Abstract
Our aim was to assess the effect of miconazole oral gel on the pharmacokinetics of oral oxycodone. In an open crossover study with two phases, 12 healthy volunteers took a single oral dose of 10 mg of immediate-release oxycodone with or without thrice-daily 85-mg miconazole oral gel treatment. The plasma concentrations of oxycodone and its oxidative metabolites were measured for 48 h. Pharmacological effects of oxycodone were recorded for 12 h. Pharmacokinetic parameters were compared by use of the geometric mean ratios (GMRs) and their 90% confidence interval (CIs). Pretreatment with miconazole oral gel caused a strong inhibition of the CYP2D6-dependent metabolism and moderate inhibition of the CYP3A4-dependent metabolism of oxycodone. The mean area under the concentration-time curve (AUC) from time zero to infinity (AUC0-∞; GMR, 1.63; 90% CI, 1.48 to 1.79) and the peak concentration of oxycodone (GMR, 1.31; 90% CI, 1.19 to 1.44) were increased. The AUC of the CYP2D6-dependent metabolite oxymorphone was greatly decreased (GMR, 0.17; 90% CI, 0.09 to 0.31) by miconazole gel, whereas that of the CYP3A4-dependent metabolite noroxycodone was increased (GMR, 1.30; 90% CI, 1.15 to 1.47) by miconazole gel. Differences in the pharmacological response to oxycodone between phases were insignificant. Miconazole oral gel increases the exposure to oral oxycodone, but the clinical relevance of the interaction is moderate. Miconazole oral gel produces a rather strong inhibitory effect on CYP2D6, which deserves further study.
Miconazole is a broad-spectrum azole antifungal agent. Due to its limited oral bioavailability and a high risk of drug interactions, the systemic use of miconazole is rare. However, miconazole is commonly used as a topical preparation (16, 21). In vitro studies have shown miconazole to be a strong inhibitor of many drug-metabolizing cytochrome P450 (CYP) enzymes (CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4) (19, 20). Compared with systemic use, the risk for drug interactions is markedly reduced when miconazole is used topically. However, there are many case reports and one controlled study indicating that clinically relevant CYP-mediated drug interactions are also possible when miconazole is used as a topical preparation, especially as an oral gel (6, 11, 23).
Oxycodone is an opioid analgesic which is increasingly used in inpatient and outpatient care (7, 10). The metabolism of oxycodone is catalyzed by CYP2D6 and CYP3A4 enzymes (14). The predominant metabolic pathway of oxycodone is CYP3A4-mediated N-demethylation to noroxycodone, while a small part of oxycodone undergoes 3-O-demethylation to oxymorphone by CYP2D6. Further oxidation of these metabolites via CYP2D6 and CYP3A4, respectively, yields noroxymorphone (13). Although the metabolites show variable μ-opioid receptor activity, it seems that the parent drug is responsible for the central opioid effects (13).
Recent studies have shown that concomitantly administered drugs which inhibit CYP enzymes may significantly alter the pharmacokinetics of oxycodone (8, 9). This study was aimed to study the possible interaction of oral oxycodone with miconazole oral gel, often prescribed for the treatment of oral fungal infections.
MATERIALS AND METHODS
Subjects.
The study protocol was approved by the Ethics Committee of the Hospital District of Southwest Finland and by the Finnish National Agency for Medicines. The study was registered to the EudraCT Clinical Trials Register (EudraCT number 2008-006834-80).
On the basis of data reported in published studies (24, 25), we calculated that 10 subjects would be required to demonstrate a 30% increase in the area under the oxycodone plasma concentration-time curve (AUC) with a type I error of 5% and a statistical power of 80%.
Twelve healthy nonsmoking volunteers (seven males and five females) participated in the study. Written informed consent was obtained from each volunteer. Before entering the study, the volunteers were ascertained to be in good health on the basis of a medical history, clinical examination, routine laboratory tests, and electrocardiogram. The risk that participants would develop opioid abuse was considered low, as evaluated by administration of the Finnish version of the Abuse Questions (17). None of the volunteers was receiving any continuous medication, including contraceptive steroids, or natural products.
Study design.
