with gray squares) from A. punctata 1 to 4 shares 83% amino acid sequence identity with the sequence of transposase_11 from A. punctata (GenBank accession no. YP_067863). Av9_1 and Av9_2 from A. veronii 9 are proteins of unknown function.
DISCUSSION
Aeromonas spp. were the predominant bacteria recovered in this study, representing 56% of the colonies obtained on ceftazidime-containing plates. The ceftazidime resistance that we have observed was mainly related to the expression of ESBLs (71%). Many ESBLs that are not frequently identified among clinically relevant Gram-negative isolates in Paris (29), namely, VEB-1a, GES-7, TLA-2, and PER-1 and PER-6, have been identified in this screening. It was actually unexpected to identify such an important diversity of class A ESBLs in Aeromonas spp., which are species known to already possess intrinsic Ambler class B, C, and D β-lactamase genes. This raises the question of why Aeromonas spp. are so often hosts for those antibiotic resistance determinants. This waterborne location may be one part of the explanation. Rivers are subjected to the effects of several human activities. We considered this environment to be a good model for studying the prevalence and molecular diversity of genes that might represent a potential risk for human health. Aeromonas spp. living in water were demonstrated to be a potent reservoir of antibiotic resistance genes.
The blaVEB-1a gene was identified in 38% of Aeromonas spp. resistant to or with reduced susceptibility to ceftazidime recovered from the Seine River, whereas blaVEB-1 has been identified in France only once in E. coli (25) and several times in A. baumannii (27, 36). The wide dissemination of this gene among so diverse Aeromonas species is of interest, since it likely indicates that the natural reservoir of that clinically relevant ESBL gene is waterborne or raises the possibility of Seine River contamination. In addition, our study emphasizes that the dissemination of blaVEB-1-like genes is related to a variety of genetic structures.
In addition, other rare β-lactamase-encoding genes were identified here, in particular, the blaTLA-2, blaPER-6, and blaGES-7 genes. The blaTLA-2 gene had already been identified on plasmid pRSB101, which had been recovered from wastewater treatment plants in Germany but which has never been reported from clinical samples (43). The blaPER-1 gene is mainly reported in Europe and Asia (45, 46, 23) and to a lesser extent in North Africa (18), but it has very rarely been identified in France (35). The blaPER-6 gene has been identified once from the Seine River in A. allosaccharophila (15). Similarly, the blaGES-7/IBC-1 gene has been detected only from clinical Enterobacteriaceae in Greece (11, 47) and in Brazil (8) but had never been identified in France.
Analysis of the genetic structures surrounding all these ESBL genes in environmental Aeromonas isolates revealed an important diversity of genetic supports and genetic environments. Noticeably, the identification of ReAv repeated elements in association with the blaPER-6 gene further underlines that those still underinvestigated genetic elements might play a relevant role in antibiotic resistance gene dissemination and mobilization.
Acknowledgments
This work was funded by a grant from INSERM (U914) and from the Ministère de l'Education Nationale et de la Recherche (UPRES-EA 3539), Université Paris XI, Paris, France, and mostly by grants from the European Community (TROCAR, HEALTH-F3-2008-223031, and TEMPOtest-QC, HEALTH-2009-241742).
Footnotes
Published ahead of print on 13 December 2010.
REFERENCES
- 1.Ambler, R. P., et al. 1991. A standard numbering scheme for the class A β-lactamases. Biochem. J. 276:269-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ash, R. J., B. Mauck, and M. Morgan. 2002. Antibiotic resistance of gram-negative bacteria in rivers, United States. Emerg. Infect. Dis. 8:713-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aubert, D., D. Girlich, T. Naas, S. Nagarajan, and P. Nordmann. 2004. Functional and structural characterization of the genetic environment of an extended-spectrum β-lactamase blaVEB gene from a Pseudomonas aeruginosa isolate obtained in India. Antimicrob. Agents Chemother. 48:3284-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aubert, D., T. Naas, M.-F. Lartigue, and P. Nordmann. 2005. Novel genetic structure associated with an extended-spectrum β-lactamase blaVEB gene in a Providencia stuartii clinical isolate from Algeria. Antimicrob. Agents Chemother. 49:3590-3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aubron, C., L. Poirel, R. J. Ash, and P. Nordmann. 2005. Carbapenemase-producing Enterobacteriaceae, U.S. rivers. Emerg. Infect. Dis. 11:260-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carattoli, A., et al. 2005. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63:219-228. [DOI] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. 2010. Performance standards for antimicrobial susceptibility testing, 19th informational supplement, M100-S19. Clinical and Laboratory Standards Institute, Wayne, PA.
