Abstract
Studies about the relationship between antibiotic consumption and carriage of antibiotic-resistant Escherichia coli in individual patients have yielded conflicting results. The goal of this study was to identify individual- and household-level factors associated with carriage of ampicillin (AMP)-resistant E. coli during consumption of a course of oral antibiotics. We enrolled outpatients and their families in a prospective household study of AMP-resistant or AMP-susceptible E. coli carriage. Two kinds of index patients were identified. Group 1 consisted of outpatients who were being initiated on a new antibiotic course at the time of a clinic visit, and group 2 consisted of outpatients not starting antibiotics. Each participant was asked to submit three stool swab samples (at baseline, week 1, and week 4) and to complete a questionnaire. Antimicrobial susceptibility testing was performed on each phenotypically distinct E. coli colony. The study included 149 group 1 households (total, 570 participants) and 38 group 2 households (total, 131 participants). AMP-resistant E. coli was recovered from 29% of stool samples. Observed associations with antibiotic exposure varied by drug class. Penicillins, which were the most frequently prescribed drug class, were associated with a modest increase in AMP-resistant E. coli carriage and a modest decrease in AMP-susceptible E. coli carriage. Neither change by itself was statistically significant. Macrolides were associated with reduced carriage of both AMP-resistant E. coli and AMP-susceptible E. coli (P < 0.05). Both AMP-resistant and AMP-susceptible E. coli demonstrated household clustering (P < 0.001). In summary, the overall effect of antibiotics on individual risk of carriage of AMP-resistant E. coli was small. However, even a modest alteration of the competitive balance between AMP-resistant and AMP-susceptible E. coli may promote population spread of resistant E. coli. Examining changes in both resistant and susceptible organisms in antibiotic-treated individuals and their close contacts improves understanding of antibiotic selection pressure.
The increase of antimicrobial resistance in multiple pathogens is a major public health concern (7). Sometimes perceived solely as a nosocomial, health care-associated problem, antimicrobial resistance is also present in many community-acquired pathogens such as Escherichia coli. Antimicrobial use at the community or hospital level has been identified as a possible cause of resistant organisms in the community, potentially creating an environment where these organisms can thrive (6, 12, 17, 19).
Establishing a direct link at the individual level between antibiotic consumption and acquisition of resistant organisms is a difficult task. On one hand, there is a clear link between the development of resistance and misuse of antibiotics for specific conditions (e.g., tuberculosis). On the other hand, less is known about bacteria that can colonize human hosts without causing disease, such as E. coli. Resistant strains may be acquired either from contaminated food or by close contact with colonized individuals, demonstrating that drug-resistant bacteria can spread to persons who are not taking antibacterial agents (5). Yet, antimicrobial consumption may accelerate acquisition of antimicrobial-resistant organisms over susceptible organisms by modifying the normal flora (14). Studies testing this hypothesis in the outpatient setting have yielded conflicting results (3, 8). For instance, acquisition of antimicrobial-resistant E. coli in travelers has been observed both in individuals taking and in individuals not taking antibiotics, with other factors, such as diet, playing an important role (9, 13). A study of children attending day care found no association between recent antibiotic use and carriage of resistant E. coli, while environmental factors played an important role (16). These studies had some limitations, such as relying on patient recall for antibiotic use without checking pharmacy data or looking at a narrow study population (e.g., travelers or children). Cross-sectional studies have additional limitations, since they do not allow assessment of the dynamics of transmission, e.g., the role of close contacts and household members in spreading resistant organisms.
We therefore undertook a study of antimicrobial resistance in E. coli in a relatively isolated, well-defined community population to: (i) determine the impact of antimicrobial use on the carriage of ampicillin-resistant (AMP-R) E. coli among individuals consuming them and their household members, (ii) assess other epidemiologic factors associated with carriage of AMP-R E. coli, and (iii) determine the impact of antimicrobial use on the carriage of ampicillin-susceptible (AMP-S) E. coli among study subjects. We reasoned that the longitudinal data and the inclusion of household members would allow us to evaluate the effects of antibiotics on carriage of resistant and susceptible E. coli strains at the household level.
MATERIALS AND METHODS
Setting and subjects.
A small, rural Idaho community (population, 35,000 persons) was chosen for the study setting because of its geographic location. Since the city is 2 h by car from another urban center, we anticipated that few residents would seek routine items of interest (such as groceries, health care, and employment) from outside this community.
Between July 2003 and April 2004, a convenience sample of persons visiting one of three targeted outpatient clinics was recruited. Techniques to solicit participation in the project included information to clinic providers about the project, distribution of flyers to potential subjects by the clinic providers, and the presence of an on-site research staff person to determine study eligibility and obtain written consent from subjects.