We used an open, randomized, balanced, crossover study design with two phases and a 4-week washout period. In the miconazole phase, subjects used miconazole oral gel (Daktarin 2% oral gel; Orion Pharma, Espoo, Finland) for 4 days. The dose of miconazole gel was 3.5 ml (approximately 85 mg) three times per day. The oral gel was kept in the mouth for 1 min and was thereafter swallowed, as instructed by the manufacturer in the summary of product characteristics (SPC). To ensure adherence to the drug dosing schedule, subjects were asked to send a mobile phone text message to the investigator after each dose. On day 3, miconazole oral gel was administered in the study facility, and 1 h after the morning dose of oral gel, the subjects ingested a single dose of 10 mg of oxycodone hydrochloride as an immediate-release formulation (Oxynorm, 10-mg capsule; Mundipharma, Bard Pharmaceuticals Ltd., Cambridge, United Kingdom). The subjects fasted overnight before oxycodone administration. Standard meals were served 4 and 8 h after oxycodone administration. The use of any other drugs, natural products, alcohol, coffee, tea, grapefruit juice, and cola drinks was not permitted on test days. In the control phase, there was no pretreatment.
Sampling and analysis of blood samples.
On the test day, a forearm vein was cannulated and blood samples were collected before and 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 24, and 48 h after oxycodone administration. Plasma was separated within 30 min and stored at −70°C until analysis.
Plasma concentrations of oxycodone, noroxycodone, oxymorphone, and noroxymorphone were determined using a validated liquid chromatography-tandem mass spectrometric (LC-MS/MS) method as previously described (18). The lower limits of quantification (LLQs) were 0.1 ng/ml for oxycodone and oxymorphone and 0.25 ng/ml for noroxycodone and noroxymorphone. The interday coefficients of variation (CVs) for oxycodone and oxymorphone were 6.3% and 9.0%, respectively, at a concentration of 0.1 ng/ml and 2.7% and 6.0%, respectively, at a concentration of 5.0 ng/ml (n = 4). The CVs for noroxycodone were 11.5% and 1.8% at 0.5 ng/ml and 5.0 ng/ml (n = 4), respectively, and those for noroxymorphone were 10.6% and 4.6% at 0.5 ng/ml and 10 ng/ml, respectively (n = 4).
Plasma concentrations of miconazole were determined with liquid chromatography, using itraconazole as the internal standard, of blood samples taken 1, 2, and 4 h after the morning dose of oral gel on the test day (4). The LLQ for miconazole was 5 ng/ml, and the day-to-day CV was 3.4% at 25 ng/ml (n = 3).
Genotyping for CYP2D6 was performed using a two-step multiplex primer extension method which allows the detection of 11 of the most relevant alleles (i.e., *1, *2, *3, *4, *6, *9, *10, *17, *29, *39, and *41) and assessment of whole-gene deletion, duplication, and allele composition of gene duplication (26).
Pharmacokinetics.
Peak plasma concentrations (Cmaxs) and the times to reach the peak concentration (Tmaxs) of oxycodone and its metabolites were observed directly from plasma concentration data. Individual terminal log-linear phases of the plasma concentration-time curves were identified visually. The elimination rate constant (kel) was determined by regression analysis of the log-linear part of the curve. The elimination half-life (t1/2) was calculated by using the equation t1/2 = ln 2/kel. The AUCs for oxycodone, noroxycodone, oxymorphone, and noroxymorphone were calculated using the linear trapezoidal rule when successive concentration values were increasing and the logarithmic trapezoidal rule when values were decreasing. For oxycodone and noroxycodone, the AUC was extrapolated to infinity (AUC0-∞) by using the respective kel value. Since kel was often impossible to determine due to the low plasma concentrations of oxymorphone and noroxymorphone, the AUC from time zero to 48 h (AUC0-48) was used instead of AUC0-∞ for these metabolites. The apparent oral clearance (CL/F) and the apparent volume of distribution of oxycodone during elimination (Vz/F) were calculated using standard noncompartmental methods. To compare the relative abundance of primary metabolites in different phases, metabolite-to-parent drug AUC ratios (AUCm/AUCp) were calculated for oxymorphone and noroxycodone. The pharmacokinetic data were analyzed with the WinNonlin pharmacokinetic program (version 4.1; Pharsight, Mountain View, CA).
Pharmacodynamics.
