Abstract
Visceral leishmaniasis (VL) caused by the parasite Leishmania donovani is a potentially fatal disease. Available limited drugs are toxic, require prolonged treatment duration, and are costly. A low-cost parenteral formulation of paromomycin sulfate (PM) has recently been approved for the treatment of VL. Monotherapy with PM runs the risk of development of resistance. Hence, efforts are needed to develop a combination therapy of PM with other drugs to shorten the duration of treatment and prolong the effective life of the drug. PM was formulated with leishmanicidal stearylamine (SA)-bearing phosphatidylcholine (PC) liposomes for low-dose therapy. In vitro and in vivo antileishmanial effects of the combination drug were determined. The immunomodulatory role of PC-SA-PM was determined using enzyme-linked immunosorbent assay (ELISA) and flow cytometry. Excluding the spleen, for which the therapeutic effect was additive, a remarkable synergistic activity toward cure and prophylaxis with a single-shot low-dose treatment with PC-SA-associated PM was achieved with BALB/c mice. PC-SA-PM showed an immunomodulatory effect on CD4+ and CD8+ T cells for gamma interferon (IFN-γ) production and downregulated disease-associated interleukin-10 (IL-10) and transforming growth factor β (TGF-β) to almost negligible levels. Such combination chemotherapy may provide a promising alternative for the cure of leishmaniasis, with a plausible conversion of the host immune response from a disease-promoting pattern to a Th1-biased response indicative of long-term resistance.
Visceral leishmaniasis (VL), or kala-azar, caused by the parasite Leishmania donovani is a potentially fatal disease with few treatment options. The available drugs, pentavalent antimonials (Sbv) and amphotericin B (AmB), are toxic, require parenteral administration, prolonged treatment duration, and hospitalization, and are thus inevitably very costly (45). The development of resistance to Sbv in patients with VL in North Bihar, India, has increased the problems of treatment. Lipid formulations of AmB have lower toxicity and a shorter duration of therapy, but a prohibitive cost. Miltefosine, the only oral drug for VL, also has long treatment durations, is costly and teratogenic, and requires monitoring for gastrointestinal toxicity, hepatic toxicity, and nephrotoxicity (45). Development of resistance against miltefosine is a major concern (45). Paromomycin sulfate (PM), an aminoglycoside antibiotic, has been reported since the 1960s to have antileishmanial activity. Promising results were found in extensive clinical trials against both cutaneous leishmaniasis (CL) and VL (13, 26), with efficacy even in Sbv-resistant areas of Bihar (43). In August 2006, the parenteral formulation of PM was approved for the treatment of VL (http://www.oneworldhealth.org/diseases/leishmaniasis.php), with future prospects to replace Sbv as the first-line drug for VL (44). However, PM has some disadvantages, such as a 3-week treatment regimen, injection site pain, and hepatic toxicities and ototoxicities (45). The mechanism of the action of PM is also not clearly understood. In Leishmania, ribosomes might be a primary target (31). It also appears to have other effects, including alterations in membrane fluidity and lipid metabolism, and may also target key mitochondrial activities (30).
Cure of leishmaniasis, even during chemotherapy, appears to be dependent upon the development of an effective immune response that activates macrophages to produce toxic nitrogen and oxygen metabolites to kill the intracellular amastigotes (4, 9, 36). This process is suppressed by the infection itself, which downregulates the requisite signaling between macrophages and T cells, such as the production of interleukin-12 (IL-12) and the presentation of major histocompatibility complex (MHC) and costimulatory molecules at the macrophage surface (5, 35).
Again monotherapy with any antileishmanial drug, including PM, runs the risk of the development of resistance in the anthroponotic foci (43). Hence, efforts are needed to develop a combination therapy of PM with other drugs that have tolerability, compatibility, and prospective immunomodulatory roles to shorten the duration of treatment, ensure the persistent therapeutic effect, and prolong the effective life of the drug (25, 29, 43).
Previous studies showed that biological immunomodulators, such as gamma interferon (IFN-γ), enhance the activity of antimonials in the treatment of VL (34, 46). In addition, if given during the early stages of infection, combined treatment with recombinant IL-12 and antimonials inhibits the appearance and progression of cutaneous lesions in BALB/c mice by promoting the development of a protective Th1 immune response (37). PM used in combination with recombinant IL-12 for topical chemotherapy of established Leishmania major infection resulted in a drastic reduction in tissue parasitism and inhibited relapses in BALB/c mice (16) probably through activation of IFN-γ production. Earlier we established the leishmanicidal activity and mechanism of the cationic liposome, composed of phosphatidylcholine (PC) and stearylamine (SA), as a monotherapy (2, 6, 14). PC-SA in combination with other antileishmanial drugs, such as SAG and AmB, not only reduced the toxicity of the drugs but also enhanced the parasite clearance in the BALB/c model to a great extent (7, 38). Moreover, beneficial immunomodulatory activities of AmB were augmented in combination with PC-SA liposomes (7).
In the present study we, for the first time, demonstrate a new combination of PM in cationic PC-SA liposome for effective treatment against experimental VL through a single-dose administration. This is also the first report showing that PM itself has Th1-inducing capability. PC-SA and PM act synergistically tilting the immunological balance in favor of protective Th1 over disease-promoting immune responses, thus resulting in profound antileishmanial activity.
MATERIALS AND METHODS
Parasite culture and maintenance.
L. donovani virulent strain AG83 (MHOM/IN/1983/AG83) was cultured as promastigotes in medium 199 (Sigma, St. Louis, MO) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% fetal calf serum (FCS) (Sigma) (M199-FCS) at 22°C. Golden hamsters and BALB/c mice, 4 weeks old and reared in institute facilities, were used for parasite maintenance and experimental purposes, respectively, with prior approval of the Animal Ethics Committee of the Indian Institute of Chemical Biology.
Entrapment of PM in liposomes.
PM (Sigma) was entrapped in liposomes as previously reported (17), with some modifications. Briefly, the lipid film prepared with PC (Sigma) and cationic lipid SA (Fluka) at a molar ratio of 7:2 was hydrated with 20 mM phosphate-buffered saline (PBS) containing 4 mg/ml of PM and sonicated for 60 s in a Microson XL-2000 (Micronix Inc., NY). Unentrapped drug was washed with PBS through ultracentrifugation at 1,00,000 × g for 1 h. PC-SA (22 mg) with entrapped PM was finally resuspended in PBS. The entrapped PM concentration was determined by an agar diffusion inhibition assay of the growth of Staphylococcus aureus (ATCC 6571) on sensitivity test agar as described previously (47). A straight-line relationship was obtained between PM concentration and zone of inhibition (mm). The amount of drug present for a vesicular sample was determined from the standard curve. To increase the entrapment of PM, lipid films were hydrated with different concentrations (1 to 10 mg/ml) of PM, and the entrapment efficiency was determined.
Toxicity determination assay.
