Abstract
Lactobacilli are known to prevent colonization by many pathogens; nevertheless, the mechanisms of their protective effect are largely unknown. In this work, we investigated the role of lactobacilli during infection of epithelial cells with group A streptococci (GAS). GAS cause a variety of illnesses ranging from noninvasive disease to more severe invasive infections, such as necrotizing fasciitis and toxic shock-like syndrome. Invasion of deeper tissues is facilitated by GAS-induced apoptosis and cell death. We found that lactobacilli inhibit GAS-induced host cell cytotoxicity and shedding of the complement regulator CD46. Further, survival assays demonstrated that lactic acid secreted by lactobacilli is highly bactericidal toward GAS. In addition, lactic acid treatment of GAS, but not heat killing, prior to infection abolishes the cytotoxic effects against human cells. Since lipoteichoic acid (LTA) of GAS is heat resistant and cytotoxic, we explored the effects of lactic acid on LTA. By applying such an approach, we demonstrate that lactic acid reduces epithelial cell damage caused by GAS by degrading both secreted and cell-bound LTA. Taken together, our experiments reveal a mechanism by which lactobacilli prevent pathogen-induced host cell damage.
Streptococcus pyogenes (group A streptococci [GAS]) is the cause of many important human diseases ranging from mild superficial skin infections to life-threatening systemic diseases. The pathogen is a common colonizer of the mucosal layers in the mouth, nose, and pharynx. Colonization by GAS is often transient and asymptomatic; however, dissemination by local infection occasionally develops into severe systemic disease, such as streptococcal toxic shock syndrome (8, 24). A pathogenicity feature of GAS is the ability to induce cytotoxicity in human cells (33, 37), which facilitates bacterial entry into deeper tissues of the body (2, 9). Invasion of host cells (17, 26, 53), as well as many virulence factors of GAS, such as the surface component lipoteichoic acid (LTA) (12, 19-22, 49, 51) and the toxins streptolysin O and streptolysin S (11, 29, 45), is reported to induce host cell damage. In addition, pathogen-induced apoptosis and necrosis lead to shedding of the cell surface complement regulator CD46 (14, 25). Shed CD46 binds to the surface of GAS and increases survival of the bacteria in the blood (23, 27).
The normal microbiota is an important first line of defense against invading pathogens. The Gram-positive lactobacilli colonize various parts of the body and are common inhabitants of the mucosal membranes in the oral tract (1, 3, 39). Lactobacilli are known to protect against colonization by many pathogens (44) and have also been reported to prevent cytotoxicity induced by bacterial pathogens (4, 5, 15, 31). In addition, in a recent study, Lactobacillus rhamnosus GG, a commonly used probiotic strain, was reported to inhibit GAS invasion of host cells (36). Lactobacilli have further been reported to inhibit streptococcal growth (15, 18, 46, 47). However, the mechanisms of the lactobacillus-mediated host cell protection and their bactericidal effects have remained enigmatic.
In this study, we investigate how lactobacilli influence infection of epithelial cells by GAS. We demonstrate that lactobacilli mediate killing of GAS by secretion of lactic acid. Further, incubation of GAS with lactic acid abolishes its cytotoxic effects against human cells. This is accomplished by lactic acid-mediated degradation of both secreted and bacterium-bound LTA. Thus, lactobacilli have two preventive strategies: one is to kill the pathogen, and the second is to inactivate and degrade the highly cytotoxic LTA.
MATERIALS AND METHODS
Bacterial strains.
L. rhamnosus Kx151A1 and Lactobacillus oris Kx112A1 were isolated from the gastric biopsy specimens from healthy human individuals described in a previous study (38). Lactobacillus reuteri MV29-2A and L. reuteri MG3, isolated from the vaginal tracts of human and monkey, respectively, were kindly provided by Biogaia AB, Stockholm, Sweden. Lactobacilli were grown on Rogosa agar plates (Oxoid) or MRS broth (Oxoid) at 37°C in 5% CO2. S. pyogenes S165 (GAS), serogroup emm6 (50), was isolated from the blood of a patient suffering from severe invasive streptococcal disease. GAS was grown on Todd-Hewitt agar plates (Acumedia) supplemented with 1.5% yeast extract at 37°C in 5% CO2.
Cell culture.
The human pharyngeal epithelial cell line FaDu (ATCC HTB-43) was cultured at 37°C and 5% CO2 in Dulbecco's modified Eagle's medium with glutamax and pyruvate (Invitrogen) and supplemented with 10% heat-inactivated fetal bovine serum (Sigma). All experiments were performed without serum, because serum has been reported to bind to LTA and thereby reduce the cytotoxic effects of bacteria (20). In all experiments, epithelial cells were seeded on cell culture plates the day before the experiments to form a monolayer overnight. Lactic acid was measured using a BioProfile Flex (Nova Biomedical) chemical cell culture analyzer.
Annexin V staining.
For annexin V staining, confluent monolayers of epithelial cells in a 6-well plate were incubated with 3 ml of GAS in cell culture medium at a multiplicity of infection (MOI) of 100 or coincubated with both GAS (MOI, 100) and different Lactobacillus strains (MOI, 100) for 16 h with a wash after 2 h to prevent overgrowth of bacteria. The flow cytometry protocol was based on a previous study (23). Briefly, the epithelial cells were washed, collected, and stained with 5% annexin V-fluorescein isothiocyanate (FITC) (BD Biosciences) for 15 min at room temperature. Samples were then washed and resuspended in phosphate-buffered saline (PBS) supplemented with 0.1% bovine serum albumin (BSA). The samples were analyzed using a FACSCalibur (BD Biosciences) and Cell Quest Pro software (BD Biosciences). Heat treatment of GAS was performed at 95°C for 30 min and lactic acid treatment (40 mM) was performed for 60 min, followed by a wash in PBS.
MTT cell viability assay.