Subjects evaluated subjective drug effects of oxycodone by using 100-mm horizontal visual analogue scales (VAS) for the following four items: no drug effect/very strong drug effect, alert/drowsy, very good performance/very poor performance, and unpleasant feeling/very pleasant feeling (2). Pupil diameter was assessed using Cogan's pupillometry under steady lighting conditions (3). The psychomotor effects of oxycodone were evaluated with the digit symbol substitution test (DSST) by recording the number of correct symbols substituted in 3 min (28). In addition, spontaneously reported adverse effects were recorded.
The analgesic effect of oxycodone was evaluated with the experimental cold pain test (27). The subject immersed his or her left hand in ice-water (temperature, 0.5 to 2°C) for 1 min. The latency from the immersion to the first sensation of pain was defined as the cold pain threshold (CPT). During the immersion, the subjects were asked to report the intensity of pain after 30 s and again after 60 s using a numerical rating scale (NRS; 0 = no pain, 100 = maximal pain).
Pharmacodynamic tests were done prior to and 1, 2, 3, 4, 5, 6, 8, 10, and 12 h after oxycodone administration. To estimate overall opioid effects during the study day, the area under the effect-time curve (AUEC) was determined by the trapezoidal rule from 0 to 6 h (AUEC0-6) and 0 to 12 h (AUEC0-12) for each pharmacodynamic variable.
Statistical analysis.
As recommended for bioequivalence testing, the geometric mean ratios (GMRs) with 90% confidence intervals (CIs) were determined for pharmacokinetic results and for AUEC values. Resultant from the calculation of geometric mean ratios, the whole data were log transformed before analysis. The results are expressed as mean values ± standard deviations (SDs) and geometric mean ratios with 90% confidence intervals, expect for Tmax, where medians and ranges are given. Bioequivalance, i.e., lack of interaction, was concluded if the 90% CI of GMR fell into the range of 0.80 to 1.25. Data were analyzed by use of the Systat for Windows statistical program (version 10.2; Systat Software, Richmond, CA) and with the Prism program for Windows (version 5; GraphPad Software, San Diego, CA).
RESULTS
All subjects completed the study. Age and body mass index ranges were 19 to 30 years and 20 to 30 kg/m2, respectively. Among the subjects, there was one poor metabolizer (PM) with genotype CYP2D6*4/*5 and one ultrarapid metabolizer (UM) with the gene duplication of CYP2D6*1/*1. The remaining 10 participants were classified as extensive metabolizers (EM).
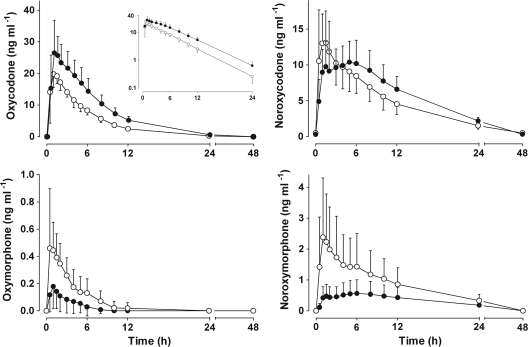
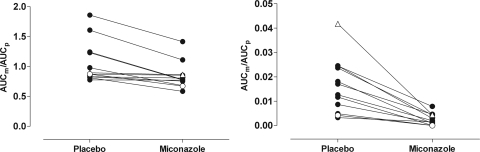
The pharmacokinetic results are presented in Fig. 1 and in Table 1. During miconazole oral gel treatment, the AUC0-∞ of oxycodone was clearly increased (GMR, 1.63; 90% CI, 1.48 to 1.79). The mean peak concentration of oxycodone was also increased during miconazole treatment (GMR, 1.31; 90% CI, 1.19 to 1.44). As shown by the different shapes of the concentration-time curves in Fig. 1, the formation, as well as the further metabolism, of the CYP3A-dependent primary metabolite noroxycodone was delayed in the miconazole phase. The inhibitory effect of miconazole oral gel appeared to be stronger on the CYP2D6 enzyme than on the CYP3A isozymes, as evidenced by the corresponding changes in AUCm/AUCp (Fig. 2). The AUC0-48 of the secondary metabolite noroxymorphone was decreased (GMR, 0.24; 90% CI, 0.10 to 0.56). The mean plasma concentration of miconazole seen 1 h after the morning dose of oral gel was 60.2 ng/ml.