We recruited two categories of index patients. The first category consisted of patients starting a course of antimicrobials for any reason, which was expected to last at least 3 days. These index patients also had to meet the following criteria: (i) no antibiotic use in the previous 3 months, (ii) residence in the study area, and (iii) residence in a household with at least one other person. The second category of index patients consisted of individuals recruited from the same clinics but not receiving a prescription for antibiotics. After consent was obtained from the index patients, household members were also recruited for the study. The households where the index subject was prescribed antibiotics were labeled “group 1 households” (also referred to as “antibiotic households”); the households without antibiotic use were labeled “group 2 households” (also referred to as “nonantibiotic households”). The study protocol was approved by the Western Institutional Review Board (IRB) and the University of Utah IRB.
Study protocol and data collection.
Consenting adults and parents of child subjects completed an exposure questionnaire, which included questions about prior antimicrobial use, travel outside the United States, consumption of animal protein, and exposure to livestock. The questionnaire addressed the prior month for dietary history and livestock contact and the prior 6 months for all other exposures. Travel outside the United States covered the prior year.
Subjects also provided consent for the researchers to obtain information on any antimicrobial prescriptions filled by the pharmacies in the community in the preceding year. Within 2 days after recruitment, research staff contacted the household by phone, and a household visit was made to obtain consents and questionnaires. For group 1 households, confirmation that the prescription had been filled and the antimicrobial started was made. Research team members reviewed the completed questionnaires and clarified any missing responses with the subjects. The data were entered into an Access database (Microsoft Corp).
For group 1 index subjects, the baseline swab was collected prior to the initiation of antimicrobial therapy or within 24 h of initiation of therapy. Either this swab was collected at the clinic or a Culture Swabs (BBL) was sent home for collection. Within 2 days, the initial stool swabs from all participating household members were also obtained, and subjects were provided with additional labeled culture swabs and instructed to use them to collect fecal material from toilet paper after wiping, from diapers, or directly from the anal area.
The research team collected all swabs and delivered them to the courier for transport, on ice, to the Idaho State Bureau of Laboratories (State Laboratory) in Boise, Idaho. This process was repeated for the second stool swab, collected approximately 7 days after the baseline swab, and for the third stool swab, collected approximately 1 month after the baseline swab.
Laboratory methods.
The Idaho State Laboratory performed primary culture and susceptibility testing for stool and confirmatory identification and susceptibility testing for clinical isolates. Stool samples were streaked directly across a regular sheep blood agar plate and a MacConkey plate, as well as three MacConkey agar plates, each containing one screening antimicrobial agent (16 mg/liter of trimethoprim-sulfamethoxazole [TMP-SMZ], 16 mg/liter of nalidixic acid, or 2 mg/liter of cefotaxime). One representative of each phenotypically distinct colony type from the antibiotic-containing plates was further analyzed. If there was no growth on the antibiotic-containing plates, distinct colonies from the MacConkey and agar plates were tested further as described below.
Putative E. coli colonies were confirmed using Microscan (Dade Behring). Susceptibility to 15 antimicrobial agents was assessed by determining the MIC, using broth microdilution (Microscan) for each of 13 drugs (amikacin, amoxicillin-clavulanate, ampicillin-sulbactam, ampicillin, cefazolin, ceftazadime, ceftriaxone, cefuroxime, gentamicin, levofloxacin, tetracycline, tobramycin, and TMP-SMZ) and Etest (AB-BIODISK) for nalidixic acid and cefoxitin. Manufacturer-specified procedures and reference strains were used along with Clinical and Laboratory Standards Institute (CLSI) guidelines. The following MIC cut points were used to classify isolates as intermediate or resistant (2): amikacin, 32; amoxicillin-clavulanate, 16/8; ampicillin-sulbactam, 16/8; ampicillin, 16; cefazolin, 16; ceftazidime, 16; ceftriaxone, 16; cefuroxime, 16; gentamicin, 8; levofloxacin, 4;, tetracycline, 8; tobramycin, 8; and TMP-SMZ, >2/38. Intermediate and resistant isolates were grouped together for the purpose of analysis.
Statistical analysis.
The multilevel nature of the data was taken into account for all statistical comparisons and estimates. The multilevel structure consisted of three repeated culture measurements nested within each individual subject and of individuals nested within households.
The two groups of households were compared with respect to household-level variables and individual subject variables. For dichotomous variables, the groups were compared using the chi-square test if no minimum expected cell frequency was less than five or using Fisher's exact test otherwise. For ordered categorical variables, the groups were compared using the Wilcoxon-Mann-Whitney test.
