Abstract
XF-73 is a dicationic porphyrin drug with rapid Gram-positive antibacterial activity currently undergoing clinical trials for the nasal decolonization of Staphylococcus aureus, including methicillin-resistant Staphylococcus aureus (MRSA). In multistep (55-passage) resistance selection studies in the presence of subinhibitory concentrations of XF-73, retapamulin, mupirocin, fusidic acid, and vancomycin against four Network on Antimicrobial Resistance in Staphylococcus aureus MRSA strains, there was no >4-fold increase in the MIC for XF-73 after 55 passages. In contrast, there was an increase in the MICs for retapamulin (from 0.25 μg/ml to 4 to 8 μg/ml), for mupirocin (from 0.12 μg/ml to 16 to 512 μg/ml), for fusidic acid (from 0.12 μg/ml to 256 μg/ml), and for vancomycin (from 1 μg/ml to 8 μg/ml in two of the four strains tested). Further investigations using S. aureus NRS384 (USA300) and daptomycin demonstrated a 64-fold increase in the MIC after 55 passages (from 0.5 μg/ml to 32 μg/ml) with a >4-fold increase in the MIC obtained after only five passages. Sequencing analysis of selected isolates confirmed previously reported point mutations associated with daptomycin resistance. No cross-resistance to XF-73 was observed with the daptomycin-resistant strains, suggesting that whereas the two drugs act on the bacterial cell membrane, their specific site of action differs. XF-73 thus represents the first in a new class of antibacterial drugs, which (unlike the comparator antibiotics) after 55 passages exhibited a ≤4-fold increase in MIC against the strains tested. Antibacterial drugs with a low propensity for inducing bacterial resistance are much needed for the prevention and treatment of multidrug-resistant bacteria both within and outside the hospital setting.
Methicillin-resistant Staphylococcus aureus (MRSA) is increasingly prevalent throughout the world and is endemic in some hospital and community settings (5, 15, 25). Community-associated MRSA (CA-MRSA) is also becoming more widespread; in the United States, 59% of skin and soft tissue infections presenting to emergency departments are caused by CA-MRSA (25). The most prevalent CA-MRSA clone in the United States, USA300, which carries the staphylococcal cassette chromosome (SCC) mec type IVa and Panton-Valentine leukocidin genes, now accounts for 61 to 87% of MRSA isolates from skin and soft tissue infections in North American emergency departments (1, 19) and is also emerging as a serious cause of health care-associated infections (14, 22, 29). There is therefore a critical need for new antibacterials, particularly those with no cross-resistance to currently available antibacterial drugs and with little or no likelihood of inducing resistance.
XF-73 is a dicationic porphyrin drug (Fig. 1), and previous studies have shown that XF-73 has potent Gram-positive in vitro bactericidal activity against a range of clinically important S. aureus isolates, including methicillin-sensitive S. aureus (MSSA), health care-associated MRSA (HA-MRSA), and CA-MRSA (minimal bactericidal concentration at which 90% of strains tested are killed [MBC90], 0.5 μg/ml) (10). XF-73 has rapid activity, with a >4-log reduction in CFU obtained after 5 min incubation in growth medium (27).
FIG. 1.
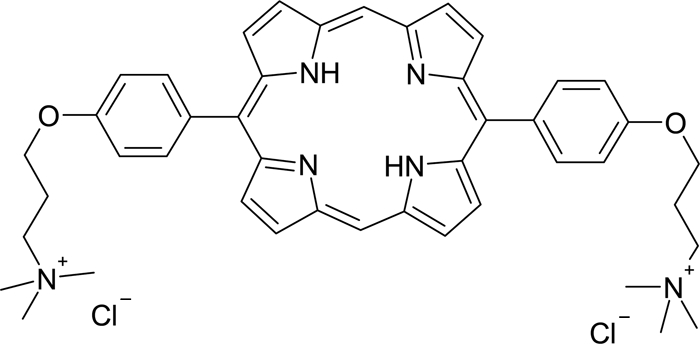
Chemical structure of XF-73.