FIG. 1.
Mean plasma concentrations (±SD) of oxycodone, noroxycodone, oxymorphone, and noroxymorphone in 12 volunteers after oral administration of 10 mg immediate-release oxycodone in control phase (open circles) or after pretreatment with miconazole oral gel (solid circles). (Inset) Same data on a semilogarithmic scale.
TABLE 1.
Pharmacokinetic parameters of oxycodone and its primary and secondary oxidative metabolites after oral administration of 10 mg immediate-release oxycodone in control phase and after pretreatment with miconazole oral gela
| Drug and parameter | Value (mean ± SD) |
Geometric mean ratio (90% CI) for miconazole/control | |
|---|---|---|---|
| Control phase | Miconazole phase | ||
| Oxycodone | |||
| Cmax(ng ml−1) | 22.5 ± 3.3 | 29.9 ± 7.6 | 1.31 (1.19, 1.44) |
| Tmax(h) | 1 (0.5-1.5) | 1.25 (0.5-3.0) | |
| AUC0-∞ (μg·min ml−1) | 7.3 ± 1.0 | 12.0 ± 2.5 | 1.63 (1.48, 1.79) |
| t1/2 (h) | 3.6 ± 0.4 | 4.0 ± 0.7 | 1.10 (1.05, 1.15) |
| % (range) of control | 100 | 110 (89-121) | |
| CL/F (liters/min) | 1.25 ± 0.16 | 0.78 ± 0.17 | 0.61 (0.56, 0.67) |
| Vz/F (liters) | 387.9 ± 64.1 | 268.4 ± 76.8 | 0.67 (0.61, 0.74) |
| Noroxycodone | |||
| Cmax (ng ml−1) | 14.6 ± 5.1 | 11.5 ± 3.1 | 0.80 (0.72, 0.89) |
| Tmax (h) | 1.0 (0.5-2.0) | 1.75 (1.0-8.0) | |
| AUC0-∞ (μg·min ml−1) | 7.7 ± 2.4 | 9.8 ± 2.4 | 1.30 (1.15, 1.47) |
| t1/2 (h) | 6.0 ± 1.1 | 7.2 ± 1.0 | 1.20 (1.11, 1.29) |
| AUCm/AUCp | 1.1 ± 0.4 | 0.8 ± 0.2 | 0.80 (0.73, 0.88) |
| Oxymorphone | |||
| Cmax (ng ml−1) | 0.56 ± 0.37 | 0.20 ± 0.16 | 0.34 (0.25, 0.46) |
| AUC0-48 (μg·min ml−1) | 0.12 ± 0.08 | 0.03 ± 0.04 | 0.17 (0.09, 0.31) |
| AUCm/AUCp | 0.02 ± 0.01 | 0.002 ± 0.002 | 0.10 (0.06, 0.18) |
| Noroxymorphone | |||
| Cmax (ng ml−1) | 2.5 ± 1.9 | 0.6 ± 0.5 | 0.27 (0.20, 0.36) |
| AUC0-48 (μg·min ml−1) | 1.6 ± 1.0 | 0.7 ± 0.5 | 0.24 (0.10, 0.56) |
Data are given as means ± SDs and geometric mean ratios (90% confidence intervals). Medians and ranges are reported for Tmax.
FIG. 2.
AUCm/AUCp for noroxycodone (left panel) and oxymorphone (right panel) after oral administration of 10 mg immediate-release oxycodone in control phase or after pretreatment with miconazole oral gel. Each circle represents one subject. The poor CYP2D6 metabolizer is depicted with open circles, and the ultrarapid CYP2D6 metabolizer is depicted with open triangles.