The main outcome of interest was carriage of AMP-R E. coli at baseline and during follow-up. Resistance was defined as presence of resistance in any strain when multiple E. coli isolates from a single stool sample were tested. Mixed-effects Poisson regression models were fit to evaluate the impact of demographic variables (including antibiotic use) on carriage of AMP-R E. coli on all three cultures, with the results expressed as prevalence ratios. In these models, two levels of clustering were specified: (i) three repeated culture measurements nested within each individual subject and (ii) individuals nested within households. Univariable models were first fitted to the list of predictor variables. Factors identified to be potential confounders, such as age and socioeconomic status, were included in subsequent multivariable models. Results of both the univariable and multivariable models are presented.
Several different methods for dividing continuous variables into multiple ordered categories were applied. Continuous variables were divided both into quintiles and into equally spaced intervals within upper and lower boundaries. The ordered categories were then included in the regression models as sets of dummy variables in order to assess the linearity of association and to evaluate evidence for nonlinear associations. A goal was to select cut points which adequately controlled for confounding. Mixed-effects Poisson regression models were also applied to examine associations between antibiotic exposure and carriage of AMP-S E. coli.
To model the acquisition of AMP-R E. coli, a multivariable mixed-effects Poisson regression was used to account for the multilevel, or nested, nature of the data as described above. In this model, the analysis was limited to individuals whose baseline culture was negative for AMP-R E. coli, and then only the two follow-up cultures were used in the model. For this model, the four subject groups shown in Table 3 and two follow-up cultures were crossed to provide eight predictor variables, leaving one out as the reference group. To compare acquisition between antibiotic household index subjects and nonantibiotic household index subjects, a postfit Wald test was performed on the adjusted acquisition probabilities, holding the remaining predictor variables at their mean values. A similar comparison was done to compare the other household members between the antibiotic and nonantibiotic households.
Household clustering of both AMP-R and AMP-S E. coli strains was also examined. The household prevalence of AMP-R and AMP-S E. coli strains was calculated separately for each of the three stool samples collected from each participant. For each individual in a household, the number of other household members with a sample positive for resistant (or susceptible) E. coli was divided by the total number of other household participants. Thus, the individual's own culture result was excluded when calculating the household prevalence of carriage of AMP-R or AMP-S E. coli.
Sample size was determined for the primary outcome and primary predictor variable. The primary outcome variable was carriage of AMP-R E. coli (presence/absence). The primary predictor variable was consumption of a course of oral antibiotics in the index patient who visited an outpatient clinic (yes/no). A 15% carriage of AMP-resistant E. coli among index patients not prescribed an oral antibiotic and a 30% carriage among index patients with a prescription were assumed, representing a prevalence ratio of 2.0. An average cluster size, or number of individuals per family, of 4 and an intraclass correlation coefficient (ICC) of 0.20 introduced by clustering of individuals within families were assumed. After applying the design effect, 1 + [(average cluster size − 1) × ICC], to the sample size required without clustering, our sample size provided approximately 80% power to detect the prevalence ratio of 2.0. The observed average cluster size and ICC were 3.75 and 0.46, respectively. Substituting these values in the calculation, our sample size had a power of 80% to detect a prevalence ratio of approximately 2.7 (15% versus 40%).
Data were analyzed using Stata release 11 statistical software (StataCorp LP, College Station, TX).
RESULTS
Enrollment.
A total of 701 subjects were enrolled, representing 149 group 1 households and 38 group 2 households (Table 1). Altogether, 2,102 stool samples were obtained, of which 99% (n = 2,082) yielded growth on blood agar or MacConkey plates. The 20 samples with no bacterial growth were excluded from subsequent analysis. All group 1 index subjects and 12 group 1 household members received antibiotics. None of the group 2 index subjects or household members received antibiotics. Initial follow-up cultures were obtained at a median of 9 days (interquartile range [IQR], 8 to 11 days) after the baseline culture; the final follow-up cultures were obtained at a median of 21 days (IQR, 18 to 22 days) later. The baseline stool sample was obtained on the same day as the antibiotic start date for 50% of group 1 index subjects.
TABLE 1.