- 8.Dropa, M., et al. 2010. Emergence of Klebsiella pneumoniae carrying the novel extended-spectrum β-lactamase gene variants blaSHV-40, blaTEM-116 and the class 1 integron-associated blaGES-7 in Brazil. Clin. Microbiol. Infect. 16:630-632. [DOI] [PubMed] [Google Scholar]
- 9.Dubois, V., et al. 2005. Prolonged outbreak of infection due to TEM-21-producing strains of Pseudomonas aeruginosa and enterobacteria in a nursing home. J. Clin. Microbiol. 43:4129-4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ford, P. J., and M. B. Avison. 2004. Evolutionary mapping of the SHV β-lactamase and evidence for two separate IS26-dependent blaSHV mobilization events from the Klebsiella pneumoniae chromosome. J. Antimicrob. Chemother. 54:69-75. [DOI] [PubMed] [Google Scholar]
- 11.Giakkoupi, P., et al. 2000. IBC-1, a novel integron-associated class A β-lactamase with extended-spectrum properties produced by an Enterobacter cloacae clinical strain. Antimicrob. Agents Chemother. 44:2247-2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Girlich, D., L. Poirel, A. Schlüter, and P. Nordmann. 2005. TLA-2, a novel Ambler class A expanded-spectrum β-lactamase. Antimicrob. Agents Chemother. 49:4767-4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girlich, D., L. Poirel, and P. Nordmann. 2010. First isolation of the blaOXA-23 carbapenemase gene from an environmental Acinetobacter baumannii isolate. Antimicrob. Agents Chemother. 54:578-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girlich, D., L. Poirel, and P. Nordmann. 2010. Novel Ambler class A carbapenem-hydrolyzing β-lactamase from a Pseudomonas fluorescens isolate from the Seine River, Paris, France. Antimicrob. Agents Chemother. 54:328-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girlich, D., L. Poirel, and P. Nordmann. 2010. PER-6, an extended-spectrum β-lactamase from Aeromonas allosaccharophila. Antimicrob. Agents Chemother. 54:1619-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henriques, I., F. Fonseca, A. Alves, M. J. Saavedra, and A. Correia. 2006. Occurrence and diversity of integrons and β-lactamase genes among ampicillin-resistant isolates from estuarine waters. Res. Microbiol. 157:938-947. [DOI] [PubMed] [Google Scholar]
- 17.Huddleston, J. R., J. C. Zak, and R. M. Jeter. 2006. Antimicrobial susceptibilities of Aeromonas spp. isolated from environmental sources. Appl. Environ. Microbiol. 72:7036-7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iabadene, H., et al. 2009. Emergence of extended-spectrum β-lactamase PER-1 in Proteus vulgaris and Providencia stuartii isolates from Algiers, Algeria. Antimicrob. Agents Chemother. 53:4043-4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarlier, V., M. H. Nicolas, G. Fournier, and A. Philippon. 1988. Extended broad-spectrum β-lactamases conferring transferable resistance to newer β-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev. Infect. Dis. 10:867-878. [DOI] [PubMed] [Google Scholar]
- 20.Kieser, T. 1984. Factors affecting the isolation or CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid 12:19-36. [DOI] [PubMed] [Google Scholar]
- 21.Kim, J., H. Y. Kang, and Y. Lee. 2008. The identification of CTX-M-14, TEM-52, and CMY-1 enzymes in Escherichia coli isolated from the Han River in Korea. J. Microbiol. 46:478-481. [DOI] [PubMed] [Google Scholar]
- 22.Ko, W. C., et al. 1996. Increasing antibiotic resistance in clinical isolates of Aeromonas strains in Taiwan. Antimicrob. Agents Chemother. 40:1260-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee, H. W., H. Y. Kang, K. S. Shin, and J. Kim. 2007. Multidrug-resistant Providencia isolates carrying blaPER-1, blaVIM-2, and armA. J. Microbiol. 45:272-274. [PubMed] [Google Scholar]
- 24.Li, D., et al. 2009. Antibiotic-resistance profile in environmental bacteria isolated from penicillin production wastewater treatment plant and the receiving river. Environ. Microbiol. 11:1506-1517. [DOI] [PubMed] [Google Scholar]
- 25.Mammeri, H., M. Van de Loo, L. Poirel, L. Martinez-Martinez, and P. Nordmann. 2005. Emergence of plasmid-mediated quinolone resistance in Escherichia coli in Europe. Antimicrob. Agents Chemother. 49:71-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marchand, S., et al. 2009. Seasonal influence on heat-resistant proteolytic capacity of Pseudomonas lundensis and Pseudomonas fragi, predominant milk spoilers isolated from Belgian raw milk samples. Environ. Microbiol. 11:467-482. [DOI] [PubMed] [Google Scholar]