Confluent monolayers of epithelial cells in 96-well plates were incubated with 100 ml of GAS alone at an MOI of 100 or coincubated with GAS (MOI, 100) and different Lactobacillus strains (MOI, 100) for 16 h. To prevent overgrowth, unbound bacteria were removed by washing the plates with medium at 2 h postinfection. At 16 h after infection, the cells were washed and incubated with 400 mg/ml gentamicin for 2 h. The cells were washed again and incubated in 0.5 mg/ml of the tetrazolium salt methylthiazolyldiphenyl-tetrazolium bromide (MTT) in cell culture medium without phenol red for 2 h. The medium was removed, and isopropanol was added. Absorbance was read at 590 nm. Relative cytotoxicity was defined as follows: 1 − absorbance of infected cells/absorbance of uninfected cells. Heat treatment of GAS, Lactobacillus supernatants, or LTA was done at 95°C for 30 min. GAS were treated with 40 mM lactic acid for 60 min followed by a wash in PBS, whereas LTA was treated with 40 mM lactic acid for 60 min followed by adjustment of the pH to 7 with NaOH. LTA purified from GAS was purchased from Sigma-Aldrich.
CD46 staining.
For CD46 staining, confluent monolayers of epithelial cells in a 6-well plate were incubated with 3 ml of GAS in cell culture medium at an MOI of 100 or coincubated with both GAS (MOI, 100) and different Lactobacillus strains (MOI, 100) for 16 h with a wash after 2 h to prevent overgrowth of bacteria. The epithelial cells were then washed, collected, and fixed in 4% paraformaldehyde for 15 min at room temperature; blocked with 2% BSA for 30 min on ice; and stained with FITC-conjugated monoclonal antibody against CD46 (5 μg/ml; BD Biosciences) for 30 min on ice, as described in a previous study (23). Samples were then washed and resuspended in 0.1% BSA in PBS. The samples were analyzed by flow cytometry using a FACSCalibur and Cell Quest Pro software.
Adherence and growth assays.
Adherence assays were performed as previously described (35). Briefly, confluent monolayers of epithelial cells were infected at an MOI uf 100 with GAS alone or together with Lactobacillus strains at an MOI of 100 or 400 μl Lactobacillus supernatants for 16 h with a wash after 2 h. Unbound bacteria were removed by washing them in medium. Infected cell layers were treated for 5 min with 1% saponin, serially diluted, and spread on plates. Adherence of GAS alone was normalized to 1. To collect supernatants, epithelial cells cultured in 48-well plates were incubated with lactobacilli and GAS for 16 h in 1 ml medium. Supernatants were sterile filtered before being used in adherence assays.
To measure growth of GAS in the presence of epithelial cells, a confluent monolayer of FaDu cells was infected with 106 CFU/ml GAS alone (MOI, 100) or together with 106 CFU/ml lactobacilli. Bacteria were serially diluted and spread on plates for viable counts at 4 h and 8 h postinfection without prior washing. All serial dilutions were performed in cell culture medium.
PI staining.
GAS resuspended to 108 CFU/ml were incubated in Lactobacillus supernatant or medium (MRS) for 30 min at 37°C. The supernatants were collected from Lactobacillus strains grown in medium (MRS) for 16 to 20 h at 37°C with shaking and then sterile filtered. GAS incubated with Lactobacillus supernatant or MRS broth were stained with propidium iodide (PI) and analyzed by flow cytometry. For PI staining, bacteria were incubated with 10% PI (BD Biosciences) for 15 min at room temperature, washed, fixed in 4% paraformaldehyde for 15 min at room temperature, washed again, and resuspended in PBS. To analyze GAS viability after coincubation with lactobacilli, samples were stained with a polyclonal antibody against GAS (50 μg/ml; Abcam) for 30 min on ice, followed by incubation with an anti-rabbit IgG antibody (Alexa 488; 5 μg/ml; Invitrogen) for 30 min on ice, after PI staining and fixation. Samples were analyzed by flow cytometry using a FACSCalibur and Cell Quest Pro software.
Bactericidal assay.
GAS at 106 CFU/ml were incubated in Lactobacillus supernatant or medium (MRS) at 37°C. To generate supernatants, Lactobacillus strains were grown in 5 ml MRS in a cell culture flask at 37°C in 5% CO2 with shaking for 16 to 20 h and then sterile filtered. The survival of GAS after incubation in Lactobacillus supernatant was assessed by serial dilutions in cell culture medium and plating. Relative survival was defined as follows: CFU after treatment/CFU before treatment. In some experiments, MRS was acidified with lactic acid or HCl to pH 4, i.e., the pH of the supernatant. In some experiments, the Lactobacillus supernatant was neutralized with NaOH to pH 6, i.e., the pH of MRS.
LTA staining.
GAS were resuspended to 108 CFU/ml and heat inactivated at 60°C for 60 min. The bacteria were then either heat treated at 95°C for 30 min, 40 mM lactic acid treated for 60 min and washed in PBS, or left untreated. Samples were washed, fixed in 4% paraformaldehyde for 15 min at room temperature, and washed again. The bacteria were stained with rabbit anti-LTA polyclonal antibody (50 μg/ml; Abcam) for 30 min on ice and with an anti-rabbit IgG antibody (Alexa 488; 5 μg/ml; Invitrogen) for 30 min on ice. The samples were then washed, resuspended in PBS, and analyzed by flow cytometry using a FACSCalibur and Cell Quest Pro software.
Enzyme-linked immunosorbent assay (ELISA).