A hemolytic assay was done in the presence of drugs as described previously (39). Briefly, normal red blood cells (RBCs) were isolated from humans and washed in PBS. Different concentrations of PC-SA-PM, free-PM, drug-free PC-SA (7:2-molar ratio) liposomes were then added to the packed RBCs at a ratio of 1:10 (vol/vol) and incubated for 60 min at 37°C. The samples were then centrifuged at 800 × g for 10 min. By measuring the A540 of the collected supernatant, the release of hemoglobin was monitored. Controls for zero hemolysis (blank) and 100% hemolysis consisted of human RBCs (hRBCs) suspended in PBS and 1% Triton X-100, respectively. For liver and kidney function tests, normal healthy mice were injected intravenously (i.v.) with a single dose of free PM (16 mg/kg body weight [bw]), PC-SA only (22 mg/mouse), or PC-SA (22 mg/mouse)-associated PM (16 mg/kg bw). Serum-alkaline phosphatase, glutamate pyruvate transaminase, urea, and creatinine levels were analyzed at day 15 after treatment using kits from Span Diagnostics Limited (Surat, India).
In vitro antileishmanial activity assay.
Macrophages were infected with promastigotes (10:1 ratio) for 3 h and treated with various doses of drug-free PC-SA, free PM, and PC-SA-associated PM for 72 h at 37°C in 5% CO2 in RPMI 1640 supplemented with 10% FCS, penicillin, and streptomycin (RPMI-FCS). Cells were then fixed and stained with Giemsa for microscopic determination of the number of intracellular parasites/200 host cells.
In vivo infection, treatment schedules, doses, and determination of parasite burden.
To compare the therapeutic efficacy of the different formulations of PM, BALB/c mice were infected i.v. via the tail vein with 2 × 107 amastigotes of AG83. Eight weeks postinfection, groups of animals (5 animals/group) were treated i.v. with a single dose of PC-SA only (22 mg/mouse), free PM (16 mg/kg bw), AmBisome (8 mg/kg bw), or PC-SA (22 mg/mouse)-associated PM (16 mg/kg bw). The spleen and liver were removed after 4 weeks of treatment. For prophylaxis, different groups of mice (5 animals/group) were treated with a single injection of the different drugs, except AmBisome, at the doses mentioned above. Mice were infected 10 days after treatment and sacrificed 3 months postinfection. Liver and spleen parasite burdens were determined from Giemsa-stained multiple impression smears, expressed as Leishman-Donovan units (LDU), and calculated as the number of parasites per 1,000 nucleated cells × organ weight in mg (42). Cure/protection can be defined as a fall in hepato-splenomegaly and elimination of parasites to negligible levels. Infection in the bone marrow was calculated as the number of parasites/1,000 host cell nuclei.
Parasite membrane antigen preparation.
Promastigote membrane antigen (LAg) was prepared as reported previously (1). Briefly, stationary-phase promastigotes, harvested after the third or fourth passage in liquid culture, were washed four times in cold PBS and resuspended at a concentration of 1.0 g of cell pellet in 50 ml of cold 5 mM Tris-HCl buffer (pH 7.6). The suspension was vortexed six times for 2 min each, with a 10-min interval of cooling on ice between each vortexing. The parasite suspension was then centrifuged at 2,310 × g for 10 min. The crude ghost membrane pellet obtained was suspended in 5 ml of 5 mM Tris buffer (pH 7.6) and sonicated three times for 1 min each at 4°C. The suspension was finally centrifuged for 30 min at 5,190 × g. The supernatant containing LAg was harvested and stored at −70°C until used. LAg was characterized by a complex of 25 to 35 polypeptides having a molecular mass in the range of 18 to 155 kDa (1). The protein content in the supernatant was measured by Lowry's method.
Determination of IgG isotype through ELISA.
Mice were bled 4 weeks after treatment, and sera were stored at −20°C until use. The specific serum IgG isotype antibody (Ab) response was measured by conventional enzyme-linked immunosorbent assay (ELISA). Wells of ELISA plates (Nunc, Thermo Fisher Scientific, Roskilde, Denmark) were coated with LAg at a concentration of 0.5 μg/well and incubated overnight at 4°C. Sera were added at 1,000-fold dilutions, followed by washing of cells and the addition of peroxidase-conjugated isotype-specific secondary Abs (goat anti-mouse IgG, IgG1, or IgG2a; BD Pharmingen, San Diego, CA). Wells were then washed and incubated with substrate solution (o-phenylenediamine dihydrochloride, 0.8 mg/ml in phosphate-citrate buffer, pH 5.0, containing 0.04% H2O2) for 30 min, and the absorbance was read on an ELISA plate reader at 490 nm.
Determination of DTH.
The delayed-type hypersensitivity (DTH) in posttreated 12-week-infected mice was determined as an index of cell-mediated immunity. The response was evaluated by measuring the difference between the footpad swelling at 24 h following intradermal inoculation of the test footpad with 50 μl (800 μg/ml) of LAg and the swelling of the control (PBS-injected) footpad.
Splenocyte proliferation assay.
Splenocytes (2 × 105 cells/well) from treated and untreated infected mice were stimulated with 2 μg/ml LAg for 48 h at 37°C in RPMI-FCS (1). Thereafter, cells were pulsed with 1 μCi [3H]thymidine/well (Amersham Life Science, Arlington Heights, IL) for 18 h before harvesting; [3H]thymidine uptake, an index of cell proliferation, was measured in a liquid scintillation counter (Tri-Carb 2100TR; Packard Bioscience Company, Meriden, CT).
Analysis of cytokines by ELISA.
Cytokine concentrations in the splenocyte culture (48-h) supernatants of differently treated normal and infected mice, in response to ConA/lipopolysaccharide (LPS) and LAg stimulations, respectively, were determined by sandwich ELISA (BD Pharmingen kit) as recommended by the manufacturer. IL-12, IFN-γ, and IL-10 were determined in normal mice, and tumor necrosis factor alpha (TNF-α) and IL-4 were measured additionally in infected mice. For total TGF-β measurement, culture supernatants were acidified with 1 N HCl for 10 min to activate latent TGF-β, neutralized with 1.2 N NaOH. TGF-β was determined using ELISA kits (R&D Systems, Minneapolis, MN).
Quantification of NO.
The splenocyte culture supernatants of differently treated normal and infected mice were analyzed for their LPS- and LAg-specific nitrite (NO2−) contents, respectively, by the Greiss method as described previously (15). Briefly, the mixture of Greiss reagent (1% sulfanilamide and 0.1% N-(1-naphthyl)ethylenediamine dehydrochloride in 2.5% H3PO4) and culture supernatant at a 1:1 ratio were incubated for 15 min at room temperature, and the optical density (OD) was determined at 550 nm by an ELISA reader. Sodium nitrite (NaNO2) diluted in culture medium served as a standard.
Flow cytometry.