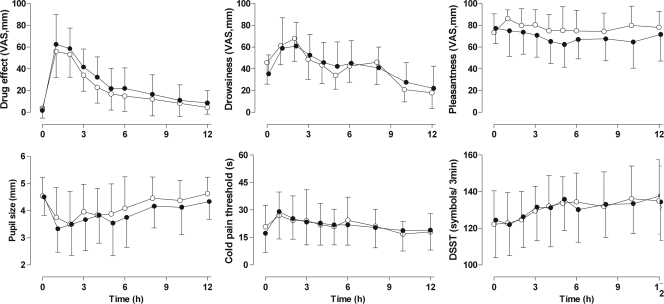
Differences in the pharmacological effects of oxycodone between study phases were insignificant (Fig. 3). The VAS scores for subjective drug effect were marginally higher in the miconazole phase. The GMR for the miconazole phase relative to the control phase was 1.23 (90% CI, 1.16 to 1.36) for the AUEC0-12 values for subjective drug effect. The differences were even smaller when the results for the first 6 h were compared. Other pharmacodynamic variables showed no changes between study phases.
FIG. 3.
Mean ± SD self-reported drug effect, drowsiness, pleasantness, pupil size, CPT, and number of digits substituted in 3 min (DSST) after oral administration of 10 mg immediate-release oxycodone in control phase (open circles) or after pretreatment with miconazole oral gel (solid circles).
Adverse events.
On the test day, one subject suffered from nausea in the afternoon while on the miconazole phase. There were no reported side effects during pretreatment periods.
DISCUSSION
Topical administration of drugs is usually considered to have a minor risk for drug interactions or systemic adverse effects. However, the miconazole oral gel, although it is primarily applied topically to the mouth cavity, is eventually swallowed, according to the dosing recommendation, and significant systemic absorption and adverse effects are therefore possible (1, 23).
In the present study, the use of miconazole oral gel increased the mean AUC0-∞ of oxycodone by 63%, with the greatest individual increase being more than 2-fold. Because the Cmax of oxycodone was also clearly increased, it is likely that miconazole not only decreased the plasma clearance of oxycodone but also decreased the first-pass metabolism of oxycodone and resulted in the higher oral bioavailability of oxycodone. The lower and more slowly appearing Cmax of noroxycodone further supports this explanation. Formation of the CYP2D6-dependent metabolite oxymorphone was substantially and consistently decreased (GMR, 0.17; 90% CI, 0.09 to 0.31), indicating significant inhibition of the CYP2D6 enzyme by miconazole. In contrast, the AUC0-48 of the CYP3A-dependent metabolite noroxycodone was increased (GMR, 1.30; 90% CI, 1.15 to 1.47) compared with that in the control phase. Still, the AUCm/AUCp of noroxycodone was decreased (GMR, 0.80; 90% CI, 0.73 to 0.88), indicating that the increase in AUC of noroxycodone was a consequence of more abundant parent drug and that CYP3A4 inhibition was, in fact, present, in spite of the increased AUC0-48 of noroxycodone. In addition, the increased noroxycodone concentrations are partly explained by the inhibition of CYP2D6 resulting in a decrease in further metabolism of noroxycodone to noroxymorphone. Previous in vivo studies have shown that miconazole may cause drug interactions with substrates of CYP2C9 and CYP3A4 enzymes (11, 22). Although the capability of miconazole to inhibit the CYP2D6 enzyme has been shown by in vitro studies (19), we are not aware of any previous study showing that in vivo.
The miconazole oral gel dosage instruction given by the manufacturer is 2.5 ml every 6 h (Daktarin oral gel SPC, 2010, Orion Pharma). To provide longer night rest for subjects, the dosing schedule for miconazole in the present study was 3.5 ml three times daily. The changed dosing schedule explains why the mean plasma concentrations of miconazole achieved 1 h after the oral gel dose (60.2 ng/ml) were higher than those reported by the manufacturer (31 to 49 ng/ml; Daktarin oral gel SPC, 2010, Orion Pharma). Absorption of miconazole from the gastrointestinal tract is known to be rather poor (5, 15). However, because some drug-metabolizing enzymes, particularly CYP3A (but not CYP2D6), are also found abundantly in the gut wall (12), the inhibitory effect by miconazole on the drug-metabolizing enzyme system can be more pronounced than one could assume on the basis of the measured plasma concentrations of miconazole. Therefore, it was somewhat surprising that the effect of miconazole oral gel was stronger on oxymorphone formation (CYP2D6) than noroxycodone formation (CYP3A).
Although this was mainly a pharmacokinetic study, we also estimated possible changes in the pharmacological response to oxycodone. As shown in Fig. 3, the pharmacokinetic interaction was too weak to cause clinically significant changes in the overall pharmacological response seen after a single small dose of oxycodone.