Microtiter plates were coated with mouse anti-LTA monoclonal antibody (5 μg/ml; MyBioSource) overnight at 4°C, washed, and blocked with 2% BSA for 1 h at room temperature. The supernatant of infected cells (untreated, heat treated, or treated with 40 mM lactic acid) or LTA purified from GAS and purchased from Sigma-Aldrich diluted in cell culture medium was added to the wells, and the plate was incubated for 1 h at room temperature. Rabbit anti-LTA polyclonal antibody (5 μg/ml; Abcam) was added, and the plate was incubated for 30 min at room temperature. The wells were washed and incubated with a horseradish peroxidase (HRP)-conjugated anti-rabbit IgG antibody (1:1,000 dilution; Bio-Rad) for 30 min at room temperature. Unbound antibodies were washed away, and bound peroxidase was detected using tetramethylbenzidine (TMB) and stop solution, and the absorbance was measured at 450 nm. The protocol is based on a method used in a previous study (16).
SDS-PAGE.
LTA purified from GAS was purchased from Sigma-Aldrich. The LTA was treated with 40 mM lactic acid for 0 or 60 min and then neutralized with NaOH. Samples were then diluted in 2× sample buffer (63 mM Tris-HCl, 2% SDS, 22% glycerol, 0.01% bromophenol blue) and analyzed on 12% acrylamide SDS-PAGE gels, followed by silver staining using a kit from Invitrogen.
Statistical analysis.
Each experiment was done at least three times in triplicate. Analysis of variance (ANOVA) and Tukey's HSD (honestly significantly different) test (XLSTAT) were used to analyze differences between groups for statistical significance. A P value below 0.05 was considered statistically significant. Error bars represent standard deviations.
RESULTS
Lactobacilli reduce cell death caused by group A Streptococcus.
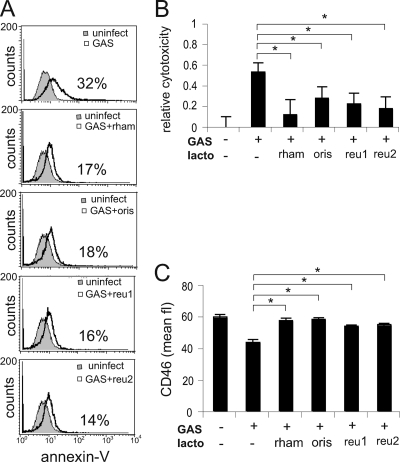
In order to investigate the role of lactobacilli during GAS infection, we coincubated pharyngeal epithelial cells with four different Lactobacillus strains. At 2 h postinfection, unbound bacteria were removed to prevent overgrowth, and at 16 h, cytotoxicity was measured. Flow cytometry analysis of annexin V-stained cells (Fig. 1A) and MTT cell viability assays (Fig. 1B) showed that coincubation of GAS with any of the Lactobacillus isolates—L. rhamnosus Kx151A1, L. oris Kx112A1, L. reuteri MV29-2A, or L. reuteri MG3—significantly reduced cytotoxicity. It has been shown that induction of cytotoxicity induces shedding of the cell surface complement regulator CD46 and that shed CD46 binds to the streptococcal surface and increases bacterial survival in blood (23, 27). To investigate the role of lactobacilli in this process, we incubated epithelial cells with GAS and lactobacilli and measured CD46 expression by flow cytometry. Coincubation with any of the Lactobacillus isolates—L. rhamnosus Kx151A1, L. oris Kx112A1, L. reuteri MV29-2A, or L. reuteri MG3—restored CD46 expression to the levels in uninfected cells (Fig. 1C). Our experiments show that lactobacilli reduce cytotoxic damage to host cells and prevent shedding of CD46 during GAS infection.
FIG. 1.
Lactobacilli reduce GAS cytotoxicity and restore epithelial cell surface expression of CD46. Epithelial cells were coincubated with GAS and different Lactobacillus strains at an MOI of 100 for 16 h, with a wash after 2 h to prevent bacterial overgrowth. The lactobacillus (lacto)/streptococcus ratio was 1:1. (A) Cytotoxicity analyzed by flow cytometry after annexin V staining. The data are presented as representative histogram plots. The percentages of annexin V-positive cells are indicated. (B) Cytotoxicity analyzed by an MTT cell viability assay. Relative cytotoxicity was defined as follows: 1 − absorbance of infected cells/absorbance of uninfected cells. (C) Flow cytometry analysis of CD46 expression after staining with a monoclonal FITC-conjugated CD46 antibody. Statistical significance was analyzed using ANOVA and Tukey's HSD test (XLSTAT). A P value below 0.05 was considered statistically significant and is marked by an asterisk. The error bars represent standard deviations. Uninfect, uninfected; rham, L. rhamnosus Kx151A1; oris, L. oris Kx112A1; reu1, L. reuteri MV29-2A; reu2, L. reuteri MG3.
Lactobacilli are bactericidal against GAS.
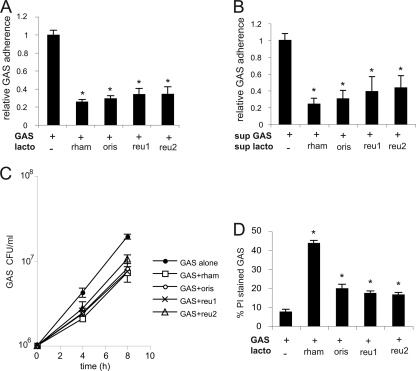
GAS cytotoxicity has previously been reported to correlate with adherence of bacteria to host cells (34). To investigate adherence upon coincubation with lactobacilli, FaDu cells were infected with GAS alone or together with lactobacilli at an MOI of 100 for 16 h, with a wash after 2 h. Attached bacteria were serially diluted and spread on plates for viable counts. All Lactobacillus strains, i.e., L. rhamnosus Kx151A1, L. oris Kx112A1, L. reuteri MV29-2A, and L. reuteri MG3, reduced GAS adherence to 25 to 40% of that in infection with GAS alone (Fig. 2A). Further, sterile-filtered supernatants of lactobacilli inhibited attachment to the same degree as live bacteria (Fig. 2B). To understand the mechanism behind the reduced attachment to cells, we measured growth of GAS in the presence of epithelial cells for 4 h and 8 h without any washing steps. Coincubation of GAS together with lactobacilli resulted in less growth than with GAS alone (Fig. 2C), indicating that lactobacilli inhibit GAS viability. To measure bacterial viability, we stained bacteria with PI and analyzed them by flow cytometry. PI staining increased in the presence of Lactobacillus strains (Fig. 2D), indicating permeabilization of membranes. Coincubation with L. rhamnosus Kx151A1 gave a higher proportion of PI-stained GAS than coincubation with the Lactobacillus isolates L. oris Kx112A1, L. reuteri MV29-2A, and L. reuteri MG3. The L. rhamnosus Kx151A1 strain grows faster than the other three Lactobacillus strains (data not shown), which could explain the difference. Taken together, these data show that lactobacilli are bactericidal against GAS, which explains the reduced number of viable GAS cells adhered to host cells in the presence of lactobacilli.