Investigations into the mechanism of action of XF-73 against S. aureus demonstrated that the drug exhibits a rapid bacterial cell membrane-perturbing activity, which is likely to be responsible for the inhibition of macromolecular synthesis and the death of staphylococci exposed to the drug (26, 27). The presence of known resistance mechanisms was found not to have any effect on the antibacterial activity of XF-73 (10), suggesting that the mechanism of action of XF-73 is novel. XF-73 has also been shown to be equally active against nongrowing S. aureus and against S. aureus within biofilms (28). XF-73 is being developed as a topical drug to prevent and treat S. aureus, and MRSA infections and clinical trials are under way to assess the potential for the nasal decolonization of S. aureus.
The purpose of this study was to investigate the propensity of XF-73 and selected comparators to select for endogenous mutational resistance during prolonged passages at subinhibitory concentrations. The topical antibiotics mupirocin, fusidic acid, and retapamulin were selected as direct comparators to XF-73, and vancomycin, which has been previously demonstrated to be refractive to the emergence of mutational resistance in multipassage studies (3, 6, 7, 20, 21), was also included. As the mechanism of action (MOA) of daptomycin (16, 30) is also proposed to act on the bacterial membrane, it was also tested against USA300, and molecular genetic studies to characterize the mechanism(s) of daptomycin resistance in isolated clones were also undertaken. Phenotypic stability of any resistant isolates obtained during the studies involving USA300 and cross-resistance with the other test compounds were also investigated.
(Part of this study was presented at the 48th Interscience Conference on Antimicrobial Agents and Chemotherapy and the 46th Annual Meeting of the Infectious Disease Society of America, a joint meeting held in Washington, DC, in 2008, and the 18th European Congress of Clinical Microbiology and Infectious Diseases, held in Barcelona, Spain, in 2008.)
MATERIALS AND METHODS
Bacteria.
Four Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) strains of MRSA, comprising prevalent and endemic HA- and CA-MRSA clones, were selected for serial passage (Table 1).
TABLE 1.
MRSA isolates used for resistance testing
| MRSA strain | Alternative name | Description | Source |
|---|---|---|---|
| NRS123 | USA400 | Multiply susceptible CA-MRSA carrying Panton-Valentine leukocidin genes and additional virulence genes distinct from those of HA-MRSA strains | NARSA |
| NRS382 | USA100 | Predominant US HA-MRSA strain, also known as the New York/Japan clone | NARSA |
| NRS384 | USA300 | CA-MRSA carrying Panton-Valentine leukocidin genes—now one of the most common isolates responsible for MRSA infections both in the community and, increasingly, in health care settings | NARSA |
| NRS387 | USA800 | Also known as the “pediatric clone,” commonly isolated from children | NARSA |
Antimicrobial agents and MIC testing.
XF-73 and retapamulin extract were provided by Destiny Pharma Ltd., Brighton, United Kingdom. Vancomycin and fusidic acid were obtained from Sigma, mupirocin was obtained from Mast, France, and daptomycin was obtained from Cubist Pharmaceuticals Inc., Cambridge, MA 01239.
MICs were determined by the broth microdilution method according to approved M7-A7E standards of the CLSI (8), except that Iso-Sensitest broth was used as the test medium. For daptomycin susceptibility testing, the medium was supplemented with calcium to a final concentration of 50 μg/ml.
Multipassage resistance selection studies.
Multipassage resistance selection studies using XF-73, mupirocin, fusidic acid, retapamulin, and vancomycin were performed with all four NARSA isolates listed in Table 1, while daptomycin was tested against S. aureus NRS384 (USA300) only. A dilution series of 10 concentrations was prepared for each test compound based on the previous MIC at 2× the required final concentration. Initial inocula (1 × 108 to 2 × 108 CFU/ml) were prepared in Iso-Sensitest broth from overnight agar cultures and diluted 100× in fresh broth, and 0.5 ml was added to 0.5 ml of each antimicrobial dilution series and the control bijou bottle (no antimicrobial). The bijou bottles were incubated at 35°C for 24 h, and the cultures were passaged daily for 55 days by using 0.5-ml inocula from the bijou bottle corresponding to 0.5× the MIC determined from the previous passage to inoculate a fresh series of drug dilutions and control bijou bottles. All drugs were passaged for the entire 55 passages, irrespective of the MIC. Isolates were saved at intervals throughout the passage experiment, and resistant mutants emerging during the course of the study were also saved and stored frozen at −70°C.