A 2.9-fold increase in exposure to oral oxycodone was noted in our previous study, where both the CYP2D6 and CYP3A4 pathways were inhibited with clinically used doses of paroxetine and itraconazole, respectively (8). In another interaction study, the antifungal agent voriconazole strongly inhibited the CYP3A4-mediated metabolism of oxycodone and caused a 3.6-fold increase in the level of exposure to oral oxycodone (9). Even though the effect of miconazole oral gel on the pharmacokinetics of oxycodone was weaker, it still is advisable to keep it in mind when miconazole is prescribed to a patient already taking oxycodone. The miconazole-induced interaction appeared to be the most pronounced in one subject classified as a UM. However, our small group size does not allow any conclusion regarding the magnitude of interaction in different genotypes to be made.
In conclusion, our results show that even a short treatment with clinically used doses of miconazole oral gel increases the exposure to oral oxycodone, but the clinical relevance of this interaction seems to be only moderate. Importantly, miconazole oral gel produced a marked inhibition of CYP2D6, which deserves further investigation with a validated substrate drug for CYP2D6.
Acknowledgments
This study was supported by EVO grant 13821 of the Hospital District of the Southwest Finland.
Kari Laine is owner and CEO for Medbase Ltd.
We thank Elina Kahra and Mia Suppanen-Olkkola for their skillful technical assistance and chemist Mikko Neuvonen for the determination of plasma oxycodone and its metabolites.
Footnotes
Published ahead of print on 20 December 2010.
REFERENCES
- 1.Ariyaratnam, S., N. S. Thakker, P. Sloan, and M. H. Thornhill. 1997. Potentiation of warfarin anticoagulant activity by miconazole oral gel. BMJ 314:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bond, A., and M. Lader. 1974. Use of analog scales in rating subjective feelings. Br. J. Med. Psychol. 47:211-218. [Google Scholar]
- 3.Cogan, D. G. 1941. Simplified entopic pupillometer. Am. J. Ophthalmol. 24:1431-1433. [Google Scholar]
- 4.Compas, D., D. J. Touw, and P. N. de Goede. 1996. Rapid method for the analysis of itraconazole and hydroxyitraconazole in serum by high-performance liquid chromatography. J. Chromatogr. B Biomed. Appl. 687:453-456. [DOI] [PubMed] [Google Scholar]
- 5.Daneshmend, T. K., and D. W. Warnock. 1983. Clinical pharmacokinetics of systemic antifungal drugs. Clin. Pharmacokinet. 8:17-42. [DOI] [PubMed] [Google Scholar]
- 6.Devaraj, A., J. P. O'Beirne, R. Veasey, and A. A. Dunk. 2002. Interaction between warfarin and topical miconazole cream. BMJ 325:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhalla, I. A., et al. 2009. Prescribing of opioid analgesics and related mortality before and after the introduction of long-acting oxycodone. Can. Med. Assoc. J. 181:891-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grönlund, J., et al. 2010. Exposure to oral oxycodone is increased by concomitant inhibition of CYP2D6 and 3A4 pathways, but not by inhibition of CYP2D6 alone. Br. J. Clin. Pharmacol. 70:78-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hagelberg, N. M., et al. 2009. Voriconazole drastically increases exposure to oral oxycodone. Eur. J. Clin. Pharmacol. 65:263-271. [DOI] [PubMed] [Google Scholar]
- 10.Hamunen, K., P. Paakkari, and E. Kalso. 2009. Trends in opioid consumption in the Nordic countries 2002-2006. Eur. J. Pain 13:954-962. [DOI] [PubMed] [Google Scholar]
- 11.Hynninen, V. V., K. T. Olkkola, P. J. Neuvonen, and K. Laine. 2009. Oral voriconazole and miconazole oral gel produce comparable effects on the pharmacokinetics and pharmacodynamics of etoricoxib. Eur. J. Clin. Pharmacol. 65:89-95. [DOI] [PubMed] [Google Scholar]