Characteristics of participating households and study subjects
| Variable | Valuea for households with index subjects: |
P value | |
|---|---|---|---|
| Receiving antibiotics (group 1) (n = 149 households and 570 individuals) | Not receiving antibiotics (group 2) (n = 38 households and 131 individuals) | ||
| Household variables | |||
| Household size, median no. of individuals (range) | 4 (2-9) | 4 (2-7) | 0.18 |
| Household size of: | |||
| 2 | 14 (9) | 8 (21) | 0.05 |
| 3-4 | 77 (52) | 20 (53) | |
| 5-9 | 58 (39) | 10 (26) | |
| Household annual income of: | |||
| ≥$50,000 | 50 (34) | 6 (17) | 0.11 |
| $20,000-$49,999 | 61 (42) | 20 (57) | |
| <$20,000 | 35 (24) | 9 (26) | |
| Household highest level of education | |||
| High school or less | 30 (20) | 7 (18) | 0.60 |
| Some college | 51 (34) | 12 (32) | |
| College graduate/postgraduate | 68 (46) | 19 (50) | |
| Household with a child ≤5 yr in day care | 65 (44) | 13 (33) | 0.29 |
| Household living on a farm | 9 (6) | 1 (3) | 0.69 |
| Household with at least one Hispanic parent | 16 (11) | 2 (5) | 0.54 |
| Individual subject variables | |||
| Female sex | 293 (51) | 75 (57) | 0.53 |
| Age, yr | |||
| <5 | 155 (27) | 38 (29) | 0.97 |
| 5-18 | 142 (25) | 28 (21) | |
| 19-39 | 197 (35) | 52 (40) | |
| ≥40 | 76 (13) | 13 (10) | |
| Class of antibiotic prescribed, n (% of 161)b | |||
| Simple penicillins | 96 (60) | 0 | |
| Beta-lactam/beta-lactamase inhibitor | 18 (11) | 0 | |
| Cephalosporins | 16 (10) | 0 | |
| Macrolides | 18 (11) | 0 | |
| Others | 13 (8) | 0 | |
Unless otherwise indicated, values are numbers (percentages) of households or individuals.
The n = 161 includes 149 index patients and 12 household members from the antibiotic households. For individuals who received more than one antibiotic prescription, the classification was made on the basis of the first antibiotic prescribed. Simple penicillins, penicillin V and amoxicillin; beta-lactam/beta-lactamase inhibitor, amoxicillin-clavulanic acid; cephalosporins, cefdinir, cephalexin, cefadroxil, cefprozil, and ceftriaxone; macrolides, azithromycin and erythromycin; others, quinolone, tetracycline, sulfonamide, and metronidazole.
Antibiotic use and other exposures.
The antibiotic class prescribed to the 161 antibiotic-treated subjects was most commonly penicillins (n = 114; 71%), followed by macrolides (n = 18; 11%), and cephalosporins (n = 16; 10%). Amoxicillin constituted 80% of penicillin-type prescriptions. The majority (73%) of antibiotic recipients were 18 years of age or younger. Recipients of penicillin-type antibiotics were younger than recipients of macrolide-type antibiotics (median, 3 years versus 30 years). The antibiotic course extended after the initial follow-up culture in 58% of instances.
Two percent of subjects reported exposure to livestock at work (n = 11), and 15% reported nonprofessional contact with livestock (n = 108) (Table 2). Twenty-four percent of patients (n = 167) self-reported eating hamburger meat more than twice a week, and one in five reported eating chicken more than twice a week (n = 139). Three percent reported travel outside the United States within the last 6 months. Overall, 61% of individuals reported a doctor visit within the last 6 months.
TABLE 2.
Adjusted probability of acquiring ampicillin-resistant E. coli by household statusa
| Subjects and culture | Probability of carriage of ampicillin-resistant E. coli (95% confidence interval) | P value |
|---|---|---|
| Index subjects | 0.65b | |
| Antibiotic households (n = 110) | ||
| Initial follow-up culture | 0.15 (0.09, 0.24) | |
| Final follow-up culture | 0.16 (0.10, 0.25) | |
| Nonantibiotic households (n = 29) | ||
| Initial follow-up culture | 0.12 (0.04, 0.37) | |
| Final follow-up culture | 0.28 (0.13, 0.59) | |
| Other household members | 0.15c | |
| Antibiotic households (n = 304) | ||
| Initial follow-up culture | 0.14 (0.10, 0.19) | |
| Initial follow-up culture | 0.22 (0.17, 0.28) | |
| Nonantibiotic households (n = 77) | ||
| Initial follow-up culture | 0.13 (0.07, 0.24) | |
| Final follow-up culture | 0.12 (0.06, 0.22) |
This table shows the results of follow-up cultures among subjects whose baseline cultures were negative for ampicillin-resistant E. coli. Individuals are divided according to household group. Probabilities of carriage of ampicillin-resistant E. coli are adjusted for age and socioeconomic status.
Index subjects in antibiotic households versus index subjects in nonantibiotic households.
Other household members in antibiotic households versus other household members in nonantibiotic households.
Stool culture results.
E. coli was recovered from 85% of specimens (n = 1,767). Ninety-five percent of study participants (n = 665) grew E. coli on at least one culture. Overall, 29% of samples yielded AMP-R E. coli and 62% grew AMP-S E. coli. AMP-S E. coli was recovered from 19% of stool samples that were also positive for AMP-R E. coli.
Among AMP-R E. coli samples, coresistance to tetracycline, TMP-SMZ, cefazolin, amoxicillin-clavulanate, and nalidixic acid was observed in 45%, 41%, 25%, 21%, and 7% of instances, respectively. In contrast, among AMP-S samples, resistance to tetracycline, TMP-SMZ, cefazolin, amoxicillin-clavulanate, and nalidixic acid was much lower (13%, 7%, 2%, 1%, and 1% of samples, respectively).