FIG. 2.
Lactobacilli kill GAS. (A) Epithelial cells were coincubated with GAS and different Lactobacillus strains at an MOI of 100 for 16 h, with a wash after 2 h. The lactobacillus/streptococcus ratio was 1:1. After incubation, the cells were washed, and viable counts of adherent bacteria were determined. Adherence of GAS alone was normalized to 1. (B) Adherence of GAS to epithelial cells in supernatants (sup) of cells coincubated with lactobacilli and GAS for 16 h. Adhered bacteria were quantified by viable counts. Adherence of GAS in the supernatant of uninfected cells was normalized to 1. (C) Recovery of viable GAS after 4 and 8 h of coincubation with lactobacilli. Viable GAS were quantified by plating viable counts without prior washing. (D) Killing of GAS after 16 h of coincubation (with a wash after 2 h) with lactobacilli. The proportion of killed GAS was analyzed by flow cytometry after staining with propidium iodide and a polyclonal GAS antibody. Statistical significance was analyzed using ANOVA and Tukey's HSD test (XLSTAT). A P value below 0.05 was considered statistically significant. The error bars represent standard deviations.
Lactobacilli produce lactic acid that kills S. pyogenes.
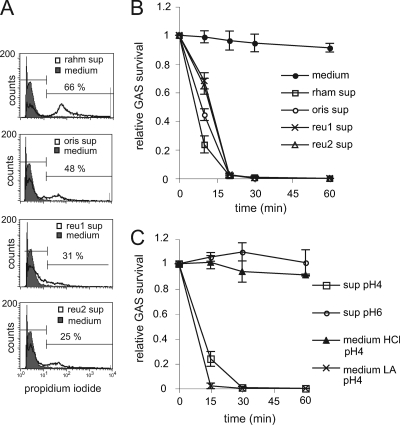
To evaluate if lactobacilli secrete a bactericidal component, we incubated GAS for 30 min in supernatants of the Lactobacillus strains. All supernatants decreased GAS viability, as determined by flow cytometry analysis of PI-stained cells (Fig. 3A). Incubation of GAS in supernatants, followed by serial dilution and plating for viable counts, confirmed that Lactobacillus supernatants kill GAS in a time-dependent manner (Fig. 3B). Since the pH of the supernatants was acidic, i.e., pH 4, we next evaluated how GAS survival was affected by pH. Adjustment of supernatants to pH 6, i.e., to the pH of the growth medium, abolished the killing of GAS (Fig. 3C). When the growth medium was adjusted to pH 4 with lactic acid, it became bactericidal to GAS. However, medium acidified with HCl did not kill GAS, suggesting that the bactericidal effect is not a direct effect of acidic pH but specific to lactic acid. Heat treatment or proteinase K treatment of the supernatant did not affect its bactericidal activity (data not shown), indicating that the bactericidal effect was not mediated by bacteriocins. These data suggest that lactic acid secreted by lactobacilli is bactericidal against GAS.
FIG. 3.
Lactic acid (LA) kills GAS. (A) PI staining of GAS after incubation in Lactobacillus supernatant or medium for 30 min. The data are presented as representative histogram plots. The percentages of propidium iodide-positive GAS are indicated. Left horizontal lines mark 95% of control. Right lines mark positiv signal. The 5% background is subtracted from the percentage shown. (B and C) GAS suspended to 106 CFU/ml was incubated in Lactobacillus supernatant; medium; Lactobacillus supernatant neutralized with NaOH to pH 6, i.e., the same pH as medium; or medium acidified with lactic acid or HCl to pH 4, i.e., the same pH as Lactobacillus supernatant. Survival was analyzed by plating serial dilutions. The survival ratio was expressed as follows: CFU after treatment/CFU before treatment. The medium used was MRS. The error bars represent standard deviations.
Streptococci killed by lactic acid are not cytotoxic to epithelial cells.
