Abstract
We report darunavir, ritonavir, and etravirine pharmacokinetics in cervicovaginal fluid and blood plasma for women from the Gender, Race and Clinical Experience (GRACE) study. Eight women received darunavir-ritonavir (600/100 mg) twice daily (b.i.d.); two also received etravirine (200 mg) b.i.d. Week 4 paired blood plasma and cervicovaginal fluid samples were collected over 12 h. Darunavir and etravirine cervicovaginal fluid exposures were higher than blood plasma exposures; ritonavir cervicovaginal fluid exposure was lower than blood plasma exposure. The high exposures of darunavir and etravirine in cervicovaginal fluid warrant further evaluation of these drugs for use in HIV-1 prevention.
Primary HIV-1 prevention involves the use of antiretroviral (ARV) therapy in an uninfected individual as pre- and postexposure prophylaxis to prevent initial viral replication. Secondary HIV-1 prevention strategies utilize ARVs in chronically infected individuals to decrease HIV-1 replication in genital secretions and reduce the risk of sexual transmission. Selection of ARV agents for either primary or secondary HIV prevention requires drugs to demonstrate both high genital tract concentrations and antiretroviral efficacy (5, 9). The protease inhibitors (PIs) and nonnucleoside reverse transcriptase inhibitors (NNRTIs) investigated to date have decreased penetration into cervicovaginal fluid (CVF) compared with that into blood plasma (BP) (5, 6). However, there is substantial intraclass and interclass variability in the degree of genital tract penetration. Therefore, the pharmacokinetics (PK) of each ARV in the genital tract compartment need to be evaluated before each drug is considered for prevention of sexual transmission of HIV-1.
The Gender, Race and Clinical Experience (GRACE) study is the largest ARV trial in North America to focus on treatment-experienced women with HIV and was designed to assess sex-based differences in efficacy, safety, and tolerability of darunavir-ritonavir (DRV/r)-based therapy (3). The PK parameters of DRV, ritonavir (RTV), and etravirine (ETR) in CVF and BP of HIV-1-infected women who participated in the genital tract substudy of the GRACE trial are reported.
MATERIALS AND METHODS
Study design.
The GRACE study (ClinicalTrials.gov identifier NCT00381303; protocol TMC114-HIV3004) was a multicenter, open-label, phase 3b study that enrolled treatment-experienced adults with HIV-1 RNA loads of at least 1,000 copies/ml (3). Subjects were excluded if, in the opinion of the investigator, they required enfuvirtide to construct a viable ARV regimen or were receiving any medication with known interactions with DRV, RTV, or ETR (3). Full inclusion and exclusion criteria have been described previously (3). Patients received DRV/r (600/100 mg) twice daily (b.i.d.), plus an investigator-selected optimized background regimen (OBR) that could include ETR. The OBR was selected based on resistance testing and included, on average, two fully active agents. This genital tract analysis was a preplanned PK substudy and was designed to enroll up to 15 premenopausal and 15 postmenopausal women. Human experimentation guidelines of the U.S. Department of Health and Human Services were followed in the conduct of this clinical research.
Patients.
Women from the GRACE study with an intact uterus who screened negative for active sexually transmitted infections and were able to abstain from sexual activity or douching for 48 h prior to genital tract sampling were eligible to participate in the substudy. All patients provided written informed consent.
Study evaluations.
Cervicovaginal fluid PK sampling was conducted in conjunction with week 4 BP sampling. Participants who were adherent, as measured by subject recall of timing of previous doses of study medication, were included. Paired BP and self-collected, directly aspirated CVF samples were obtained immediately predose and at 1, 2, 3, 4, 6, 9, and 12 h postdose. For premenopausal women, CVF PK sampling was performed 5 to 10 days after completion of menses; for postmenopausal women, genital tract sampling was not coordinated with menses. A minimum of 50 μl was required for analysis. Antiretroviral concentrations were measured by validated liquid chromatography-mass spectrometry assays (2, 10) with an intra- and interday variability below 10% and a dynamic range of 2 to 2,000 ng/ml. Data were analyzed by noncompartmental methods, using WinNonlin (version 5.2.1). HIV-1 RNA in genital secretions was measured using the Abbott Realtime HIV-1 0.6-ml protocol (Abbott Laboratories, Abbott Park, IL). Plasma viral load was measured by quantitative PCR (Amplicor HIV-1 Monitor v1.5; Roche Diagnostics, Branchburg, NJ). Since the substudy was not powered for statistical comparisons, descriptive statistics are reported. Unless otherwise noted, data are presented as medians (interquartile ranges [IQR]).