After 55 passages, the stability of acquired resistance in the S. aureus NRS384 (USA300) passage experiment was investigated by MIC determinations after 10 successive passages on drug-free agar for cultures isolated from four different passage numbers. The cross-resistance of the isolates derived from USA300 was evaluated by determining the MIC against the other test compounds and comparing them to the baseline (passage 0).
In this study, resistance was defined as a >4-fold increase in the initial MIC (4, 7, 21).
Molecular characterization of daptomycin-resistant clones.
Three isolates with reduced susceptibility for daptomycin, identified during the daptomycin serial passage, were selected for genetic characterization to determine the mechanism of resistance. DNA from the three isolates, wild-type USA300, and the control strain MW2 was extracted and amplified by PCR. Nine target DNA sequences, previously identified as sites where mutations resulted in elevated MICs for daptomycin, were investigated (11).
Genomic DNA purification.
DNA was extracted from the isolates using the DNA template preparation kit (Roche) according to the manufacturer's instructions. An additional cell lysis solution (250 U lysozyme/ml, 25 U lysostaphin, 10 mM EDTA, 10 mM Tris [pH 8.0]) was used to facilitate cell lysis.
PCR amplification and DNA sequencing.
PCR and sequencing primers were as used in a previous investigation into the genetic changes correlating with reduced susceptibility by daptomycin by S. aureus (11) with the exception of two additional primer sequences for the intergenic region upstream from rspU (1654504C-T AltF [GTTTTAACGGCTTCACAA], located at gene position 1654708-1654725, and 1654504C-T AltR [GAGGGAGTCAACAGAGGTA], located at gene position 1654291-1654309). PCR amplification and sequencing were undertaken by Quotient Bioresearch Ltd. as a service for a fee.
RESULTS
For XF-73, compared with the initial MIC values, there was a 2-fold increase for three strains, and a 4-fold increase for the fourth, in the MIC after 55 passages (Table 2). During the course of the passage experiment, MIC values for XF-73 showed no more than a 4-fold increase from the starting MIC value, indicating that no resistance (defined as a >4-fold increase in MIC [4, 7, 21]) developed during the 55 passages (Table 2).
TABLE 2.
Multipassage resistance selection results
| Strain | Antibacterial | Initial MIC (μg/ml) | Passage at which resistancea emerges | Final MIC (μg/ml) at passage 55 |
|---|---|---|---|---|
| S. aureus NRS123 (USA400; MRSA) | XF-73 | 0.5 | No resistance | 1 |
| Vancomycin | 1 | No resistance | 4 | |
| Retapamulin | 0.25 | 3 | 8 | |
| Fusidic acid | 0.12 | 5 | 256 | |
| Mupirocin | 0.12 | 7 | 32 | |
| S. aureus NRS382 (USA100; MRSA) | XF-73 | 0.25 | No resistance | 1 |
| Vancomycin | 1 | 32 (35b) | 8 | |
| Retapamulin | 0.25 | 9 (13b) | 8 | |
| Fusidic acid | 0.12 | 2 | 256 | |
| Mupirocin | 0.12 | 2 | 16 | |
| S. aureus NRS384 (USA300; MRSA) | XF-73 | 0.25 | No resistance | 0.5 |
| Vancomycin | 1 | 20 (46b) | 8 | |
| Daptomycin | 0.5 | 5 | 32 | |
| Retapamulin | 0.25 | 16 (19b) | 4 | |
| Fusidic acid | 0.12 | 2 | 256 | |
| Mupirocin | 0.12 | 2 | 512 | |
| S. aureus NRS387 (USA800; MRSA) | XF-73 | 0.25 | No resistance | 0.5 |
| Vancomycin | 1 | No resistance | 4 | |
| Retapamulin | 0.25 | 15 | 8 | |
| Fusidic acid | 0.12 | 1 | 256 | |
| Mupirocin | 0.12 | 2 | 256 |
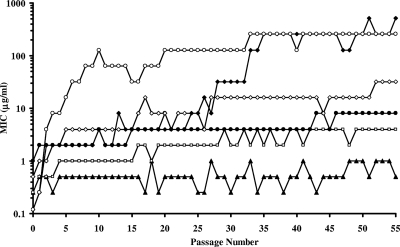
For mupirocin, fusidic acid, and daptomycin, a >4-fold increase in the original MIC was observed for all four strains tested within seven repeat passages (Table 2). For vancomycin, a >4-fold increase was observed for two of the four strains tested (Table 2).