Two-color flow cytometry was performed for intracellular analysis of IFN-γ-producing CD4+ and CD8+ T lymphocytes at the single-cell level. Splenocytes from differently treated infected mice were stimulated with 5 μg/ml of LAg for 20 to 24 h. Brefeldin A (10 μg/ml) was added to the cultures 2 h before harvest. The cells were washed in PBS containing 0.1% NaN3-1% FCS at 4°C, stained with either fluorescein isothiocyanate (FITC)-conjugated anti-CD4 or anti-CD8 monoclonal antibody (MAb), permeabilized by treatment with fluorescence-activated cell sorter (FACS) permeable solution (BD Pharmingen), stained with phycoerythrin (PE)-conjugated anti-IFN-γ or isotype-matched control MAbs, and analyzed on a flow cytometer (FACSCalibur) using the CellQuest program on at least 10,000 events.
Statistical analyses.
The in vitro experiments were performed at least in triplicate. A minimum of 5 mice/group were used for any in vivo experiment. The statistical significance of differences between groups was determined as described in the figure legends by the use of the program GraphPad Prism, and a P value of <0.05 was considered statistically significant. Error bars represent the standard error of the mean (SEM).
RESULTS
PM in PC-SA liposome: a new combination drug.
In order to prepare the new formulation, entrapment of PM in PC-SA was attempted at increasing hydrating PM concentrations. Maximum entrapment was achieved at 4 mg/ml, at which 22 mg of PC-SA liposomes contained 400 μg of PM (7:2:0.175, molar ratio), with an entrapment efficacy of approximately 10%.
PC-SA-associated PM elicits remarkable leishmanicidal activity in vitro and in vivo without any toxic effect.
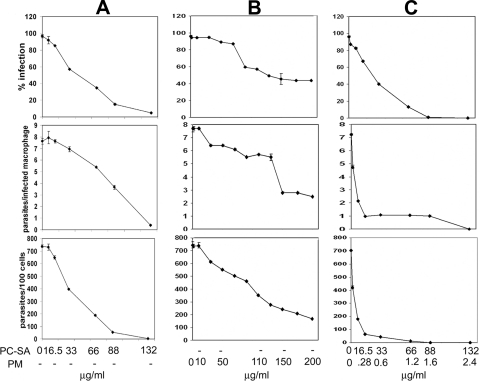
Previously we showed the antileishmanial activity of PC-SA as monotherapy (2, 14) as well as combination therapy (7, 38). Here, the efficacy of these leishmanicidal liposomes was determined as a delivery tool, as well as in combination with PM. PC-SA-associated PM was significantly more potent than either of the monotherapies in vitro (Fig. 1). The 50% effective doses (ED50) for the suppression of parasite infection in macrophages, calculated using sigmoidal regression analysis (Microsoft Excel), for empty PC-SA and free PM were 38 and 105 μg/ml, respectively, whereas only 0.072 μg of entrapped PM in 5 μg of PC-SA/ml induced an equal effect.
FIG. 1.
Activities of empty PC-SA liposomes, free PM, and PC-SA-encapsulated PM against L. donovani amastigotes in resident peritoneal macrophages from BALB/c mice. Cells were infected with L. donovani promastigotes and incubated with increasing concentrations of PC-SA (A), PM (B), and PC-SA-PM (C) for 72 h at 37°C. Levels of infection efficiency (percent infected cells [top]), intracellular growth of parasites (parasites per infected cell [middle]), and parasite survival (parasites per 100 cells [bottom]) of drug treated cells are shown. Infected control macrophages contained 7.38 ± 1.45 amastigotes per macrophage. The bars show the standard errors for three replicates and are representative of two independent experiments.
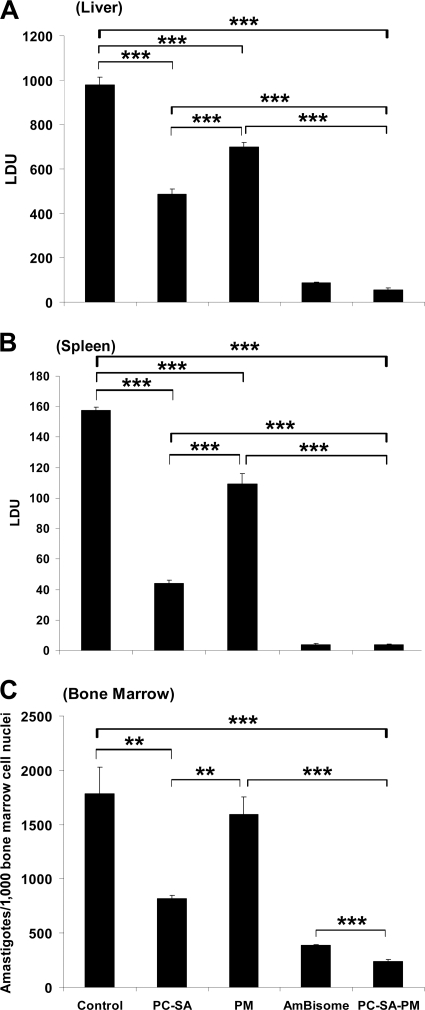
PC-SA liposomes (22 mg/mouse) in combination with 16 mg/kg bw (i.e., 400 μg/mouse) of PM through a single-dose treatment caused 94%, 98%, and 88% (P < 0.0001) reductions of parasite burden in liver, spleen, and bone marrow, respectively, compared to controls (Fig. 2 A to C). Equivalent doses of empty PC-SA and free PM, however, suppressed infection only by 50% and 29% in liver, 72% and 31% in spleen, and 52% and 12% in bone marrow, respectively. Although AmBisome (8 mg/kg bw) showed a parasite killing effect almost equal to that of PC-SA-PM in liver and spleen, PC-SA-PM was more effective than AmBisome in bone marrow.
FIG. 2.
Effect of PC-SA-associated PM treatment on established L. donovani infection in BALB/c mice. Effect of PC-SA (molar ratio of 7:2, 22 mg/mouse)-associated PM (400 μg/mouse) was compared with those of treatment with drug-free PC-SA (22 mg/mouse) liposomes, free PM (400 μg/mouse), and AmBisome (8 mg/kg bw) on 12-week-infected mice. Untreated, infected mice were considered controls. Hepatic (A) and splenic (B) parasite burdens were determined by stamp-smear method and expressed as Leishman-Donovan units (LDU; defined in Materials and Methods), and bone marrow parasite load (C) was determined with cell smear prepared from femur bone marrow and expressed as amastigotes/1,000 bone marrow nuclei. Data represent the mean ± SE for five animals per group. Data were tested by ANOVA. Differences between means were assessed for statistical significance by Tukey's test (**, P < 0.001; ***, P < 0.0001). Results are one representative of three experiments.
The in vitro effective doses of PC-SA-PM were nontoxic toward human RBC. Estimation of serum alkaline phosphatase and glutamate pyruvate transaminase for liver dysfunction and the levels of serum urea and creatinine for kidney dysfunction 15 days postinjection of PC-SA-PM i.v. in normal BALB/c mice demonstrated normal levels, indicating no in vivo toxicity.
Humoral immune response in BALB/c mice after cure with PC-SA-associated PM treatment.