Overall, 28% of individuals who did not have AMP-R E. coli at baseline had AMP-R E. coli recovered from one or both of their follow-up cultures. Conversely, 84% of individuals who had AMP-R E. coli at baseline had AMP-R E. coli recovered from one of their follow-up cultures. AMP-R E. coli was 3.92-fold more likely to be detected in follow-up cultures when the baseline culture grew AMP-R E. coli than when it did not (P < 0.001).
Among individuals with negative baseline cultures, 14% had AMP-R E. coli in initial follow-up culture and 20% had AMP-R E. coli in the final follow-up culture (Table 2). Thus, the prevalence of AMP-R E. coli cultures was modestly higher in the final follow-up culture than in the first follow-up culture. The trend itself was statistically significant (P = 0.03). However, acquisition of AMP-R E. coli did not differ across household groups (likelihood ratio P value for nested models = 0.44). Baseline carriage of AMP-R E. coli and acquisition of AMP-R E. coli did not vary across groups of index patients divided according to timing of initiation of antibiotics relative to collection of the first stool sample.
Epidemiologic factors associated with carriage of AMP-R E. coli and AMP-S E. coli.
A trend between older age and decreased carriage of AMP-R E. coli was observed (Table 3). Lower socioeconomic status was associated with carriage of AMP-R E. coli (P = 0.02 for income of less than $20,000 per year). The magnitudes of these associations were similar for baseline cultures and follow-up cultures. None of the occupational or dietary factors surveyed were associated with carriage of AMP-R E. coli either at baseline or at follow-up (Table 3).
TABLE 3.
Univariate analysis of exposures and carriage of ampicillin-resistant E. coli during baseline or follow-up cultures
| Exposure | No. (%) of individuals with exposure | Prevalence ratioa (95% confidence interval) | P value |
|---|---|---|---|
| Antibiotics | |||
| Antibiotic prescription (any type) | 161 (23) | 1.05 (0.87-1.28) | 0.60 |
| Antibiotic class | |||
| None | 538 (77) | 1 | |
| Penicillin | 114 (16) | 1.17 (0.95-1.45) | 0.16 |
| Macrolide | 18 (3) | 0.18 (0.04-0.76) | 0.02 |
| Other | 29 (4) | 0.92 (0.57-1.48) | 0.72 |
| Membership in group 1 household | 565 (81) | 1.31 (0.87-1.96) | 0.19 |
| Self-reported antibiotic use within 6 mo | 259 (37) | 1.01 (0.83-1.23) | 0.90 |
| Food preparation and diet | |||
| Regular cook (age ≥15 yrb) | 238 (66) | 0.83 (0.63-1.09) | 0.18 |
| Regular shopper (age ≥15 yrb) | 244 (67) | 0.90 (0.67-1.20) | 0.46 |
| Food eaten >2 times/wk | |||
| Chicken | 139 (20) | 1.00 (0.75-1.33) | >0.99 |
| Pork | 22 (3) | 1.07 (0.56-2.06) | 0.83 |
| Hamburger | 167 (24) | 1.14 (0.86-1.51) | 0.35 |
| Steak | 33 (5) | 0.98 (0.60-1.60) | 0.94 |
| Animals | |||
| Work with livestock (age ≥15 yrb) | 11 (3) | 1.21 (0.58-2.55) | 0.60 |
| Exposure to farm animals | 108 (16) | 0.97 (0.07-1.34) | 0.86 |
| Demographics | |||
| Annual household income of: | |||
| ≥$50,000 | 210 (31) | 1 | |
| $20,000--$49,999 | 313 (46) | 1.17 (0.82-1.65) | 0.39 |
| <$20,000 | 159 (23) | 1.61 (1.09-2.39) | 0.02 |
| Household size of: | |||
| 2 | 43 (6) | 1 | |
| 3-4 | 311 (45) | 1.05 (0.59-1.85) | 0.87 |
| 5-9 | 342 (49) | 1.24 (0.69-2.22) | 0.46 |
| Age (yr) | |||
| <5 | 192 (28) | 1 | |
| 5-18 | 169 (24) | 0.97 (0.76-1.23) | 0.77 |
| 19-39 | 248 (36) | 0.84 (0.69-1.04) | 0.11 |
| ≥40 | 87 (12) | 0.67 (0.47-0.96) | 0.027 |
| Health care contact and social exposures | |||
| Attend day care (<6 yrc) | 100 (46) | 0.96 (0.69-1.34) | 0.82 |
| Travel outside USA in past 6 mo | 22 (3) | 1.27 (0.74-2.18) | 0.32 |
| Visited doctor in past 6 mo | 427 (61) | 1.08 (0.88-1.32) | 0.45 |
| Hospitalized in past 6 mo | 64 (9) | 1.30 (0.98-1.73) | 0.073 |
Prevalence ratios estimated by mixed-effects Poisson regression, with n = 3 cultures nested in each of n = 701 individuals nested in n = 187 households.