For retapamulin, the initial MIC of the extracted sample corresponded well with previously reported MIC values (0.25 μg/ml compared to an MIC90 of 0.12 μg/ml [17, 32]), confirming the validity of the sample. A >4-fold increase over the initial MIC was observed after 3 to 16 repeat passages (Table 2) for all four strains tested.
Daptomycin showed a steady increase in MIC during the course of the passage experiment in S. aureus NRS384 (USA300), with a final MIC of 32 μg/ml obtained (Table 2).
Representative resistance development profiles for XF-73 and comparators versus S. aureus NRS384 (USA300) are presented in Fig. 2.
FIG. 2.
Plot of MICs (μg/ml) of XF-73, mupirocin, fusidic acid, retapamulin, daptomycin, and vancomycin for S. aureus NRS384 (USA300) during 55 serial passages at 0.5 MIC (determined by the previous passage): ▴, XF-73; □, retapamulin; •, vancomycin; ⋄, daptomycin; ⧫, mupirocin; ○, fusidic acid.
Stability of acquired resistance.
The elevated MICs for mupirocin, fusidic acid, and daptomycin after 5, 24 (25 for fusidic acid), and 55 passages were found to be stable (defined as having an MIC after 10 drug-free passages within one dilution of the MIC before the drug-free passages [7, 21]).
Cross-resistance of acquired mutations.
The cross-resistance profiles of XF-73-, mupirocin-, fusidic acid-, or daptomycin-incubated S. aureus NRS384 (USA300) isolates from passages 0, 5, 24 (25 for fusidic acid), and 55 were determined against a range of antimicrobial comparators: XF-73, mupirocin, fusidic acid, daptomycin, and two glycopeptide antibiotics, vancomycin and teicoplanin, after 10 passages in nonselective conditions on drug-free agar.
There was no evidence of cross-resistance with XF-73 in any of the resistant isolates tested. With one exception, none of the resistant isolates showed any cross-resistance with the other antibiotic comparators tested. Daptomycin-resistant isolates from passage 55 showed a >16-fold increase in teicoplanin MIC (passage 0 MIC, 0.25 μg/ml; passage 55 MIC, 4 μg/ml).
Genetic characterization of isolates of S. aureus NRS384 (USA300) with elevated daptomycin MICs.
DNA sequences obtained from selected isolates of S. aureus NRS384 (USA300) obtained during the daptomycin 55-passage study with increasing daptomycin MIC values were compared to those of the parent strain [S. aureus NRS384 (USA300), no serial passages] for each of the targets selected (11). DNA sequences obtained from MW2 were compared to the published complete genome of MW2 (NCBI accession number BA000033) as a sequencing control.
S. aureus NRS384 (USA300) at passage 5, with a raised daptomycin MIC of 2 μg/ml (8-fold increase in initial MIC), was found to have an amino acid change at location A302V in the mprF gene. By passage 24, the daptomycin MIC had increased to 8 μg/ml (16-fold increase in initial MIC) and a further amino acid change in the mprF gene at location N352K and in the yycG gene at location L331S was detected. At passage number 55, with a daptomycin MIC of 32 μg/ml (64-fold increase in original MIC), no further amino acid changes were observed in the targets sequenced in this investigation.
DISCUSSION
No increase of the XF-73 MIC >4-fold was observed for any of the four MRSA strains tested in this study after 55 successive passages at subinhibitory concentrations. In contrast, there was a significant increase in the MIC for three of the comparators against all the strains tested and two of four strains for vancomycin using the same conditions. XF-73 has a very rapid bactericidal activity (26, 27) and acts on both dividing and nongrowing bacterial cells (28), and investigations suggest that it has a novel MOA (26, 27). The results reported in this study confirm that such an antibacterial profile is likely to reduce the potential for the emergence of bacterial mutational resistance.