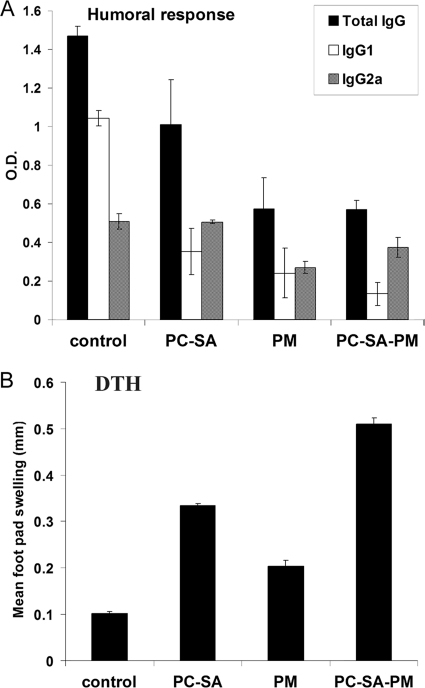
Since cure of leishmaniasis is associated with an effective immune response, we investigated the possible immunological alterations induced by the treatment of PC-SA-PM in L. donovani-infected mice at cure. We assessed leishmanial antigen-specific IgG and its isotype (IgG1 and IgG2a) levels in the sera of mice following cure with different drug forms. Control-infected animals exhibited higher IgG1 than IgG2a, and the difference was statistically significant (P < 0.0001) (Fig. 3 A). The highest IgG2a/IgG1 ratio was found in PC-SA-associated PM-treated mice (3.09 ± 0.229), followed by the PC-SA-only treatment group (1.48 ±0.170) and free-PM-treated mice (0.685 ± 0.103). Since cytokines such as IFN-γ and IL-4 direct immunoglobulin class switching for IgG2a and IgG1 (12), our data indicate that disease-protective immunity is generated by PC-SA-associated PM treatment.
FIG. 3.
LAg-specific antibody and DTH responses in differently treated infected mice. (A) Sera from treated animals were analyzed for LAg-specific anti-IgG, anti-IgG1, and anti-IgG2a levels by ELISA. (B) DTH response was evaluated by measuring the difference between the footpad swelling at 24 h following intradermal inoculation of the test footpad with 50 μl (800 μg/ml) of LAg and the swelling of the control (PBS-injected) footpad. Data represent the mean ± SE for five animals per group.
PC-SA-PM in L. donovani-infected BALB/c mice induces leishmanial membrane antigen-specific DTH and lymphoproliferation in the splenocytes at cure.
Chemotherapeutic intervention and cure are generally associated with the acquisition of a delayed-type hypersensitivity (DTH) response and consequently “classical” cell-mediated immunity (3). Hence, we investigated LAg-induced DTH responses in infected BALB/c mice after cure with PC-SA-PM treatment, as an index of cell-mediated immunity. PC-SA-PM-treated mice showed the strongest DTH response (Fig. 3B), and empty-liposome- and free-PM-treated mice followed. Active VL is characterized by marked T-cell anergy toward leishmanial antigens (11, 21, 23). We observed the highest proliferative response (P < 0.0001, versus untreated infected controls) with splenocytes from mice treated with PC-SA-PM, followed by those treated with free PM (P < 0.001). PC-SA treatment induced marginal proliferation (Fig. 4 A).
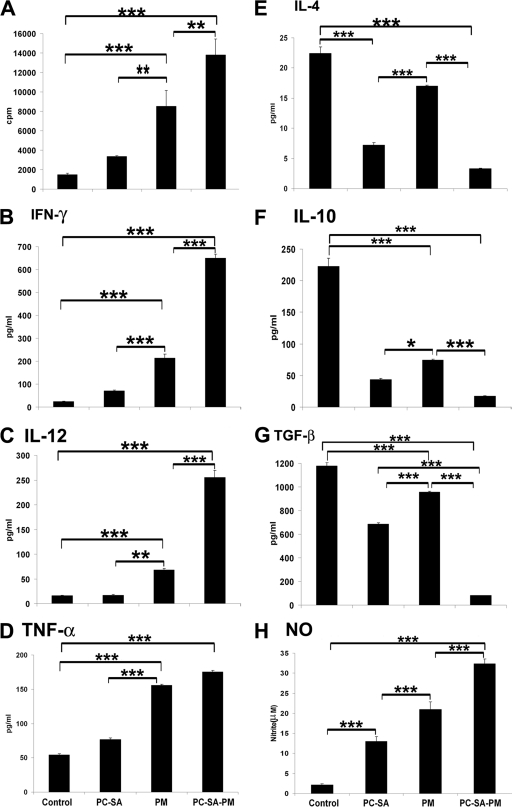
FIG. 4.
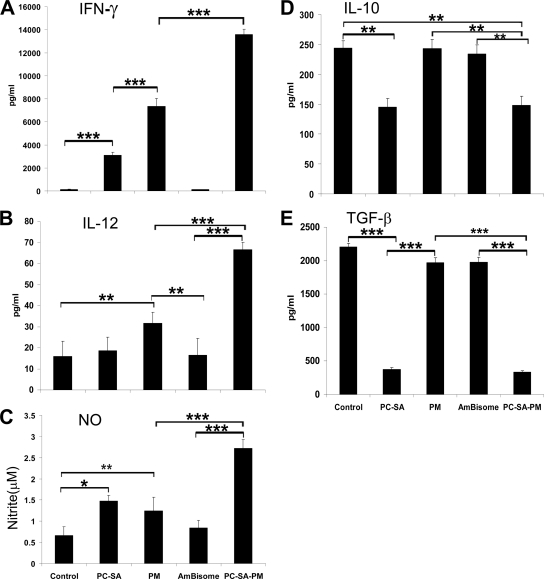
LAg-specific lymphoproliferation, cytokine, and NO levels in differently treated infected mice. Spleen cells of indicated treated animals were isolated 4 weeks posttreatment, plated aseptically (2 × 105 cells/well), and stimulated with LAg at 2 μg/ml for 48 h. (A) LAg-specific in vitro proliferation of spleen cells of differently treated animals was determined. IFN-γ (B), IL-12 (C), TNF-α (D), IL-4 (E), IL-10 (F), TGF-β (G), and NO (H) in spleen cell culture supernatants of indicated treatment groups were determined by ELISA (for panels B to G) and the Greiss assay method (for panel H). Data represent the mean ± SE for five animals per group. Data were tested by ANOVA. Differences between means were assessed for statistical significance by Tukey's test (**, P < 0.01; ***, P < 0.001).
PC-SA-PM treatment elicits a Th1-like response and induces nitrite production in cured mice.