RESULTS
Patient population and baseline characteristics.
A total of eight ARV-experienced women enrolled in the GRACE genital tract substudy. Although the substudy was designed to enroll 30 women (15 premenopausal and 15 postmenopausal), the enrollment target was not met due to a delay in site activation. Therefore, the planned comparison between premenopausal and postmenopausal women could not be performed. Results presented henceforth are the combined results for the 6 premenopausal and 2 postmenopausal subjects. All subjects received DRV/r (600/100 mg) b.i.d., and two patients received ETR (200 mg) b.i.d. in their OBR. The median (IQR) age was 44 (43 to 49) years, and the median (IQR) body mass index was 25 (22 to 34) kg/m2. Seven women were black, and one was Hispanic.
Pharmacokinetics of darunavir, ritonavir, and etravirine in blood plasma and cervicovaginal fluid.
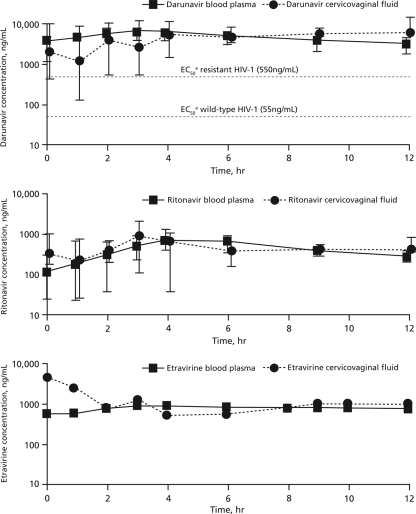
DRV, ETR, and RTV were detected in 90%, 95%, and 86% of CVF samples, respectively. Pharmacokinetic profiles can be found in Fig. 1, and PK parameters are described in Table 1. DRV and ETR exposures in CVF were higher than those in BP, and RTV exposure in CVF was slightly lower than that in BP. Drug exposures in CVF were compared to those in BP by using the median and IQR for the areas under the concentration-time curves (AUC). The median AUCCVF:BP ratios (IQR) were 1.5 (1.0 to 1.6) for DRV, 1.3 for ETR, and 0.8 (0.6 to 1.6) for RTV.
FIG. 1.
Median (interquartile range) concentrations of darunavir (n = 8) (top), ritonavir (n = 8) (middle), and etravirine (n = 2) (bottom) in blood plasma and cervicovaginal fluid. The points and whiskers on the graphs represent the median values and interquartile ranges, respectively. a, protein-adjusted half-maximal effective concentration.
TABLE 1.
Darunavir, ritonavir, and etravirine exposures in blood plasma and cervicovaginal fluid
| PK parametera | Median value (IQR) |
|||||
|---|---|---|---|---|---|---|
| Darunavir (n = 8) |
Ritonavir (n = 8) |
Etravirineb (n = 2) |
||||
| BP | CVF | BP | CVF | BP | CVF | |
| Cmax (ng/ml) | 7,045 (4,954-8,015) | 12,020 (7,951-14,032) | 696 (547-1,134) | 735 (612-1,603) | 873 | 4,558 |
| Tmax (h) | 2.5 (2-3) | 5 (3-7) | 4 (4-6) | 7 (3-10) | 4 | 5 |
| AUC12h (ng·h/ml) | 63,371 (40,604-67,641) | 73,037 (52,414-101,752) | 4,572 (3,505-7,385) | 5,601 (3,306-6,959) | 8,870 | 11,963 |
| CVF/BP AUC12h ratioc | 1.5 (1.0-1.6) | 0.8 (0.6-1.6) | 1.3 | |||
Cmax, maximum plasma concentration; Tmax, time to reach Cmax; AUC12h, area under the concentration-time curve at 12 h.