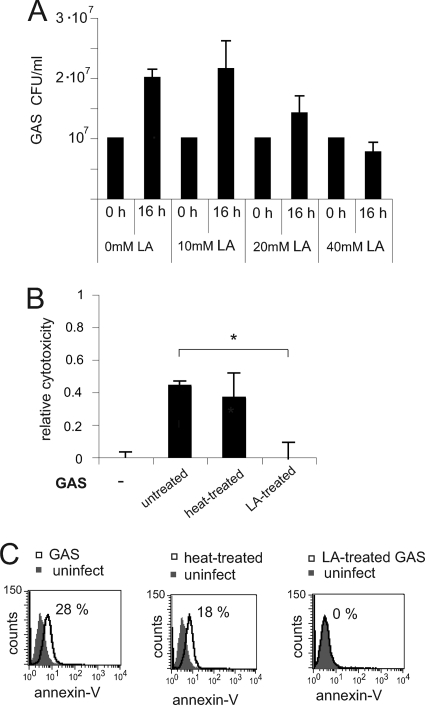
The lactic acid concentration in epithelial cell supernatants after coincubation with lactobacilli and GAS for 16 h was measured to 40 mM, and with GAS alone to 9 mM, using a Bioprofile Flex chemical analyzer. To evaluate if 40 mM lactic acid exhibited killing activity against GAS, we incubated bacteria in 40 mM lactic acid in cell culture medium for 16 h and determined viable counts. As shown in Fig. 4A, 40 mM lactic acid significantly reduced bacterial growth, whereas 10 mM lactic acid did not. We next incubated GAS with 40 mM lactic acid for 60 min, washed them, and overlaid epithelial cells for 16 h. Lactic acid treatment abolished the cytotoxic effects of GAS, whereas heat treatment of bacteria at 95°C for 30 min did not reduce cytotoxicity, as determined by an MTT viability assay (Fig. 4B) and annexin V staining (Fig. 4C). To exclude the possibility that invasion contributed to cytotoxicity in this work, we did an invasion assay. S. pyogenes strain S165 did not invade the pharyngeal epithelial cell line FaDu, as determined by plating viable counts after killing extracellular bacteria with gentamicin (data not shown). This is in accordance with previous reports showing that GAS invasion of eukaryotic cells correlates with the isolation site (17, 26, 53) and that invasive blood isolates invade poorly (30). Taken together, these results indicate that lactic acid treatment, but not heat killing, abolishes the cytotoxic effect of GAS.
FIG. 4.
Lactic acid abolishes cytotoxicity of GAS. (A) Effect of 40 mM lactic acid on streptococcal growth after 16 h of incubation in cell culture medium. Viable GAS were quantified by plating viable counts. (B and C) Epithelial cells were infected with live GAS or GAS killed by heat treatment or lactic acid treatment at an MOI of 100. The incubation time was 16 h, with a wash after 2 h for cells incubated with live GAS. (B) Cytotoxicity analyzed by an MTT cell viability assay. Relative cytotoxicity was defined as follows: 1 − absorbance of infected control cells/absorbance of uninfected cells. (C) Cytotoxicity analyzed by flow cytometry after annexin V staining. The data are presented as representative histogram plots. The percentages of annexin V-positive cells are indicated. Statistical significance was analyzed using ANOVA and Tukey's HSD test (XLSTAT). A P value below 0.05 was considered statistically significant. The error bars represent standard deviations.
Lactic acid degrades the toxic component lipoteichoic acid.
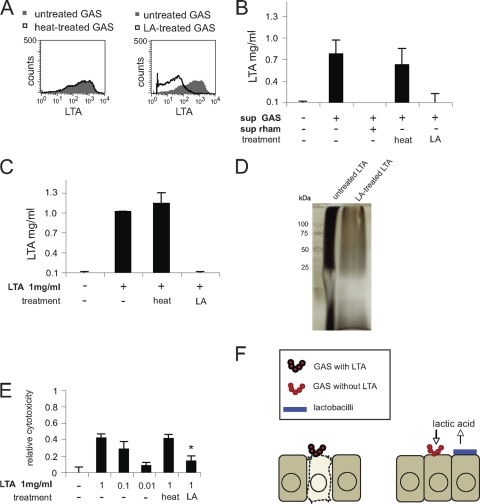
The finding that GAS mediate cytotoxicity by a component sensitive to lactic acid treatment but resistant to heat treatment drew our attention to LTA, which is a heat-stable structure previously reported to cause damage to host cells (12, 19-22, 49, 51). To study this, we measured LTA expression in GAS by flow cytometry after treatment of the bacteria with lactic acid or heat at 95°C. As shown in Fig. 5A, lactic acid dramatically altered the LTA expression of GAS, whereas heat had no effect. Since LTA may be released into the extracellular environment (22), we collected supernatants of GAS-infected cells. The supernatants were analyzed by ELISA and found to contain 0.75 mg/ml LTA (Fig. 5B). Coincubation of the supernatants with 40 mM lactic acid reduced LTA to undetectable levels, whereas heat treatment of the supernatants did not affect LTA levels (Fig. 5B). Supernatants from cells coincubated with GAS and L. rhamnosus did not contain detectable LTA. To verify the degradation of LTA, we treated purified LTA with lactic acid or with heat. As shown in Fig. 5C, LTA was not detectable in the supernatants after 30 min of incubation with lactic acid, whereas the LTA level was unaffected by heat treatment. To further confirm the degradation of LTA, lactic acid-treated and untreated LTA was analyzed by SDS-PAGE. It was previously reported that LTA migrates as a smear on SDS-PAGE gels (28). Silver staining of gels revealed that lactic acid degrades LTA (Fig. 5D). Finally, we tested the ability of LTA to induce host cell cytotoxicity. Pretreatment of purified LTA with lactic acid reduced the cytotoxic effects, whereas heat treatment did not, as determined by an MTT viability assay (Fig. 5E). In control experiments, the cytotoxic effect of 0.01 to 1 mg/ml LTA on epithelial cells was tested; 1 mg/ml LTA was highly cytotoxic, and 0.01 mg/ml was not cytotoxic. In summary, these data suggest that lactobacilli mediate reduction of host cell toxicity by producing lactic acid that degrades the LTA of GAS (Fig. 5F).
FIG. 5.