Model limited to subjects 15 years or older (n = 362 individuals) so variable would not be confounded by adult status.
Model limited to subjects less than 6 years old (n = 217 individuals) so variable would not be confounded by young age.
Receipt of an antibiotic was not associated with increased carriage of AMP-R E. coli (prevalence ratio = 1.05; P = 0.6) but was associated with a modest decrease in carriage of AMP-S E. coli (prevalence ratio = 0.84; P = 0.015). The prevalence ratios for receipt of an antibiotic were similar following adjustment for age and socioeconomic status (adjusted prevalence ratios for AMP-R E. coli and AMP-S E. coli of 0.99 and 0.84, respectively). Further, there was no interaction between age and antibiotic use with respect to carriage of AMP-R E. coli or AMP-S E. coli (P > 0.5).
Observed associations varied across different drug classes. The macrolide drug class was associated with both decreased carriage of AMP-R E. coli and decreased carriage of AMP-S E. coli (prevalence ratios of 0.18 and 0.47, respectively). These associations were statistically significant (P < 0.05) despite the small number of individuals who received macrolide antibiotics.
Changes in E. coli carriage following receipt of penicillin-class drugs were small in comparison. Although penicillins were associated with a modest increase in AMP-R E. coli carriage (prevalence ratio = 1.17) and a modest decrease in AMP-S E. coli (prevalence ratio = 0.91), neither change was statistically significant (P = 0.16 and 0.23, respectively). Changes in carriage of AMP-R E. coli were most pronounced at the time of the first follow-up culture. Among recipients of penicillin-class drugs, AMP-R E. coli carriage rose from 31% at baseline to 40% at the first follow-up culture, and AMP-S E. coli carriage dropped from 62% to 51%. Neither finding was statistically significant by itself. However, when directly comparing exposure at the time of first follow-up culture, the prevalence of treatment with penicillin-class drugs was 1.68-fold higher among individuals who carried AMP-R E. coli than among individuals who carried AMP-S E. coli (P = 0.01).
Household clustering.
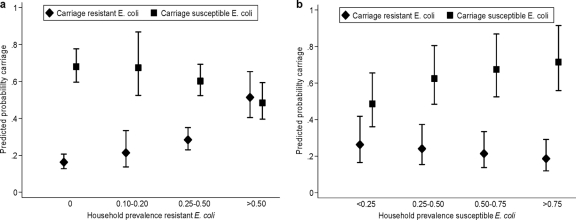
Both AMP-R and AMP-S E. coli strains demonstrated significant household clustering. The probability of carriage of AMP-R E. coli correlated strongly with household prevalence of AMP-R E. coli (P < 0.001) (Fig. 1a). An individual's estimated risk of carrying AMP-R E. coli was 51% when the prevalence of AMP-R in other household members exceeded 50%, compared to 16% when none of the other participating household members carried AMP-R E. coli. Similarly, carriage of AMP-S E. coli was positively correlated with household prevalence of AMP-S E. coli and negatively correlated with household prevalence of AMP-R E. coli (Fig. 1b) (P = 0.001).
FIG. 1.
(a) Relationship between household prevalence of ampicillin-resistant E. coli and predicted age-adjusted probability of either ampicillin-susceptible E. coli or ampicillin-resistant E. coli. Error bars represent 95% confidence intervals. (b) Relationship between household prevalence of ampicillin-susceptible E. coli and predicted probability of either ampicillin-susceptible E. coli or ampicillin-resistant E. coli. Error bars represent 95% confidence intervals.