Data are medians only.
Data represent the medians(IQR) of the individual ratios rather than the median concentrations.
HIV-1 RNA in blood plasma and cervicovaginal fluid.
For the eight study participants, the median HIV-1 RNA load in BP was 5.1 (range, 3.2 to 5.6) log10 copies/ml at baseline and 2.7 (range, 1.7 to 3.3) log10 copies/ml at week 4. All subjects had a decrease in HIV RNA (range, 0.5 to 3.2 log10 copies/ml). Among the eight participants, baseline HIV-1 RNA loads in CVF were available for five women (range, 2.5 to 6.1 log10 copies/ml, or 320 to 1,184,080 copies/ml). Only two subjects had evaluable CVF at week 4, and both of their HIV-1 RNA loads were <2.8 log10 copies/ml (or 640 copies/ml). For the remaining six participants, either the participants had less than the required 800 μl of CVF needed for the HIV-1 RNA assay or the reverse transcription-PCR (RT-PCR) was inhibited for their samples. Due to the small numbers of CVF samples that were available for HIV-1 RNA analyses, no correlation analysis between drug exposure and HIV RNA response was performed.
DISCUSSION
This study investigated DRV, RTV, and ETR exposures in BP and CVF. Our data show that DRV and ETR achieved more penetration into CVF than other PIs and NNRTIs evaluated to date (4, 5; A. J. Jones et al., presented at the 10th International Workshop on Clinical Pharmacology of HIV Therapy, 15 to 17 April 2009, Amsterdam, Netherlands). DRV exposure in the female genital tract was 1.5-fold higher than that in BP. All concentrations of DRV in CVF were above the protein-adjusted 50% effective concentration (EC50) for wild-type virus (55 ng/ml) or resistant virus (550 ng/ml) (1, 11).
This relative CVF exposure was 3- to 20-fold higher than what has been reported for other PIs. For example, the CVF exposures of atazanavir and lopinavir are <20% and <10%, respectively, of those in BP (6). Indinavir is the only PI that shows higher CVF and BP exposures than darunavir (200% versus 150%, respectively). Etravirine exposure in CVF was 30% higher than that in BP, in contrast to the case for efavirenz, which shows minimal penetration into CVF (<1% of BP penetration) (5). However, the ETR data from this study were based on a small sample size (n = 2), which limits any forecasting of its use in the prevention arena until more data are available. Ritonavir exposure in the female genital tract was higher in this study than in previous evaluations, in which RTV exposure in CVF was 25% that in BP (5), although RTV concentrations in the CVF in this study were still lower than those in the BP (80% of BP exposure).
This substudy of the GRACE study did not reach the target enrollment of 15 premenopausal and 15 postmenopausal women, predominantly due to a delay in substudy initiation. There were, however, sufficient numbers of premenopausal women who received DRV to evaluate steady-state genital tract exposure in this population. In general, the high penetration level of DRV into the female genital tract suggests that DRV may be beneficial in preventing the sexual transmission of HIV-1 and in preventing the development of compartmental viral resistance. A direct association between increased ARV penetration into the female genital tract and eradication of infectiousness has not been demonstrated. However, the low level of penetration into the female genital tract of the first-generation NNRTIs has been linked with increased HIV-1 shedding at the cervix compared with that with PIs (with and without low-dose RTV), which have better penetration (7).
Large volumes of CVF are required for HIV-1 RNA quantification (>800 μl is required to detect a level of 200 copies/ml). Despite pooling of all remaining CVF after PK analyses for each individual, this study was not able to adequately evaluate cervical shedding of HIV-1 RNA in all subjects. Therefore, no correlation between drug exposure and infectiousness can be made. Understanding this correlation in the female genital tract is important as treatment guidelines evolve. Further evaluation of the pharmacokinetic-pharmacodynamic relationship and the development of viral resistance among individual ARVs is necessary. While this substudy evaluated ARV penetration under steady-state conditions, further investigation will also be needed to assess single-dose PK of DRV and ETR in order to understand their role in primary prevention. Nonetheless, the CVF penetration of DRV was similar to those of other ARVs (including tenofovir, which has a CVF/BP penetration ratio of 110%) that are being investigated for primary prophylaxis (5, 8).