Lactic acid degrades LTA. (A) Flow cytometry of LTA expression in GAS preincubated for 60 min with 40 mM lactic acid or in 95°C heat for 30 min. The data are presented as representative histogram plots. (B) ELISA analysis of LTA concentrations in supernatants of GAS-infected epithelial cells in the absence and presence of L. rhamnosus. The supernatants were treated with 40 mM lactic acid for 60 min, or 95°C heat for 30 min. Relative cytotoxicity is defined as follows: 1 − absorbance of infected control cells/absorbance of uninfected cells. (C) ELISA analysis of purified LTA pretreated with 40 mM lactic acid for 60 min or 95°C heat for 30 min. (D) Lactic acid degradation of lipoteichoic acid analyzed by SDS-PAGE and silver staining. Lanes: left, molecular mass ladder; middle, untreated LTA; right, LA-treated LTA. (E) Epithelial cells were incubated with LTA (untreated, heat treated, or lactic acid treated). Cytotoxicity was analyzed by an MTT cell viability assay. Relative cytotoxicity is defined as follows: 1 − absorbance of infected control cells/absorbance of uninfected cells. (F) Schematic model showing lactobacillus-mediated reduction of host cell toxicity by producing lactic acid that degrades GAS lipoteichoic acid. The error bars represent standard deviations.
DISCUSSION
In this work, we studied how lactobacilli interfere with infection of pharyngeal cells by GAS. It is well established that GAS trigger host cell damage and cell death, which facilitates dissemination of the bacteria into deeper tissues (2, 9). Here, we demonstrated that the presence of Lactobacillus strains reduces cell cytotoxicity during GAS infection. Coincubation with lactobacilli reduces attachment of GAS to epithelial cells as a result of growth inhibition. Analysis of supernatants revealed that lactic acid secreted by lactobacilli is bactericidal against GAS. Further, pretreatment of GAS with lactic acid abolished induction of host cell damage. In addition, lactic acid reduces secreted LTA to noncytotoxic levels. Thus, other cytotoxic mediators that lactobacilli are ineffective against, potentially streptolysin S, mediated the remaining cytotoxicity in coincubation experiments. The contributions of other organic acids, such as acetic acid, to the bactericidal effect and degradation of LTA cannot be excluded. Organic acids, such as malic acid, citric acid, lactic acid, and acetic acid, were previously reported to be bactericidal to GAS (10). However, the measured concentration of lactic acid after coculture was sufficient to mediate these effects.
Since heat-killed GAS are still cytotoxic, viability is not a prerequisite for cytotoxicity. Indeed, it has been demonstrated that killed GAS induce high levels of inflammatory cytokines, such as tumor necrosis factor (TNF) (32). The strong inflammatory response to invasive GAS infection may result in severe disease symptoms, such as toxic shock. In this work, we demonstrated that lactic acid degrades LTA, which causes damage to epithelial cells and is very potent in inducing inflammation through induction of TNF (13, 43). Degradation of LTA might be an important mechanism by which lactobacilli protect against severe inflammation. The ability of acids to degrade LTA through hydrolyzation of phosphodiester bonds and acyl linkages is well established (6, 42, 43, 52). LTA is a surface component of GAS that has been reported to contribute to adherence to epithelial cells by binding fibronectin (48). GAS lipoteichoic acid has further been reported to be important for virulence in vivo (7). However, lipoteichoic acid is not an essential structure for GAS viability (7), which excludes the possibility that GAS die from degradation of lipoteichoic acid.
An intact epithelium is an important barrier that protects underlying tissues from bacterial invasion. Damaged epithelial cells shed the surface complement regulator CD46. Soluble CD46 signals that the epithelium is damaged and stimulates epithelial cells to secrete interleukin 8 (IL-8) (25), which triggers inflammatory responses. Shed CD46 has been reported to bind to the surface of S. pyogenes and protect against immune recognition (23, 27). In accordance with previous reports, infection with GAS reduced CD46 at the epithelial cell surface. Coincubation with GAS and lactobacilli restored CD46 expression to the levels in uninfected epithelial cells. Thus, by reducing the cytotoxicity of the epithelial cells, lactobacilli also reduce shedding of CD46.
In this study, four different Lactobacillus isolates were used: two gastric isolates and two vaginal isolates. The vaginal strains were isolated from humans and monkeys, but they were equally efficient in reducing cytotoxicity. Lactobacillus inhibition of pathogens has previously been reported to be strain specific (40, 54). However, even though coincubation with L. rhamnosus was more bactericidal to GAS than coincubation with the other Lactobacillus strains, the four Lactobacillus strains tested in this study all reduce cytotoxicity to the same level, which could be explained by the fact that lactic acid production is a general feature of lactobacilli. The production of lactic acid from GAS themselves did not reach bactericidal concentrations.
The protective role of the normal microbiota has been used for development of probiotics—live microorganisms that confer health benefits on the host (41). Lactobacillus is the most studied genus of bacteria for probiotic use (41). However, the mechanisms by which lactobacilli prevent colonization by pathogens are largely unknown. The current study gives insight into one mechanism by which lactobacilli protect against a Gram-positive pathogen. Taken together, we show that lactobacilli degrade GAS lipoteichoic acid, a cytotoxic inflammation inducer, and thereby reduce host cell damage caused by GAS. Future experiments will employ in vivo models to evaluate the ability of lactobacilli to prevent colonization and dissemination of GAS.
Acknowledgments
This work was supported by the Swedish Research Council, the Swedish Cancer Society, Knut and Alice Wallenbergs Stiftelse, and Torsten and Ragnar Söderbergs Stiftelse.
We thank Lili Ostovar for assistance with adhesion experiments.