The results obtained with mupirocin and fusidic acid in this study are in line with results reported in previous passage studies (3, 20). Mupirocin is widely used for the elimination of S. aureus nasal carriage and the prophylactic intranasal eradication of MRSA (23, 33, 35). However, this has led to increased resistance rates and calls to limit its use to outbreak situations only, rather than for routine decolonization of S. aureus (18, 24, 34). Recent estimates suggest that mupirocin resistance rates in HA-MRSA are in the order of 15 to 18% (9, 31, 36). Of particular concern is the recent finding that high-level mupirocin-resistant MRSA strains are more likely to be associated with CA-MRSA, tend to occur more commonly in MRSA-colonized patients, and are more likely to have coresistance to fusidic acid (31). A recent demonstration (2) that nasal decolonization of MSSA using mupirocin of elective surgical hospital patients can have a significant positive impact on MSSA infection rates is encouraging. However, the use of mupirocin as a prophylactic for decolonization of the much larger numbers of patients carrying nasal MSSA (30 to 40%) might be considered a strategy which may drive mupirocin resistance rates even higher and to the point of impotence.
Retapamulin is a new topical antibiotic approved in 2007, but increases in retapamulin MICs in in vitro passage studies were reported in 2006 (20): three strains were chosen for prolonged passage to the maximum 50 passages, at which point the MICs for retapamulin were found to be 4 to 16 μg/ml, a 67- to 533-fold increase over the initial MIC. Subsequent studies have suggested that there may be a fitness cost associated with mutations that result in a reduction of activity of retapamulin (12), but clinical isolates with MICs of ≥2 μg/ml for retapamulin have already been reported (13).
Vancomycin has been used as a comparator in a significant number of multipassage studies (3, 4, 6, 7, 20, 21), and the emergence of resistance has been reported in only one of these (3). In our study, the initial MIC for vancomycin increased >4-fold in two of the four strains tested. In comparison, using the same strains, no such increase in the MIC of XF-73 was observed.
As the proposed antibacterial MOA for daptomycin (16, 30) is similar to that proposed for XF-73 (26, 27), both of which appear to target the bacterial membrane, resulting in membrane depolarization and loss of intracellular components, it was also included in this study. In vitro reduced susceptibility to daptomycin in S. aureus NRS384 (USA300) emerged after only five repeat passages and remained stable after 10 serial passages in drug-free conditions. This result is in accordance with results reported in previous passage studies (3, 4, 21).
The genetic characterization of the mutations that had occurred which resulted in a reduced susceptibility to daptomycin confirmed that the initial mutations were located in previously identified sites in the genome (11). The mutations which were responsible for the elevation of the daptomycin MIC from 8 μg/ml to 32 μg/ml were not located in any of the nine locations investigated in this study, suggesting that mutations can occur at other sites. The final MIC obtained in our study (32 μg/ml) was higher than that reported in the source reference (11), and thus it is plausible that the mutations responsible were not identified in the previous study.
No effect on the antibacterial activity of XF-73 was observed with any of the daptomycin isolates tested from different passage numbers, suggesting that while XF-73 and daptomycin may both act on the bacterial cell membrane, XF-73 does not appear to share any common target sites with daptomycin.
The results of this multistep passage resistance study clearly demonstrate that XF-73 has potential significant advantages over existing topical antibacterial compounds with regards to the emergence of mutational resistance at subinhibitory concentrations. No >4-fold increase in MICs for XF-73 was observed after 55 successive passages at subinhibitory concentrations for four NARSA strains. XF-73 may potentially provide a significant addition to the antibacterial armamentarium for topical bacterial infection prophylaxis and treatment. XF-73 is currently undergoing clinical trials for the nasal decolonization of S. aureus, including MRSA.
Acknowledgments
The NARSA strains were obtained through the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) program, supported under NIAID/NIH contract number HHSN272200700055C.
Footnotes
Published ahead of print on 13 December 2010.
