Burkholderia cepacia complex (BCC), a group of nonfermenting Gram-negative bacilli (NFGNB), is emerging as an important nosocomial pathogen. It causes a wide variety of infections, such as pneumonia (especially in patients with cystic fibrosis), meningitis, and septicemia. These infections are often difficult to treat due to high intrinsic antimicrobial resistance in BCC and the lack of effective antibiotics. BCC is resistant to aminoglycosides, antipseudomonal penicillins, and cephalosporins (4). Hence, the need for new agents active against this pathogen has become critical for patient care.
In recent years, carbapenems have assumed a greater therapeutic role for multidrug-resistant (MDR) isolates. Doripenem is a new broad-spectrum intravenous 1-β-methyl carbapenem. It has high activity against many Gram-positive and -negative organisms, including NFGNB. Against Pseudomonas aeruginosa, doripenem exhibits rapid bactericidal activity, with 2- to 4-fold-lower MICs than those of meropenem. However, the activity of doripenem against BCC has not been reported much, and present susceptibility reports are mainly from isolates of cystic fibrosis patients (6). Isolation of BCC from blood of non-CF septicemic patients is on the rise in our region (3), and the in vitro activity of doripenem against these isolates has been studied.
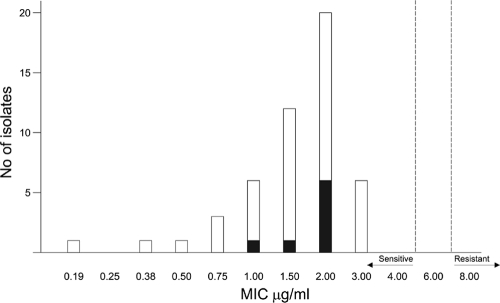
Fifty blood isolates of BCC obtained between May 2007 and January 2010 were included in this study. These isolates were identified using standard biochemical tests and confirmed using recA PCR-based restriction fragment length polymorphism (RFLP) analysis as described by Mahenthiralingam et al. to identify species (5). B. cenocepacia (genomovar IIIA) was the most prevalent species of BCC obtained. Pure cultures of the isolates were lyophilized and stored at 4°C. The meropenem susceptibilities of these 50 isolates were determined by the disk diffusion method (Astra Zeneca Pvt. Ltd., India) according to CLSI guidelines, with 8 isolates (16%) being meropenem resistant. In vitro susceptibility to doripenem (Ranbaxy Intensiva, India) was determined by the Etest. Etest strips (bioMérieux India Pvt. Ltd.) were used per the instructions of the manufacturer. In addition, Escherichia coli ATCC 25922 and P. aeruginosa ATCC 27853 were tested for quality control, and MICs obtained for these organisms were within the ranges recommended by the CLSI (2a). A distribution of the MICs of doripenem determined by the Etest for 50 strains of BCC is shown in Fig. 1.
FIG. 1.
Distribution of the MICs of doripenem determined by Etest for 50 strains of BCC. Black bars represent meropenem-resistant isolates.
The MIC values classifying isolates as susceptible or resistant to doripenem suggested by Bhavnani et al. (2) and Andes et al. (1) indicate susceptibility at ≤4 mg/liter and resistance at ≥16 mg/liter, with intermediate resistance being inferred as 8 mg/liter. Since there are no FDA- or CLSI-approved breakpoints at this time for doripenem, the MIC values obtained were interpreted based on the above criteria, with all 50 isolates (100%) of BCC being susceptible to doripenem (MIC ≤ 4 mg/liter). A greater proportion of strains among meropenem-resistant BCC were inhibited by doripenem at 2 mg/liter (Fig. 1).
Therefore, the in vitro susceptibility profile of BCC to doripenem is comparable to those of BCC to meropenem, and it can be an effective therapy for BCC strains resistant to meropenem.
Footnotes
Published ahead of print on 28 December 2010.
REFERENCES
- 1.Andes, D. R., S. Kiem, and W. A. Craig. 2003. In vivo pharmacodynamic activity of a new carbapenem, doripenem (DOR), against multiple bacteria in a murine thigh infection model, abstr. A-308, p. 10. Prog. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL. American Society for Microbiology, Washington, DC.
- 2.Bhavnani, S. M., J. P. Hammel, and B. B. Cirincioni. 2003. PK-PD target attainment with Monte Carlo simulation as decision support of phase 2/3 dosing strategies for clinical development of doripenem (DOR), abstr. A-11, p. 2. Prog. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., Chicago, IL. American Society for Microbiology, Washington, DC.
- 2a.CLSI. 2010. Performance standards for antimicrobial susceptibility testing. Document M100-S20. CLSI, Wayne, PA.
- 3.Gautam, V., et al. 2010. Burkholderia cepacia complex in septicaemic non-cystic fibrosis cases from two tertiary care hospitals in north India. Indian J. Med. Res. 131:829-832. [PubMed] [Google Scholar]
- 4.LiPuma, J. J., B. J. Currie, G. D. Lum, and P. Vandamme. 2007. Burkholderia, Stenotrophomonas, Ralstonia, Cupriavidus, Pandoraea, Brevundimonas, Comamonas, Delftia and Acidovorax, p. 749-769. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. L. Landry, and M. A. Pfaller (ed.), Manual of clinical microbiology, 9th ed. ASM Press, Washington, DC.
- 5.Mahenthiralingam, E., et al. 2000. DNA-based diagnostic approaches for identification of Burkholderia cepacia complex, Burkholderia vietnamiensis, Burkholderia multivorans, Burkholderia stabilis, and Burkholderia cepacia genomovars I and III. J. Clin. Microbiol. 38:3165-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Traczewski, M. M., and S. D. Brown. 2006. In vitro activity of doripenem against Pseudomonas aeruginosa and Burkholderia cepacia isolates from both cystic fibrosis and non-cystic fibrosis patients. Antimicrob. Agents Chemother. 50:819-821. [DOI] [PMC free article] [PubMed] [Google Scholar]