Footnotes
Published ahead of print on 18 January 2011.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Ahrne, S., et al. 1998. The normal Lactobacillus flora of healthy human rectal and oral mucosa. J. Appl. Microbiol. 85:88-94. [DOI] [PubMed] [Google Scholar]
- 2.Andreadis, S. T., K. E. Hamoen, M. L. Yarmush, and J. R. Morgan. 2001. Keratinocyte growth factor induces hyperproliferation and delays differentiation in a skin equivalent model system. FASEB J. 15:898-906. [DOI] [PubMed] [Google Scholar]
- 3.Badet, C., and N. B. Thebaud. 2008. Ecology of lactobacilli in the oral cavity: a review of literature. Open Microbiol. J. 2:38-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banerjee, P., G. J. Merkel, and A. K. Bhunia. 2009. Lactobacillus delbrueckii ssp. bulgaricus B-30892 can inhibit cytotoxic effects and adhesion of pathogenic Clostridium difficile to Caco-2 cells. Gut Pathog. 1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burkholder, K. M., and A. K. Bhunia. 2009. Salmonella enterica serovar Typhimurium adhesion and cytotoxicity during epithelial cell stress is reduced by Lactobacillus rhamnosus GG. Gut Pathog. 1:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Button, D., and N. L. Hemmings. 1976. Lipoteichoic acid from Bacillus licheniformis 6346 MH-1. Comparative studies on the lipid portion of the lipoteichoic acid and the membrane glycolipid. Biochemistry 15:989-995. [DOI] [PubMed] [Google Scholar]
- 7.Cox, K. H., et al. 2009. Inactivation of DltA modulates virulence factor expression in Streptococcus pyogenes. PLoS One 4:e5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham, M. W. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13:470-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cywes Bentley, C., A. Hakansson, J. Christianson, and M. R. Wessels. 2005. Extracellular group A Streptococcus induces keratinocyte apoptosis by dysregulating calcium signalling. Cell Microbiol. 7:945-955. [DOI] [PubMed] [Google Scholar]
- 10.Daglia, M., et al. 2007. Antibacterial activity of red and white wine against oral streptococci. J. Agric. Food Chem. 55:5038-5042. [DOI] [PubMed] [Google Scholar]
- 11.Datta, V., et al. 2005. Mutational analysis of the group A streptococcal operon encoding streptolysin S and its virulence role in invasive infection. Mol. Microbiol. 56:681-695. [DOI] [PubMed] [Google Scholar]
- 12.DeVuono, J., and C. Panos. 1978. Effect of L-form Streptococcus pyogenes and of lipoteichoic acid on human cells in tissue culture. Infect. Immun. 22:255-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Draing, C., et al. 2008. Cytokine induction by Gram-positive bacteria. Immunobiology 213:285-296. [DOI] [PubMed] [Google Scholar]
- 14.Elward, K., et al. 2005. CD46 plays a key role in tailoring innate immune recognition of apoptotic and necrotic cells. J. Biol. Chem. 280:36342-36354. [DOI] [PubMed] [Google Scholar]
- 15.Hafez, M. 2007. Interference between lactobacilli and group A Streptococcus pyogenes: an expansion to the concept of probiotics. N. Engl. J. Med. 17:262-284. [Google Scholar]
- 16.Jones, A., M. Georg, L. Maudsdotter, and A. B. Jonsson. 2009. Endotoxin, capsule, and bacterial attachment contribute to Neisseria meningitidis resistance to the human antimicrobial peptide LL-37. J. Bacteriol. 191:3861-3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klenk, M., et al. 2005. Global epithelial cell transcriptional responses reveal Streptococcus pyogenes Fas regulator activity association with bacterial aggressiveness. Cell Microbiol. 7:1237-1250. [DOI] [PubMed] [Google Scholar]
- 18.Koll, P., et al. 2008. Characterization of oral lactobacilli as potential probiotics for oral health. Oral Microbiol. Immunol. 23:139-147. [DOI] [PubMed] [Google Scholar]
- 19.Leon, O., and C. Panos. 1983. Cytotoxicity and inhibition of normal collagen synthesis in mouse fibroblasts by lipoteichoic acid from Streptococcus pyogenes type 12. Infect. Immun. 40:785-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leon, O., and C. Panos. 1985. Effect of streptococcal lipoteichoic acid on prolyl hydroxylase activity as related to collagen formation in mouse fibroblast monolayers. Infect. Immun. 50:745-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leon, O., and C. Panos. 1987. An electron microscope study of kidney basement membrane changes in the mouse by lipoteichoic acid from Streptococcus pyogenes. Can. J. Microbiol. 33:709-717. [DOI] [PubMed] [Google Scholar]
- 22.Leon, O., and C. Panos. 1990. Streptococcus pyogenes clinical isolates and lipoteichoic acid. Infect. Immun. 58:3779-3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lovkvist, L., et al. 2008. CD46 contributes to the severity of group A streptococcal infection. Infect. Immun. 76:3951-3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luca-Harari, B., et al. 2009. Clinical and microbiological characteristics of severe Streptococcus pyogenes disease in Europe. J. Clin. Microbiol. 47:1155-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahtout, H., F. Chandad, J. M. Rojo, and D. Grenier. 2009. Porphyromonas gingivalis mediates the shedding and proteolysis of complement regulatory protein CD46 expressed by oral epithelial cells. Oral Microbiol. Immunol. 24:396-400. [DOI] [PubMed] [Google Scholar]
- 26.Marouni, M. J., and S. Sela. 2004. Fate of Streptococcus pyogenes and epithelial cells following internalization. J. Med. Microbiol. 53:1-7. [DOI] [PubMed] [Google Scholar]
- 27.Matsui, H., et al. 2009. CD46 transgenic mouse model of necrotizing fasciitis caused by Streptococcus pyogenes infection. Infect. Immun. 77:4806-4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maurer, J. J., and S. J. Mattingly. 1991. Molecular analysis of lipoteichoic acid from Streptococcus agalactiae. J. Bacteriol. 173:487-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michos, A., et al. 2006. Enhancement of streptolysin O activity and intrinsic cytotoxic effects of the group A streptococcal toxin, NAD-glycohydrolase. J. Biol. Chem. 281:8216-8223. [DOI] [PubMed] [Google Scholar]