REFERENCES
- 1.Al-Rawahi, G. N., et al. 2008. Community-associated CMRSA-10 (USA-300) is the predominant strain among methicillin-resistant Staphylococcus aureus strains causing skin and soft tissue infections in patients presenting to the emergency department of a Canadian tertiary care hospital. J. Emerg. Med. 38:6-11. [DOI] [PubMed] [Google Scholar]
- 2.Bode, L. G., et al. 2010. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N. Engl. J. Med. 362:9-17. [DOI] [PubMed] [Google Scholar]
- 3.Bogdanovich, T., L. M. Ednie, S. Shapiro, and P. C. Appelbaum. 2005. Antistaphylococcal activity of ceftobiprole, a new broad-spectrum cephalosporin. Antimicrob. Agents Chemother. 49:4210-4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogdanovich, T., et al. 2007. Antistaphylococcal activity of CG400549, a new experimental FabI inhibitor, compared with that of other agents. Antimicrob. Agents Chemother. 51:4191-4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. MRSA in healthcare settings. Centers for Disease Control and Prevention, Atlanta, GA. http://www.cdc.gov/ncidod/dhqp/pdf/ar/mdroGuideline2006.pdf.
- 6.Clark, C., et al. 2009. Antistaphylococcal activity of dihydrophthalazine antifolates, a family of novel antibacterial drugs. Antimicrob. Agents Chemother. 53:1353-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark, C., et al. 2009. Resistance selection studies comparing the activity of razupenem (PTZ601) to vancomycin and linezolid against eight methicillin-resistant and two methicillin-susceptible Staphylococcus aureus strains. Antimicrob. Agents Chemother. 53:3118-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute (CLSI). 2007. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard—seventh edition, M7-A7. CSLI, Wayne, PA.
- 9.Cuevas, O., et al. 2007. Molecular epidemiology of methicillin-resistant Staphylococcus aureus in Spain: a multicentre prevalence study (2002). Clin. Microbiol. Infect. 13:250-256. [DOI] [PubMed] [Google Scholar]
- 10.Farrell, D. J., M. Robbins, W. Rhys-Williams, and W. G. Love. 2010. In vitro activity of XF-73, a novel antibacterial agent, against antibiotic-sensitive and -resistant Gram-positive and Gram-negative bacterial species. Int. J. Antimicrob. Agents 35:531-536. [DOI] [PubMed] [Google Scholar]
- 11.Friedman, L., J. D. Alder, and J. A. Silverman. 2006. Genetic changes that correlate with reduced susceptibility to daptomycin in Staphylococcus aureus. Antimicrob. Agents Chemother. 50:2137-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gentry, D. R., S. F. Rittenhouse, L. McCloskey, and D. J. Holmes. 2007. Stepwise exposure of Staphylococcus aureus to pleuromutilins is associated with stepwise acquisition of mutations in rplC and minimally affects susceptibility to retapamulin. Antimicrob. Agents Chemother. 51:2048-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gentry, D. R., et al. 2008. Genetic characterization of Vga ABC proteins conferring reduced susceptibility to pleuromutilins in Staphylococcus aureus. Antimicrob. Agents Chemother. 52:4507-5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez, B. E., et al. 2006. Community-associated strains of methicillin-resistant Staphylococcus aureus as the cause of healthcare-associated infection. Infect. Control Hosp. Epidemiol. 27:1051-1056. [DOI] [PubMed] [Google Scholar]
- 15.Health Protection Agency. 2007. Surveillance of healthcare associated infections report 2007. Health Protection Agency, London, United Kingdom. http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1196942169446.