To evaluate the immune alterations, cytokine production in splenocyte supernatants was estimated by ELISA with the three treatment groups (Fig. 4B to G). PC-SA-PM induced the highest levels of IFN-γ, IL-12, and TNF-α and the lowest levels of IL-4, IL-10, and TGF-β. Interestingly, free PM, at 16 mg/kg only, induced significantly elevated levels of IFN-γ, IL-12, and TNF-α (P < 0.0001) and reduced levels of IL-4, IL-10, and TGF-β (P < 0.0001) compared to untreated infected controls. Notably, although drug-free PC-SA led to the marginal elevation of IFN-γ, IL-12, and TNF-α, it could significantly reduce IL-4, IL-10, and TGF-β (P < 0.0001) compared to controls as well as to free PM (Fig. 4B to G). All together, the cytokine profiles indicate that PM, having an IFN-γ-inducing effect, and PC-SA, having IL-10- and TGF-β-reducing capabilities, showed profound Th1 stimulation when PM was associated with PC-SA. In addition, the highest production of nitrite was demonstrated in PC-SA-PM-treated mice (Fig. 4H), followed by free-PM- and empty-PC-SA-treated mice (P < 0.0001, versus control). These results show the striking potential of PC-SA-PM as a proper effector to induce protective immune responses.
PC-SA-PM treatment elicits a high frequency of IFN-γ-secreting CD4+ and CD8+ T cells.
It is well established that MHC class II-restricted CD4+ T cells are dominant during the development of immunity against Leishmania (27). Despite these interpretations, a few studies point to an essential role for CD8+ cells in immunity to primary infection with L. major (8) and also in the induction of long-term, vaccine-induced resistance against many intracellular pathogens (22). A low frequency of CD4+ (1.73 ± 1.129) and CD8+ (1.19 ± 1.124) T cells producing IFN-γ was detected in the spleens of mice with established L. donovani infection (Fig. 5). The frequency of IFN-γ-producing CD4+ and CD8+ T cells increased 4 weeks after treatment with PC-SA (5.417 ± 2.716 and 4.927 ± 1.586, respectively) but increased more with free PM (7.473 ± 0.753 and 5.663 ± 0.241, respectively). The magnitude was, however, highest with PC-SA-PM treatment (9.387 ± 0.233 and 7.633 ± 1.021, respectively) (Fig. 5). These findings demonstrate a prominent inclination toward Th1 effector function and the involvement of both CD4+ and CD8+ T cells at cure.
FIG. 5.
Percentage of LAg-stimulated CD4+ and CD8+ T cells of differently treated and untreated infected BALB/c mice producing IFN-γ. Splenocytes were stimulated with LAg (5 μg/ml). Surface phenotyping and intracellular staining were performed as described in Materials and Methods, and cells were examined by flow cytometry. Mean percentages of CD4+ and CD8+ cells producing IFN-γ in each group of untreated and cured (n = 3) BALB/c mice are presented. The significance of differences between the means was determined by Student's t test (*, P <0.05).
Immunomodulatory effects of PC-SA-PM on splenocytes of normal mice in vivo.
The remarkable leishmanicidal effect with T-cell-mediated immunomodulation induced by PC-SA-PM made us curious to investigate whether PC-SA-PM could induce any protective immunomodulation in normal animals. For this, normal healthy mice were treated with a single dose of PC-SA-PM, empty PC-SA, free PM, or AmBisome. Ten days after treatment, mice were sacrificed, and the production of different cytokines and NO from splenocytes was evaluated in vitro. PC-SA-PM induced significant elevation of ConA-specific IFN-γ (P < 0.0001) and LPS-specific IL-12 and NO (P < 0.001) in comparison to untreated controls (Fig. 6 A to C). Lower but significant induction of IFN-γ was also observed with free PM and PC-SA (P < 0.001)-treated mice. Free PM and PC-SA also induced some IL-12 and NO. Notably, PC-SA-PM and free PC-SA were found equally effective in reducing IL-10 (P < 0.001) and TGF-β (P < 0.0001) (Fig. 6D and E), whereas free PM was unable to significantly reduce these cytokines in comparison to untreated controls. AmBisome, however, did not show any immunomodulatory effect.
FIG. 6.
Immunomodulatory effect of PC-SA-PM on splenocytes of normal mice in vivo. (A to E) Normal mice were treated once with PC-SA (22 mg/mouse), PM (400 μg/mouse), AmBisome (8 mg/kg bw), and PC-SA-associated PM (22 mg/mouse and 400 μg/mouse, respectively). Spleen cells of differently treated animals were isolated 10 days after treatment, plated aseptically (2.5 × 105 cells/well), and stimulated with ConA (suboptimal dose, 2.5 μg/ml) or LPS (2.5 μg/ml) for 48 h. Levels of ConA-specific IFN-γ and LPS-specific IL-12, NO, IL-10, and TGF-β were determined by ELISA. Data represent the mean ± SE for five animals per group. Data were tested by ANOVA. Differences between means were assessed for statistical significance by Tukey's test (*, P < 0.05; **, P < 0.001; ***, P < 0.0001).
PC-SA-associated PM induces a prophylactic effect.
To further ascertain that the combination regimen induces protective immunity, BALB/c mice were treated with different drug forms prior to infection, and prophylaxis was compared 12 weeks postinfection. Empty liposomes and free PM could suppress parasite burden to 12.97% and 46.96% in the liver and 8.5% and 20.5% in spleen, respectively. The prophylactic effect of PC-SA-associated PM was most pronounced, reducing the parasite burden by 80.6% in liver and 92.54% in spleen, in comparison to untreated controls (P < 0.0001) (Fig. 7 A and B).
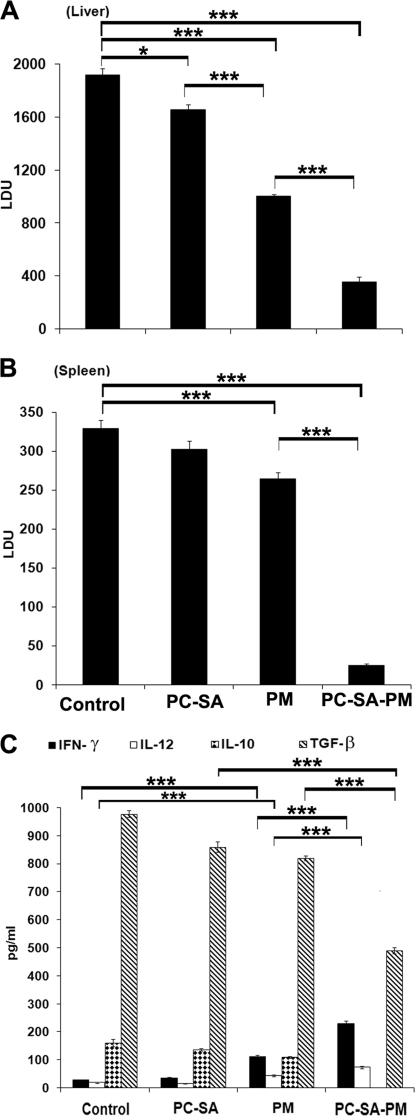
FIG. 7.