DISCUSSION
Despite a large body of research about the influence of antibiotic exposure on the emergence and selection of resistant E. coli in the gut flora, no study has specifically assessed, after adjustment for individual- and group-level confounders and clustering effects, the independent effects of household carriage, family transmission, and antibiotic use. Our results do not confirm the strong association previously reported by other investigators who examined the effect of other agents, such as TMP-SMZ or fluoroquinolones, at the individual level (3, 4, 14, 15). This lack of association between antibiotic consumption and short-term carriage of AMP-R E. coli is intriguing. One explanation may be that the penicillin antibiotics taken by the study subjects did not exert a substantial selection effect in the colon, due to factors such as adsorption by stool and enzymatic inactivation by beta-lactamases. However, the transient decrease in carriage of AMP-S organisms observed in study participants taking antibiotics suggests that there was indeed an antibiotic effect on the fecal flora. Another explanation may be that penicillin and amoxicillin use may not have the same effect on AMP-R E. coli as suggested by the relationship between TMP-SMZ and fluoroquinolone use and resistance in E. coli (18). Finally, previous investigations (3, 15) have shown that the effect of antibiotic use rapidly disappears, so it is not surprising to observe that, at least in the third follow-up culture 3 to 4 weeks after antibiotic use, no significant increase in resistant pathogens could be observed. A recent longitudinal study from Germany investigated short-term changes in E. coli resistance patterns during and after antibiotic therapy in a cohort of more than 500 patients with a febrile infection (15). This study found that with the exception of TMP-SMZ resistance, the prevalence of resistance returned to baseline levels within 2 weeks after the cessation of antibiotic therapy. However, in this study, transmission patterns including household members, changes in the susceptible flora of antibiotic-treated patients, and other clinically important agents (e.g., fluoroquinolones) were not studied in detail.
Another interesting finding from our study was the suggestion that both susceptible and resistant E. coli strains may be transmitted between household members. In particular, both individual and household member E. coli results for the previous culture (the latter only in households where the index subject took antimicrobial agents) were associated with AMP-R E. coli carriage on subsequent cultures in the same household. This suggests that susceptible and resistant E. coli strains compete for niches within the gastrointestinal tract. Exposure to antibiotics such as amoxicillin is able to promote dissemination of resistant E. coli by affecting this competitive balance, even in the absence of a demonstrable individual-level effect on carriage of resistant E. coli. Thus, antibiotic consumption may disturb the ecology of the gut flora in the entire household, not just the treated subject, and continuous intrafamilial transmission of resistant E. coli may contribute significantly to the persistence of AMP-R E. coli in a household or community. Other recent investigations have confirmed the importance of household transmission in the spread of antibiotic-resistant E. coli (10, 11, 20).
There are several limitations to our study. First, we performed only two cultures in the period during and after antibiotic treatment. Therefore, we may have missed low-level carriage of resistant E. coli. Second, at each time point, fewer than 85% of specimens grew E. coli. This is somewhat surprising, since it is assumed that 100% of the population have E. coli in the fecal flora. On the other hand, previous studies have reported recovery rates for E. coli in stool samples of as low as 60% in human volunteers (11, 21). Moreover, only 20 swabs yielded no culture at all, suggesting that our participants respected the study protocol. Second, there may have been a lack of sensitivity of our culture protocol: working up only one specimen from each distinct colony per sample may have resulted in false-negative results for AMP-R E. coli. However, other studies have suggested that this approach is the method of choice if the primary interest is to monitor trends in carriage of resistant organisms in a population, as opposed to individual case ascertainment (1). Finally, the small number of nonantibiotic households also limited our ability to make statistical comparisons across household groups.
Our study also had several significant strengths, including the systematic sampling approach. All study participants submitted three stool samples, with the exception of one participant who left the area before completion of the study. All stool samples were processed in a single laboratory, reducing the chances of laboratory variability. Antibiotic data were obtained prospectively and were checked against pharmacy data, therefore avoiding potential recall bias. Recruiting household members was also very important. It allowed us to adjust for clustering in the data analysis and made it possible to explore the influence of close contacts on carriage of antibiotic-resistant E. coli.
In conclusion, antibiotic consumption was not associated with an individual increase in AMP-R E. coli carriage in our study but was associated with a decrease of AMP-S E. coli carriage. The strongest predictor for carrying AMP-R E. coli on follow-up cultures was an individual's status at baseline. Our interpretation is that consumption of antimicrobial agents disturbs the ecology of the flora of the household, not just the treated subject. These changes may be mediated through effects on susceptible organisms and may not be manifest as increased carriage of resistant bacteria in the treated subject.
Acknowledgments
Funding was provided by the Centers for Disease Control and Prevention, grant number RS1 CCR820631 (M.H.S.).
We thank all members of the IMPART working group for their collaborative effort and all patients and their family members for active participation.
The authors have no conflicts of interest.
We dedicate this paper to Theodor Escherich, the German-Austrian pediatrician who discovered the bacterium Escherichia coli. This year marks the centennial of his death.
Footnotes
Published ahead of print on 29 November 2010.
REFERENCES
- 1.Brun, E., G. Holstad, H. Kruse, and J. Jarp. 2002. Within-sample and between-sample variation of antimicrobial resistance in fecal Escherichia coli isolates from pigs. Microb. Drug Resist. 8:385-391. [DOI] [PubMed] [Google Scholar]
- 2.Clinical Laboratory Standards Institute. 2007. Performance standards for antimicrobial susceptibility testing, vol. 27: 17th informational supplement. CLSI document M100-S17. Clinical and Laboratory Standards Institute, Wayne, PA.