- 27.Naas, T., et al. 2006. VEB-1 extended-spectrum β-lactamase-producing Acinetobacter baumannii, France. Emerg. Infect. Dis. 12:1214-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naas, T., D. Aubert, T. Lambert, and P. Nordmann. 2006. Complex genetic structures with repeated elements, a sul-type class 1 integron, and the blaVEB extended-spectrum β-lactamase gene. Antimicrob. Agents Chemother. 50:1745-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naas, T., L. Poirel, and P. Nordmann. 2008. Minor extended-spectrum β-lactamases. Clin. Microbiol. Infect. 14:42-52. [DOI] [PubMed] [Google Scholar]
- 30.Nagano, N., Y. Nagano, C. Cordevant, N. Shibata, and Y. Arakawa. 2004. Nosocomial transmission of CTX-M-2 β-lactamase-producing Acinetobacter baumannii in a neurosurgery ward. J. Clin. Microbiol. 42:3978-3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nordmann, P., et al. 1993. Characterization of a novel extended-spectrum β-lactamase from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 37:962-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Picão, C. R., et al. 2008. Expanded-spectrum β-lactamase PER-1 in an environmental Aeromonas media isolate from Switzerland. Antimicrob. Agents Chemother. 52:3461-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poirel, L., I. Le Thomas, T. Naas, A. Karim, and P. Nordmann. 2000. Biochemical sequence analyses of GES-1, a novel class A extended-spectrum β-lactamase, and the class 1 integron In52 from Klebsiella pneumoniae. Antimicrob. Agents Chemother. 44:622-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poirel, L., L. Brinas, A. Verlinde, L. Ide, and P. Nordmann. 2005. BEL-1, a novel clavulanic acid-inhibited extended-spectrum β-lactamase, and the class 1 integron In120 in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 49:3743-3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poirel, L., L. Cabanne, H. Vahaboglü, and P. Nordmann. 2005. Genetic environment and expression of the extended-spectrum β-lactamase blaPER-1 gene in gram-negative bacteria. Antimicrob. Agents Chemother. 49:1708-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poirel, L., O. Menuteau, N. Agoli, C. Cattoen, and P. Nordmann. 2003. Outbreak of extended-spectrum β-lactamase VEB-1-producing isolates of Acinetobacter baumannii in a French hospital. J. Clin. Microbiol. 41:3542-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poirel, L., et al. 2009. ISCR2, another vehicle for blaVEB gene acquisition. Antimicrob. Agents Chemother. 53:4940-4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poirel, L., T. Naas, and P. Nordmann. 2008. Genetic support of extended-spectrum β-lactamases. Clin. Microbiol. Infect. 14:75-81. [DOI] [PubMed] [Google Scholar]
- 39.Poirel, L., et al. 1999. Molecular and biochemical characterization of VEB-1, a novel class A extended-spectrum β-lactamase encoded by an Escherichia coli integron gene. Antimicrob. Agents Chemother. 43:573-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pontes, D. S., et al. 2009. Multiple antimicrobial resistance of Gram-negative bacteria from natural oligotrophic lakes under distinct anthropogenic influence in a tropical region. Microb. Ecol. 58:762-772. [DOI] [PubMed] [Google Scholar]
- 41.Quinteira, S., and L. Peixe. 2006. Multiniche screening reveals the clinically relevant metallo-β-lactamase VIM-2 in Pseudomonas aeruginosa far from the hospital setting: an ongoing dispersion process? Appl. Environ. Microbiol. 72:3743-3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 43.Szczepanowski, R., et al. 2004. Antibiotic multiresistance plasmid pRSB101 isolated from a wastewater treatment plant is related to plasmids residing in phytopathogenic bacteria and carries eight different resistance determinants including a multidrug transport system. Microbiology 150:3613-3630. [DOI] [PubMed] [Google Scholar]
- 44.Tenover, F. C., et al. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vahaboglü, H., et al. 1997. Widespread detection of PER-1-type extended-spectrum β-lactamase among nosocomial Acinetobacter and Pseudomonas aeruginosa isolates in Turkey: a nationwide multicenter study. Antimicrob. Agents Chemother. 41:2265-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vahaboglü, H., et al. 1996. Characterization of multiple-antibiotic-resistant Salmonella typhimurium strains: molecular epidemiology of PER-1-producing isolates and evidence for nosocomial plasmid exchange by a clone. J. Clin. Microbiol. 34:2942-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vourli, S., et al. 2003. Characterization of In111, a class 1 integron that carries the extended-spectrum β-lactamase gene blaIBC-1. FEMS Microbiol. Lett. 225:149-153. [DOI] [PubMed] [Google Scholar]
- 48.Zong, Z., S. R. Partridge, and J. R. Iredell. 2009. A blaVEB-1 variant, blaVEB-6, associated with repeated elements in a complex genetic structure. Antimicrob. Agents Chemother. 53:1693-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]