Evaluation of prophylactic effect of PC-SA-associated PM. Normal mice were treated once with PC-SA, PM, and PC-SA-PM at doses described in the legend to Fig. 6. Animals were infected with parasites 10 days after treatment and sacrificed after 12 weeks of infection. Liver (A) and spleen (B) parasite burden. (C) IFN-γ and IL-12 were induced, and IL-10 and TGF-β were inhibited in mice prophylactically treated with PC-SA-PM. Spleen cell culture supernatants of 12-week-infected BALB/c mice after indicated treatments were analyzed for LAg-specific cytokine secretion through ELISA. Data represent the mean ± SE for five animals per group. Data were tested by ANOVA. Differences between means were assessed for statistical significance by Tukey's test (**, P < 0.01; ***, P < 0.001).
Cytokine correlates of protective immunity.
Our aforesaid data demonstrated that PC-SA-PM therapy remarkably induced IFN-γ and IL-12 and inhibited both IL-10 production and TGF-β production in the spleen cell culture supernatants of infected BALB/c mice. To evaluate the role of these cytokines in immunoprophylaxis, we cultured splenocytes of differently treated mice 12 weeks postchallenge. Empty PC-SA liposomes failed to induce IFN-γ and IL-12 or inhibit IL-10 to significant levels, except for TGF-β, which was significantly reduced by this treatment (P < 0.0001, versus untreated control). Free PM treatment could induce IFN-γ and IL-12 (P < 0.0001, versus untreated control) and inhibit TGF-β and IL-10 to significant levels in comparison to untreated infected controls (P < 0.001). Interestingly, PC-SA-associated PM treatment showed the highest induction of IFN-γ and IL-12 (P < 0.0001), almost complete inhibition of IL-10, and significant suppression of TGF-β (P < 0.0001) compared to untreated infected controls (Fig. 7C).
DISCUSSION
This is the first report of a combination of PM with a leishmanicidal liposome, PC-SA, against experimental VL. PC-SA-PM showed profound parasite killing activity both in vitro and in vivo, coupled with T-cell-mediated immunomodulation without any adverse effect on the host. This combination was also successful in almost completely eliminating the parasites with a low- and single-dose treatment.
Previous reports showed that early treatment of Leishmania infantum-infected BALB/c mice with PM in combination with meglumine antimoniate cleared parasites from the liver but was ineffective in the spleen (20). Williams et al. (47) demonstrated parasite clearance only from the liver of L. donovani-infected mice, with single- or double-dose treatment with nonionic-surfactant vesicular formulations of PM (47). Recently, liposomal formulations of PM were used as a topical treatment against CL to show the enhanced skin permeation and retention capacity of PM (10, 24). We, for the first time, used PC-SA liposome-associated PM as a combination drug against experimental VL to achieve 88 to 98% parasite clearance from bone marrow, the liver, and the spleen, with a single-shot delivery.
Chemotherapy against leishmaniasis is dependent upon the development of an effective immune response (4, 9, 36). In the present understanding of murine and human VL, significance is placed on the role of IL-10 and TGF-β rather than a strict Th2 response, in terms of IL-4 during susceptibility (33, 41). In contrast, CL progression in BALB/c mice correlates with the production of Th2 cytokines, in particular, IL-4 and IL-10 (40). Human CL is characterized by a strong Th2 response in severe disease, while mild manifestations remain associated with a predominant Th1 response (18). Despite differences between CL and VL, resistance to disease in both forms of leishmaniasis is marked by a dominant Th1 response (41). Apart from the leishmanicidal activities of the drugs, immune-response skewing protocols that shift the prevailing disease-promoting immune response to Th1-mediated protection may therefore facilitate healing. Hence, we investigated whether PC-SA-PM played any immunomodulatory role, which could be responsible for its remarkable parasite clearance ability. Here, PC-SA-PM switched LAg-specific disease-resolving humoral as well as cell-mediated immunity over that of the disease-promoting immune response in L. donovani-infected BALB/c mice. PC-SA-PM induced the highest elevation of DTH, lymphoproliferation, IL-12, IFN-γ, TNF-α, and NO, followed by PM and PC-SA, and maximally reduced IL-4, IL-10, and TGF-β, followed by PC-SA and PM. Thus, the combination drug augments the host protective immune mechanisms induced by the monotherapies. Notably, the induction of lymphoproliferation, IFN-γ, and IL-12 and the suppression of TGF-β by PC-SA-PM were synergistic activities of PC-SA and PM, though the elevation of NO and downregulation of IL-4 and IL-10 were additive effects. Drug-free PC-SA, although unable to induce Th1 cytokines, could significantly reduce IL-10, TGF-β, and IL-4 to a greater extent than free PM. Nonetheless, it could induce NO. Recently Liu et al. (28) demonstrated that in the absence of IL-10 even a slight enhancement of IFN-γ could prevent disease pathology in the case of L. major infection. This might explain why PC-SA could enhance NO in the presence of low IFN-γ. Again, flow-cytometric analysis revealed the simultaneous involvement of CD4+ and CD8+ T cells in IFN-γ production in the spleen even 3 months posttreatment with PC-SA-PM. These data were further supported by the in vitro depletion of CD4+ and CD8+ cells resulting in reduced IFN-γ production (data not shown), favoring a role for these cells in promoting a durable response. In this study, cytokine profiles after cure with different drugs correlated well with their respective leishmanicidal activities, reconfirming that reduction of IL-10 and TGF-β was indeed helpful for parasite clearance.
It could be argued that downregulation of IL-10 and TGF-β and upregulation of IL-12, IFN-γ, and NO are the outcome of parasite reduction. To prove that PC-SA-PM itself has immunomodulatory activity, we performed cytokine analysis of splenic supernatants from uninfected BALB/c mice 10 days after treatment with respective drugs. Our results, for the first time, showed that free-PM treatment induced ConA-specific IFN-γ and LPS-specific IL-12 and NO production in normal mouse splenocytes, although it failed to reduce LPS-specific IL-10 and TGF-β. Previously PM was shown to have prophylactic effect against infection with Cryptosporidium parvum and an influence on the development of immunity (32). Similar therapeutic and prophylactic effects were observed with a combination regimen of PM and recombinant IL-12 against C. parvum, probably through activation of IFN-γ production (19). This combination regimen was also found to be effective against established L. major infection, further inhibiting disease relapse in susceptible mice (16). The present study showed that PC-SA at a molar ratio of 7:2 significantly enhanced the levels of IL-12, IFN-γ, and NO and reduced significantly not only IL-10 but also TGF-β, corroborating our previous observations (7). Maximum induction of IL-12, IFN-γ, and NO was observed with PC-SA-PM treatment in normal mice. The induction of IL-12 and IFN-γ appeared synergistic, while NO production was additive, with respect to free-PC-SA and PM treatment (Fig. 6). However, almost equal reductions of IL-10 and TGF-β by PC-SA-PM and drug-free PC-SA indicated that our drug formulation had retained the beneficial capacity of PC-SA for reducing IL-10 and TGF-β. All together, the findings make it evident that our present combination therapy incorporated both the IL-12-, IFN-γ-, and NO-promoting capacities of PM and the IL-10- and TGF-β-reducing capacity of PC-SA.