- 3.Donnan, P. T., et al. 2004. Presence of bacteriuria caused by trimethoprim resistant bacteria in patients prescribed antibiotics: multilevel model with practice and individual patient data. BMJ 328:1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fantin, B., et al. 2009. Ciprofloxacin dosage and emergence of resistance in human commensal bacteria. J. Infect. Dis. 200:390-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grenet, K., et al. 2004. Antibacterial resistance, Wayampis Amerindians, French Guyana. Emerg. Infect. Dis. 10:1150-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harbarth, S., A. D. Harris, Y. Carmeli, and M. H. Samore. 2001. Parallel analysis of individual and aggregated data on antibiotic exposure and resistance in gram-negative bacilli. Clin. Infect. Dis. 33:1462-1468. [DOI] [PubMed] [Google Scholar]
- 7.Harbarth, S., and M. H. Samore. 2005. Antimicrobial resistance determinants and future control. Emerg. Infect. Dis. 11:794-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hay, A. D., et al. 2005. The relationship between primary care antibiotic prescribing and bacterial resistance in adults in the community: a controlled observational study using individual patient data. J. Antimicrob. Chemother. 56:146-153. [DOI] [PubMed] [Google Scholar]
- 9.Huang, D. B., Z. D. Jiang, C. D. Ericsson, J. Adachi, and H. L. Dupont. 2001. Emergence of trimethoprim-resistant Escherichia coli in healthy persons in the absence of prophylactic or therapeutic antibiotics during travel to Guadalajara, Mexico. Scand. J. Infect. Dis. 33:812-814. [DOI] [PubMed] [Google Scholar]
- 10.Johnson, J. R., K. Owens, A. Gajewski, and C. Clabots. 2008. Escherichia coli colonization patterns among human household members and pets, with attention to acute urinary tract infection. J. Infect. Dis. 197:218-224. [DOI] [PubMed] [Google Scholar]
- 11.Lietzau, S., E. Raum, H. von Baum, R. Marre, and H. Brenner. 2007. Household contacts were key factor for children's colonization with resistant Escherichia coli in community setting. J. Clin. Epidemiol. 60:1149-1155. [DOI] [PubMed] [Google Scholar]
- 12.Lipsitch, M., and M. H. Samore. 2002. Antimicrobial use and antimicrobial resistance: a population perspective. Emerg. Infect. Dis. 8:347-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray, B. E., J. J. Mathewson, H. L. DuPont, C. D. Ericsson, and R. R. Reves. 1990. Emergence of resistant fecal Escherichia coli in travelers not taking prophylactic antimicrobial agents. Antimicrob. Agents Chemother. 34:515-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray, B. E., E. R. Rensimer, and H. L. DuPont. 1982. Emergence of high-level trimethoprim resistance in fecal Escherichia coli during oral administration of trimethoprim or trimethoprim-sulfamethoxazole. N. Engl. J. Med. 306:130-135. [DOI] [PubMed] [Google Scholar]
- 15.Raum, E., S. Lietzau, H. von Baum, R. Marre, and H. Brenner. 2008. Changes in Escherichia coli resistance patterns during and after antibiotic therapy: a longitudinal study among outpatients in Germany. Clin. Microbiol. Infect. 14:41-48. [DOI] [PubMed] [Google Scholar]
- 16.Reves, R. R., M. Fong, L. K. Pickering, A. Bartlett III, M. Alvarez, and B. E. Murray. 1990. Risk factors for fecal colonization with trimethoprim-resistant and multiresistant Escherichia coli among children in day-care centers in Houston, Texas. Antimicrob. Agents Chemother. 34:1429-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samore, M. H., et al. 2001. High rates of multiple antibiotic resistance in Streptococcus pneumoniae from healthy children living in isolated rural communities: association with cephalosporin use and intrafamilial transmission. Pediatrics 108:856-865. [DOI] [PubMed] [Google Scholar]
- 18.Sundqvist, M., et al. 2010. Little evidence for reversibility of trimethoprim resistance after a drastic reduction in trimethoprim use. J. Antimicrob. Chemother. 65:350-360. [DOI] [PubMed] [Google Scholar]
- 19.Tacconelli, E. 2009. Antimicrobial use: risk driver of multidrug resistant microorganisms in healthcare settings. Curr. Opin. Infect. Dis. 22:352-358. [DOI] [PubMed] [Google Scholar]
- 20.Valverde, A., et al. 2008. High rate of intestinal colonization with extended-spectrum-beta-lactamase-producing organisms in household contacts of infected community patients. J. Clin. Microbiol. 46:2796-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van de Mortel, H. J., et al. 1998. The prevalence of antibiotic-resistant faecal Escherichia coli in healthy volunteers in Venezuela. Infection 26:292-297. [DOI] [PubMed] [Google Scholar]