- 12.Kato, M. 2008. Intestinal first-pass metabolism of CYP3A4 substrates. Drug Metab. Pharmacokinet. 23:87-94. [DOI] [PubMed] [Google Scholar]
- 13.Lalovic, B., et al. 2006. Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: role of circulating active metabolites. Clin. Pharmacol. Ther. 79:461-479. [DOI] [PubMed] [Google Scholar]
- 14.Lalovic, B., B. Phillips, L. L. Risler, W. Howald, and D. D. Shen. 2004. Quantitative contribution of CYP2D6 and CYP3A to oxycodone metabolism in human liver and intestinal microsomes. Drug Metab. Dispos. 32:447-454. [DOI] [PubMed] [Google Scholar]
- 15.Männistä, P. T., R. Mäntylä, S. Nykänen, U. Lamminsivu, and P. Ottoila. 1982. Impairing effect of food on ketoconazole absorption. Antimicrob. Agents Chemother. 21:730-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez-Beneyto, Y., P. Lopez-Jornet, A. Velandrino-Nicolas, and V. Jornet-Garcia. 2010. Use of antifungal agents for oral candidiasis: results of a national survey. Int. J. Dent. Hyg. 8:47-52. [DOI] [PubMed] [Google Scholar]
- 17.Michna, E., et al. 2004. Predicting aberrant drug behavior in patients treated for chronic pain: importance of abuse history. J. Pain Symptom Manage. 28:250-258. [DOI] [PubMed] [Google Scholar]
- 18.Neuvonen, M., and P. J. Neuvonen. 2008. Determination of oxycodone, noroxycodone, oxymorphone, and noroxymorphone in human plasma by liquid chromatography-electrospray-tandem mass spectrometry. Ther. Drug Monit. 30:333-340. [DOI] [PubMed] [Google Scholar]
- 19.Niwa, T., S. Inoue-Yamamoto, T. Shiraga, and A. Takagi. 2005. Effect of antifungal drugs on cytochrome P450 (CYP) 1A2, CYP2D6, and CYP2E1 activities in human liver microsomes. Biol. Pharm. Bull. 28:1813-1816. [DOI] [PubMed] [Google Scholar]
- 20.Niwa, T., T. Shiraga, and A. Takagi. 2005. Effect of antifungal drugs on cytochrome P450 (CYP) 2C9, CYP2C19, and CYP3A4 activities in human liver microsomes. Biol. Pharm. Bull. 28:1805-1808. [DOI] [PubMed] [Google Scholar]
- 21.Oliver, R. J., H. S. Dhaliwal, E. D. Theaker, and M. N. Pemberton. 2004. Patterns of antifungal prescribing in general dental practice. Br. Dent. J. 196:701-703. [DOI] [PubMed] [Google Scholar]
- 22.O'Reilly, R. A., et al. 1992. Mechanisms of the stereoselective interaction between miconazole and racemic warfarin in human subjects. Clin. Pharmacol. Ther. 51:656-667. [DOI] [PubMed] [Google Scholar]
- 23.Pemberton, M. N., R. J. Oliver, and E. D. Theaker. 2004. Miconazole oral gel and drug interactions. Br. Dent. J. 196:529-531. [DOI] [PubMed] [Google Scholar]
- 24.Pöyhiä, R., K. T. Olkkola, T. Seppälä, and E. Kalso. 1991. The pharmacokinetics of oxycodone after intravenous injection in adults. Br. J. Clin. Pharmacol. 32:516-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pöyhiä, R., T. Seppälä, K. T. Olkkola, and E. Kalso. 1992. The pharmacokinetics and metabolism of oxycodone after intramuscular and oral administration to healthy subjects. Br. J. Clin. Pharmacol. 33:617-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sistonen, J., S. Fuselli, A. Levo, and A. Sajantila. 2005. CYP2D6 genotyping by a multiplex primer extension reaction. Clin. Chem. 51:1291-1295. [DOI] [PubMed] [Google Scholar]
- 27.Staahl, C., and A. M. Drewes. 2004. Experimental human pain models: a review of standardised methods for preclinical testing of analgesics. Basic Clin. Pharmacol. Toxicol. 95:97-111. [DOI] [PubMed] [Google Scholar]
- 28.Stone, B. M. 1984. Pencil and paper tests—sensitivity to psychotropic drugs. Br. J. Clin. Pharmacol. 18(Suppl. 1):15S-20S. [DOI] [PMC free article] [PubMed] [Google Scholar]