Again, PC-SA-PM induced a prophylactic effect through the enhanced production of IFN-γ and IL-12 and downregulation of IL-10 and TGF-β. Contrary to the therapeutic activity observed herein, free PM showed better prophylactic effect than free PC-SA (Fig. 2 and 7A and B). PM, 10 days after pretreatment, resulted in better augmentation of IL-12, IFN-γ, and NO, which eventually cleared the parasite more efficiently. Here, unlike therapy, the IL-10-reducing efficacy of free PC-SA probably had no significant role in parasite clearance since it was administered in parasite-free mice having a basal IL-10 level. Notably, the synergistic rise of IL-12 and IFN-γ, followed by the decline of IL-10 (absolute decline) and TGF-β, after 3 months of challenge infection suggestively explains the synergistic prophylactic effect of the combination drug.
Taken together, these findings indicate that the efficacy of PC-SA-PM for cure as well as for acquiring resistance against L. donovani infection is dependent simultaneously on the direct parasite-killing activity of PC-SA and PM and on the switching-on of Th1-biased protective cell-mediated immunity. PM in combination with PC-SA can emerge as a prospective antileishmanial agent for several reasons. The remarkable activity toward cure and prophylaxis with a single-shot low-dose treatment is a significant achievement. The combination is nontoxic and, with it being a liposomal formulation, also advantageous for targeted delivery. Additionally, with its ability to induce protective immunity, the combination therapy is expected to be more effective than AmBisome (lacking an immunomodulatory role) in inducing long-term protection. The risk of the development of drug resistance against PM as a monotherapeutic regimen can also be resolved through the patronage of this single-shot combination therapy.
Acknowledgments
This work was supported through grants from the Indian Council of Medical Research, the government of India. A.B. was a Senior Research Fellow supported by the Council of Scientific and Industrial Research, the government of India.
We gratefully acknowledge Samiran Saha for his valuable scientific input during preparation of the manuscript and Debaprasad Mandal and Shankar Bhattacharyya for flow cytometric analysis.
The authors do not have a commercial or other association that might pose a conflict of interest.
Footnotes
Published ahead of print on 10 January 2011.
REFERENCES
- 1.Afrin, F., et al. 2002. Characterization of Leishmania donovani antigens encapsulated in liposomes that induce protective immunity in BALB/c mice. Infect. Immun. 70:6697-6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afrin, F., T. Dey, K. Anam, and N. Ali. 2001. Leishmanicidal activity of stearylamine-bearing liposomes in vitro. J. Parasitol. 87:188-193. [DOI] [PubMed] [Google Scholar]
- 3.Alexander, J., K. C. Carter, N. Al-Fasi, A. Satoskar, and F. Brombacher. 2000. Endogenous IL-4 is necessary for effective drug therapy against visceral leishmaniasis. Eur. J. Immunol. 30:2935-2943. [DOI] [PubMed] [Google Scholar]
- 4.Alvar, J., et al. 1997. Leishmania and human immunodeficiency virus coinfection: the first 10 years. Clin. Microbiol. Rev. 10:298-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antoine, J. C., E. Prina, N. Courret, and T. Lang. 2004. Leishmania spp.: on the interactions they establish with antigen-presenting cells of their mammalian hosts. Adv. Parasitol. 58:1-68. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee, A., J. Roychoudhury, and N. Ali. 2008. Stearylamine-bearing cationic liposomes kill Leishmania parasites through surface exposed negatively charged phosphatidylserine. J. Antimicrob. Chemother. 61:103-110. [DOI] [PubMed] [Google Scholar]
- 7.Banerjee, A., M. De, and N. Ali. 2008. Complete cure of experimental visceral leishmaniasis with amphotericin B in stearylamine-bearing cationic liposomes involves down-regulation of IL-10 and favorable T cell responses. J. Immunol. 181:1386-1398. [DOI] [PubMed] [Google Scholar]
- 8.Belkaid, Y., et al. 2002. CD8+ T cells are required for primary immunity in C57BL/6 mice following low-dose, intradermal challenge with Leishmania major. J. Immunol. 168:3992-4000. [DOI] [PubMed] [Google Scholar]
- 9.Berhe, N., et al. 1999. HIV viral load and response to antileishmanial chemotherapy in co-infected patients. AIDS 13:1921-1925. [DOI] [PubMed] [Google Scholar]
- 10.Carneiro, G., et al. 2010. Topical delivery and in vivo antileishmanial activity of paromomycin-loaded liposomes for treatment of cutaneous leishmaniasis. J. Liposome Res. 20:16-23. [DOI] [PubMed] [Google Scholar]
- 11.Carvalho, E. M., et al. 1994. Restoration of IFN-γ production and lymphocyte proliferation in visceral leishmaniasis. J. Immunol. 152:5949-5956. [PubMed] [Google Scholar]
- 12.Coffman, R. L., D. A. Lebman, and P. Rothman. 1993. Mechanism and regulation of immunoglobulin isotype switching. Adv. Immunol. 54:229-270. [DOI] [PubMed] [Google Scholar]
- 13.Croft, S. L., and V. Yardley. 2002. Chemotherapy of leishmaniasis. Curr. Pharm. Des. 8:319-342. [DOI] [PubMed] [Google Scholar]
- 14.Dey, T., K. Anam, F. Afrin, and N. Ali. 2000. Antileishmanial activities of stearylamine-bearing liposomes. Antimicrob. Agents Chemother. 44:1739-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding, A. H., C. F. Nathan, and D. J. Stuehr. 1988. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages: comparison of activating cytokines and evidence for independent production. J. Immunol. 141:2407-2412. [PubMed] [Google Scholar]
- 16.Fernandes, A. P., et al. 2001. Combined interleukin-12 and topical chemotherapy for established Leishmaniasis drastically reduces tissue parasitism and relapses in susceptible mice. J. Infect. Dis. 183:1646-1652. [DOI] [PubMed] [Google Scholar]
- 17.Ferreira, L. S., G. A. Ramaldes, E. A. Nunan, and L. A. Ferreira. 2004. In vitro skin permeation and retention of paromomycin from liposomes for topical treatment of the cutaneous leishmaniasis. Drug Dev. Ind. Pharm. 30:289-296. [DOI] [PubMed] [Google Scholar]
- 18.Gaafar, A., et al. 1995. Dichotomy of the T cell response to Leishmania antigens in patients suffering from cutaneous leishmaniasis; absence or scarcity of Th1 activity is associated with severe infections. Clin. Exp. Immunol. 100:239-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gamra, M. M., and L. M. el-Hosseiny. 2003. Comparative study of the prophylactic and therapeutic effects of paromomycin, recombinant IL-12 alone or in combination against Cryptosporidium parvum infection in immunosuppressed mice. J. Egypt. Soc. Parasitol. 33:109-122. [PubMed] [Google Scholar]
- 20.Gangneux, J. P., A. Sulahian, Y. J. Garin, and F. Derouin. 1997. Efficacy of aminosidine administered alone or in combination with meglumine antimoniate for the treatment of experimental visceral leishmaniasis caused by Leishmania infantum. J. Antimicrob. Chemother. 40:287-289. [DOI] [PubMed] [Google Scholar]
- 21.Gifawesen, C., and J. P. Farrell. 1989. Comparison of T-cell responses in self-limiting versus progressive visceral Leishmania donovani infections in golden hamsters. Infect. Immun. 57:3091-3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gurunathan, S., et al. 2000. Requirements for the maintenance of Th1 immunity in vivo following DNA vaccination: a potential immunoregulatory role for CD8+ T cells. J. Immunol. 165:915-924. [DOI] [PubMed] [Google Scholar]
- 23.Haldar, J. P., S. Ghose, K. C. Saha, and A. C. Ghose. 1983. Cell-mediated immune response in Indian kala-azar and post-kala-azar dermal leishmaniasis. Infect. Immun. 42:702-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaafari, M. R., et al. 2009. Effect of topical liposomes containing paromomycin sulfate in the course of Leishmania major infection in susceptible BALB/c mice. Antimicrob. Agents Chemother. 53:2259-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jha, T. K. 2006. Drug unresponsiveness & combination therapy for kala-azar. Indian J. Med. Res. 123:389-398. [PubMed] [Google Scholar]
- 26.Klaus, S. N., and D. Kafka. 1992. Topical paromomycin: a safe and effective therapy for cutaneous leishmaniasis, abstr. p. 410-411. Dermatology: progress and perspective. Abstr. Proc. 18th World Congr. Dermatol.