- 16.Hobbs, J. K., K. Miller, A. J. O'Neill, and I. Chopra. 2008. Consequences of daptomycin-mediated membrane damage in Staphylococcus aureus. J. Antimicrob. Chemother. 62:1003-1008. [DOI] [PubMed] [Google Scholar]
- 17.Jones, R. N., T. R. Fritsche, H. S. Sader, and J. E. Ross. 2006. Activity of retapamulin (SB-275833), a novel pleuromutilin, against selected resistant gram-positive cocci. Antimicrob. Agents Chemother. 50:2583-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kauffman, C. A., et al. 1993. Attempts to eradicate methicillin-resistant Staphylococcus aureus from a long-term-care facility with the use of mupirocin ointment. Am. J. Med. 94:371-378. [DOI] [PubMed] [Google Scholar]
- 19.King. M. D., et al. 2006. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft-tissue infections. Ann. Intern. Med. 144:309-317. [DOI] [PubMed] [Google Scholar]
- 20.Kosowska-Shick, K., et al. 2006. Single- and multistep resistance selection studies on the activity of retapamulin compared to other agents against Staphylococcus aureus and Streptococcus pyogenes. Antimicrob. Agents Chemother. 50:765-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kosowska-Shick, K., et al. 2009. Activity of telavancin against staphylococci and enterococci determined by MIC and resistance selection studies. Antimicrob. Agents Chemother. 53:4217-4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kourbatova, E. V., et al. 2005. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA 300 clone as a cause of health care-associated infections among patients with prosthetic joint infections. Am. J. Infect. Control 33:385-391. [DOI] [PubMed] [Google Scholar]
- 23.Laupland, K. B., and J. M. Conly. 2003. Treatment of Staphylococcus aureus colonization and prophylaxis for infection with topical intranasal mupirocin: an evidence-based review. Clin. Infect. Dis. 37:933-938. [DOI] [PubMed] [Google Scholar]
- 24.Miller, M. A., A. Dascal, J. Portnoy, and J. Mendelson. 1996. Development of mupirocin resistance among methicillin-resistant Staphylococcus aureus after widespread use of nasal mupirocin ointment. Infect. Control Hosp. Epidemiol. 17:811-813. [DOI] [PubMed] [Google Scholar]
- 25.Moran, G. J., et al. 2006. EMERGEncy ID Net Study Group. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 355:666-674. [DOI] [PubMed] [Google Scholar]
- 26.Ooi, N., K. Miller, W. Rhys-Williams, W. G. Love, and I. Chopra. 2009. Comparison of bacterial membrane active novel porphyrin and metalloporphyrin antimicrobials. Clin. Microbiol. Infect. 15:S287-S288. [Google Scholar]
- 27.Ooi, N., et al. 2009. XF-73, a novel antistaphylococcal membrane-active agent with rapid bactericidal activity. J. Antimicrob. Chemother. 64:735-740. [DOI] [PubMed] [Google Scholar]
- 28.Ooi, N., et al. 2010. XF-70 and XF-73, novel antibacterial agents active against slow-growing and non-dividing cultures of Staphylococcus aureus including biofilms. J. Antimicrob. Chemother. 65:72-78. [DOI] [PubMed] [Google Scholar]
- 29.Seybold, U., et al. 2006. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin. Infect. Dis. 42:647-656. [DOI] [PubMed] [Google Scholar]
- 30.Silverman, J. A., N. G. Perlmutter, and H. M. Shapiro. 2003. Correlation of daptomycin bactericidal activity and membrane depolarization in Staphylococcus aureus. Antimicrob. Agents Chemother. 47:2538-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simor, A. E., et al. 2007. Mupirocin-resistant, methicillin-resistant Staphylococcus aureus strains in Canadian hospitals. Antimicrob. Agents Chemother. 51:3880-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Traczewski, M. M., and S. D. Brown. 2008. Proposed MIC and disk diffusion microbiological cutoffs and spectrum of activity of retapamulin, a novel topical antimicrobial agent. Antimicrob. Agents Chemother. 52:3863-3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trautmann, M., J. Stecher, W. Hemmer, K. Luz, and H. T. Panknin. 2008. Intranasal mupirocin prophylaxis in elective surgery. A review of published studies. Chemotherapy 54:9-16. [DOI] [PubMed] [Google Scholar]
- 34.Upton, A., S. Lang, and H. Heffernan. 2003. Mupirocin and Staphylococcus aureus: a recent paradigm of emerging antibiotic resistance. J. Antimicrob. Chemother. 51:613-617. [DOI] [PubMed] [Google Scholar]
- 35.van Rijen, M. M., M. Bonten, R. P. Wenzel, and J. A. Kluytmans. 2008. Intranasal mupirocin for reduction of Staphylococcus aureus infections in surgical patients with nasal carriage: a systematic review. J. Antimicrob. Chemother. 61:254-261. [DOI] [PubMed] [Google Scholar]
- 36.Vivoni, A. M., et al. 2005. Mupirocin for controlling methicillin-resistant Staphylococcus aureus: lessons from a decade of use at a university hospital. Infect. Control Hosp. Epidemiol. 26:662-667. [DOI] [PubMed] [Google Scholar]