The high exposures of DRV and ETR in the female genital tract suggest that both ARVs may be effective in primary and secondary HIV-1 prevention strategies beyond reduction of plasma HIV-1 RNA. Further research is warranted to define the effects of DRV and ETR on HIV-1 RNA, and thus infectiousness, in the female genital tract.
Acknowledgments
Funding for the GRACE study, including the genital tract substudy, and editorial support were provided by Tibotec Therapeutics. This work was also supported in part by NIH Career Development Award AI077355 (K.P.), Research Center grant AI50410 (A.K. and S.J.), and Investigator-Initiated Clinical Trial planning grant AI087065 (A.K.).
We thank the study sites, the patients and their families, and the principal investigators for their participation in the trial. We acknowledge Gilead for supplying emtricitabine, tenofovir, and emtricitabine-tenofovir, and we thank the internal study support staff, as well as Cali Howitt, Medicus International New York, for drafting and editorial assistance.
Footnotes
Published ahead of print on 20 December 2010.
REFERENCES
- 1.Boffito, M., D. Miralles, and A. Hill. 2008. Pharmacokinetics, efficacy, and safety of darunavir/ritonavir 800/100 mg once-daily in treatment-naive and -experienced patients. HIV Clin. Trials 9:418-427. [DOI] [PubMed] [Google Scholar]
- 2.Bouche, M. P., L. Michielsen, M. Piot, and P. Timmerman. 2006. Swift and simultaneous determination of darunavir (TMC114) and ritonavir in human plasma using LC-MS/MS, poster TuP-042. Abstr. 17th Int. Mass Spectrom. Conf., Prague, Czech Republic, 27 August to 1 September 2006.
- 3.Currier, J. D., et al. Sex-based outcomes of darunavir-ritonavir therapy: a single-group trial. Ann. Intern. Med. 153:349-357. [DOI] [PMC free article] [PubMed]
- 4.Dumond, J. B., et al. 2009. Maraviroc concentrates in the cervicovaginal fluid and vaginal tissue of HIV-negative women. J. Acquir. Immune Defic. Syndr. 51:546-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dumond, J. B., et al. 2007. Antiretroviral drug exposure in the female genital tract: implications for oral pre- and post-exposure prophylaxis. AIDS 21:1899-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Min, S. S., et al. 2004. Protease inhibitor and nonnucleoside reverse transcriptase inhibitor concentrations in the genital tract of HIV-1-infected women. J. Acquir. Immune Defic. Syndr. 37:1577-1580. [DOI] [PubMed] [Google Scholar]
- 7.Neely, M. N., et al. 2007. Cervical shedding of HIV-1 RNA among women with low levels of viremia while receiving highly active antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 44:38-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patterson K. N., et al. 2009. Darunavir, ritonavir, and etravirine steady-state pharmacokinetics in the cervicovaginal fluid and blood plasma of HIV-infected women enrolled in the GRACE study, poster 270. Abstr. 47th Annu. Meet. Infect. Dis. Soc. Am. (IDSA), Philadelphia, PA, 29 October to 1 November 2009.
- 9.Reddy, Y. S., A. Kashuba, J. Gerber, and V. Miller. 2003. Roundtable report: importance of antiretroviral drug concentrations in sanctuary sites and viral reservoirs. AIDS Res. Hum. Retroviruses 19:167-176. [DOI] [PubMed] [Google Scholar]
- 10.Rezk, N. L., N. R. White, S. H. Jennings, and A. D. Kashuba. 2009. A novel LC-ESI-MS method for the simultaneous determination of etravirine, darunavir and ritonavir in human blood plasma. Talanta 79:1372-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tibotec Therapeutics. 2009. Prezista (darunavir) tablet package insert. Tibotec Therapeutics, Titusville, NJ.