- 27.Lang, T., P. Ave, M. Huerre, G. Milon, and J. C. Antoine. 2000. Macrophage subsets harbouring Leishmania donovani in spleens of infected BALB/c mice: localization and characterization. Cell. Microbiol. 2:415-430. [DOI] [PubMed] [Google Scholar]
- 28.Liu, D., et al. 2009. The p110delta isoform of phosphatidylinositol 3-kinase controls susceptibility to Leishmania major by regulating expansion and tissue homing of regulatory T cells. J. Immunol. 183:1921-1933. [DOI] [PubMed] [Google Scholar]
- 29.Loiseau, P. M., and C. Bories. 2006. Mechanisms of drug action and drug resistance in Leishmania as basis for therapeutic target identification and design of antileishmanial modulators. Curr. Top. Med. Chem. 6:539-550. [DOI] [PubMed] [Google Scholar]
- 30.Maarouf, M., F. Lawrence, S. Brown, and M. Robert-Gero. 1997. Biochemical alterations in paromomycin-treated Leishmania donovani promastigotes. Parasitol. Res. 83:198-202. [DOI] [PubMed] [Google Scholar]
- 31.Maarouf, M., F. Lawrence, S. L. Croft, and M. Robert-Gero. 1995. Ribosomes of Leishmania are a target for the aminoglycosides. Parasitol. Res. 81:421-425. [DOI] [PubMed] [Google Scholar]
- 32.Mancassola, R., J. M. Reperant, M. Naciri, and C. Chartier. 1995. Chemoprophylaxis of Cryptosporidium parvum infection with paromomycin in kids and immunological study. Antimicrob. Agents Chemother. 39:75-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McMahon-Pratt, D., and J. Alexander. 2004. Does the Leishmania major paradigm of pathogenesis and protection hold for New World cutaneous leishmaniases or the visceral disease? Immunol. Rev. 201:206-224. [DOI] [PubMed] [Google Scholar]
- 34.Murray, H. W., J. D. Berman, and S. D. Wright. 1988. Immunochemotherapy for intracellular Leishmania donovani infection: gamma interferon plus pentavalent antimony. J. Infect. Dis. 157:973-978. [DOI] [PubMed] [Google Scholar]
- 35.Murray, H. W., J. D. Berman, C. R. Davies, and N. G. Saravia. 2005. Advances in leishmaniasis. Lancet 366:1561-1577. [DOI] [PubMed] [Google Scholar]
- 36.Murray, H. W., M. J. Oca, A. M. Granger, and R. D. Schreiber. 1989. Requirement for T cells and effect of lymphokines in successful chemotherapy for an intracellular infection in experimental visceral leishmaniasis. J. Clin. Invest. 83:1253-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nabors, G. S., L. C. Afonso, J. P. Farrell, and P. Scott. 1995. Switch from a type 2 to a type 1 T helper cell response and cure of established Leishmania major infection in mice is induced by combined therapy with interleukin-12 and Pentostam. Proc. Natl. Acad. Sci. U. S. A. 92:3142-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pal, S., R. Ravindran, and N. Ali. 2004. Combination therapy using sodium antimony gluconate in stearylamine-bearing liposomes against established and chronic Leishmania donovani infection in BALB/c mice. Antimicrob. Agents Chemother. 48:3591-3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papagiannaros, A., C. Bories, C. Demetzos, and P. M. Loiseau. 2005. Antileishmanial and trypanocidal activities of new miltefosine liposomal formulations. Biomed. Pharmacother. 59:545-550. [DOI] [PubMed] [Google Scholar]
- 40.Sacks, D., and N. Noben-Trauth. 2002. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2:845-858. [DOI] [PubMed] [Google Scholar]
- 41.Saha, S., et al. 2006. Immune reponses in kala-azar. Indian J. Med. Res. 123:245-266. [PubMed] [Google Scholar]
- 42.Stauber, L. A., E. M. Franchino, and J. Grun. 1958. An eight-day method for screening compounds against Leishmania donovani in the golden hamster. J. Protozool. 5:269-273. [Google Scholar]
- 43.Sundar, S., and J. Chakravarty. 2008. Paromomycin in the treatment of leishmaniasis. Expert Opin. Investig. Drugs 17:787-794. [DOI] [PubMed] [Google Scholar]
- 44.Sundar, S., and M. Rai. 2005. Treatment of visceral leishmaniasis. Expert. Opin. Pharmacother. 16:2821-2829. [DOI] [PubMed] [Google Scholar]
- 45.Sundar, S., T. K. Jha, C. P. Thakur, P. K. Sinha, and S. K. Bhattacharya. 2007. Injectable paromomycin for visceral leishmaniasis in India. N. Engl. J. Med. 356:2571-2581. [DOI] [PubMed] [Google Scholar]
- 46.Sundar, S., V. P. Singh, S. Sharma, M. K. Makharia, and H. W. Murray. 1997. Response to interferon-γ plus pentavalent antimony in Indian visceral leishmaniasis. J. Infect. Dis. 176:1117-1119. [DOI] [PubMed] [Google Scholar]
- 47.Williams, D., A. B. Mullen, and A. J. Baillie. 1998. Comparison of the efficacy of free and non-ionic-surfactant vesicular formulations of paromomycin in a murine model of visceral leishmaniasis. J. Pharm. Pharmacol. 50:1351-1356. [DOI] [PubMed] [Google Scholar]