- 30.Molinari, G., and G. S. Chhatwal. 1998. Invasion and survival of Streptococcus pyogenes in eukaryotic cells correlates with the source of the clinical isolates. J. Infect. Dis. 177:1600-1607. [DOI] [PubMed] [Google Scholar]
- 31.Moorthy, G., M. R. Murali, and S. Niranjali Devaraj. 2010. Lactobacilli inhibit Shigella dysenteriae 1 induced pro-inflammatory response and cytotoxicity in host cells via impediment of Shigella-host interactions. Dig Liver Dis. 42:33-39. [DOI] [PubMed] [Google Scholar]
- 32.Müller-Alouf, H., et al. 1994. Comparative study of cytokine release by human peripheral blood mononuclear cells stimulated with Streptococcus pyogenes superantigenic erythrogenic toxins, heat-killed streptococci, and lipopolysaccharide. Infect. Immun. 62:4915-4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakagawa, I., M. Nakata, S. Kawabata, and S. Hamada. 2004. Transcriptome analysis and gene expression profiles of early apoptosis-related genes in Streptococcus pyogenes-infected epithelial cells. Cell Microbiol. 6:939-952. [DOI] [PubMed] [Google Scholar]
- 34.Ofek, I., D. Zafriri, J. Goldhar, and B. I. Eisenstein. 1990. Inability of toxin inhibitors to neutralize enhanced toxicity caused by bacteria adherent to tissue culture cells. Infect. Immun. 58:3737-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plant, L., et al. 2006. Lipooligosaccharide structure contributes to multiple steps in the virulence of Neisseria meningitidis. Infect. Immun. 74:1360-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Princivalli, M. S., et al. 2009. Lactobacillus rhamnosus GG inhibits invasion of cultured human respiratory cells by prtF1-positive macrolide-resistant group A streptococci. Lett. Appl. Microbiol. 48:368-372. [DOI] [PubMed] [Google Scholar]
- 37.Quinn, R. W., and P. N. Lowry. 1967. Effect of Streptococcus pyogenes on tissue cells. J. Bacteriol. 93:1825-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roos, S., L. Engstrand, and H. Jonsson. 2005. Lactobacillus gastricus sp. nov., Lactobacillus antri sp. nov., Lactobacillus kalixensis sp. nov. and Lactobacillus ultunensis sp. nov., isolated from human stomach mucosa. Int. J. Syst. Evol. Microbiol. 55:77-82. [DOI] [PubMed] [Google Scholar]
- 39.Ross, P. W. 1971. Quantitative studies on the salivary flora. J. Clin. Pathol. 24:717-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryan, K. A., P. Daly, Y. Li, C. Hooton, and P. W. O'Toole. 2008. Strain-specific inhibition of Helicobacter pylori by Lactobacillus salivarius and other lactobacilli. J. Antimicrob. Chemother. 61:831-834. [DOI] [PubMed] [Google Scholar]
- 41.Schrezenmeir, J., and M. de Vrese. 2001. Probiotics, prebiotics, and synbiotics—approaching a definition. Am. J. Clin. Nutr. 73:361S-364S. [DOI] [PubMed] [Google Scholar]
- 42.Seo, H. S., R. T. Cartee, D. G. Pritchard, and M. H. Nahm. 2008. A new model of pneumococcal lipoteichoic acid structure resolves biochemical, biosynthetic, and serologic inconsistencies of the current model. J. Bacteriol. 190:2379-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seo, H. S., and M. H. Nahm. 2009. Lipoprotein lipase and hydrofluoric acid deactivate both bacterial lipoproteins and lipoteichoic acids, but platelet-activating factor-acetylhydrolase degrades only lipoteichoic acids. Clin. Vaccine Immunol. 16:1187-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Servin, A. L. 2004. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol. Rev. 28:405-440. [DOI] [PubMed] [Google Scholar]
- 45.Sierig, G., C. Cywes, M. R. Wessels, and C. D. Ashbaugh. 2003. Cytotoxic effects of streptolysin O and streptolysin S enhance the virulence of poorly encapsulated group a streptococci. Infect. Immun. 71:446-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silva, M., N. V. Jacobus, C. Deneke, and S. L. Gorbach. 1987. Antimicrobial substance from a human Lactobacillus strain. Antimicrob. Agents Chemother. 31:1231-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simark-Mattsson, C., R. Jonsson, C. G. Emilson, and K. Roos. 2009. Final pH affects the interference capacity of naturally occurring oral Lactobacillus strains against mutans streptococci. Arch. Oral Biol. 54:602-607. [DOI] [PubMed] [Google Scholar]
- 48.Simpson, W. A., J. B. Dale, and E. H. Beachey. 1982. Cytotoxicity of the glycolipid region of streptococcal lipoteichoic acid for cultures of human heart cells. J. Lab. Clin. Med. 99:118-126. [PubMed] [Google Scholar]
- 49.Simpson, W. A., and E. H. Beachey. 1983. Adherence of group A streptococci to fibronectin on oral epithelial cells. Infect. Immun. 39:275-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sjölinder, H., et al. 2008. The ScpC protease of Streptococcus pyogenes affects the outcome of sepsis in a murine model. Infect. Immun. 76:3959-3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tomlinson, K., O. Leon, and C. Panos. 1983. Morphological changes and pathology of mouse glomeruli infected with a streptococcal L-form or exposed to lipoteichoic acid. Infect. Immun. 42:1144-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toon, P., P. E. Brown, and J. Baddiley. 1972. The lipid-teichoic acid complex in the cytoplasmic membrane of Streptococcus faecalis N.C.I.B. 8191. Biochem. J. 127:399-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsai, P. J., Y. S. Lin, C. F. Kuo, H. Y. Lei, and J. J. Wu. 1999. Group A Streptococcus induces apoptosis in human epithelial cells. Infect. Immun. 67:4334-4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vielfort, K., H. Sjolinder, S. Roos, H. Jonsson, and H. Aro. 2008. Adherence of clinically isolated lactobacilli to human cervical cells in competition with Neisseria gonorrhoeae. Microbes Infect. 10:1325-1334. [DOI] [PubMed] [Google Scholar]