Abstract
Studies were conducted to determine if there is a mechanistic basis for reports of suboptimal virologic responses and concerns regarding the safety of regimens containing the combination of tenofovir (TFV) disoproxil fumarate (TDF) and didanosine (ddI) by assessing the pharmacokinetic consequences of coadministration of these drugs on intracellular nucleotides. This was a prospective and longitudinal study in HIV-1-infected patients of adding either TDF or ddI to a stable antiretroviral regimen containing the other drug. Intracellular concentrations of the nucleotide analogs TFV diphosphate (TFV-DP) and ddATP and the endogenous purine nucleotides dATP and 2′-dGTP in peripheral blood mononuclear cells were measured. A total of 16 patients were enrolled into the two study arms and a study extension. Intracellular TFV-DP concentrations (median, 120 fmol/106 cells) and ddATP concentrations (range, 1.50 to 7.54 fmol/106 cells in two patients) were unaffected following addition of ddI or TDF to a stable regimen containing the other drug. While coadministration of ddI and TDF for 4 weeks did not appear to impact dATP or dGTP concentrations, cross-sectional analysis suggested that extended therapy with ddI-containing regimens, irrespective of TDF coadministration, may decrease dATP and ddATP concentrations. Addition of TDF or ddI to a stable regimen including the other drug, in the context of ddI dose reduction, did not adversely affect the concentration of dATP, dGTP, TFV-DP, or ddATP. The association between longer-term ddI therapy and reduced intracellular nucleotide concentrations and this observation's implication for the efficacy and toxicity of ddI-containing regimens deserve further study.
Use of the combination of the nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) didanosine (ddI) and tenofovir (TFV; given as the oral prodrug TFV disoproxil fumarate [TDF]) as part of antiretroviral treatment regimens for HIV infection has remained controversial due to reported pharmacokinetic and pharmacodynamic drug-drug interactions. Coadministration results in up to a 60% increase in ddI plasma exposure (as measured by the area under the concentration-time curve [AUC] at steady state) with no change in the TFV AUC (24). There is evidence that the mechanism for this pharmacokinetic interaction is the inhibition of the purine nucleoside phosphorylase (PNP)-dependent phosphorolysis of ddI by phosphorylated metabolites of TFV (36). When it is coadministered with TDF, it is therefore recommended that the ddI dose be reduced from 400 to 250 mg once a day, that more vigilant safety monitoring for ddI-associated toxicities be undertaken, and that virologic and immunologic responses be followed more closely (Videx package insert; Bristol-Myers Squibb).
Despite viral suppression, patients on TDF and non-dose-reduced ddI have been reported to have paradoxical CD4+ cell declines (4, 32, 33) or reduced CD4+ cell recovery (30). Administration of non-dose-adjusted ddI and TDF has also been associated with an increased incidence of pancreatitis and hyperglycemia (13, 28), and use of this combination has been reported in case reports of renal adverse events (9, 15, 19). The mechanism for these findings appears to be ddI-related mitochondrial toxicity compounded by increased intracellular concentrations of the active triphosphate analog ddATP (9, 26, 31, 43). Consistent with this hypothesis, the incidence of adverse events has been observed to be decreased with ddI dose reduction (3, 8, 23, 41, 45).
Some recent results have raised questions regarding the efficacy of regimens containing TDF-ddI. Triple-NRTI-only regimens including TDF-ddI in combination with either abacavir (ABC) or lamivudine (3TC) were found to have high rates of treatment nonresponse, virologic failure, and selection of the K65R resistance mutation (12, 21, 44). However, the suboptimal performance of triple-NRTI-only regimens is not limited to combinations containing TDF-ddI and may reflect class-related limitations in distribution to certain sites of infection and overlapping resistance profiles (42). For example, ABC-ddI-stavudine was also reported to have low efficacy and high rates of selection of the K65R resistance mutation (14, 39). There have also been reports of higher rates of virologic failure when the combination of TDF and dose-reduced ddI was given with a non-nucleoside reverse transcriptase inhibitor (1, 25, 34).
The pharmacology of the interaction between ddI and TFV has been extensively studied in vitro (29, 36, 38, 42, 43), and intracellular concentrations of the active metabolites of TFV and ddI have been reported in a cross-sectional study in patients receiving TDF and ddI either alone or in combination (35). In this prospective and longitudinal study, we sought to determine the intracellular consequences on active NRTI metabolites and endogenous purine nucleotides of adding TDF or ddI to a stable antiviral regimen containing the other NRTI. Intracellular nucleotide concentrations were determined at multiple times pre- and postaddition of the second NRTI. The study was extended to include additional patients on long-term ddI therapy to understand if ddI treatment may have consequences on intracellular nucleotide concentrations.
(This work was presented in part at the 10th International Workshop on Clinical Pharmacology of HIV Therapy, 15 to 17 April 2009, Amsterdam, Netherlands [16].)
MATERIALS AND METHODS
Study design.
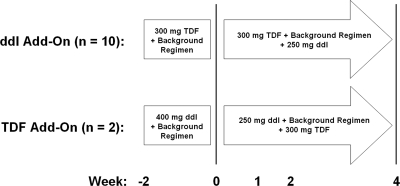
This was a prospective, longitudinal, open-labeled, and two-arm study of the effects on intracellular nucleotides of adding either TDF or ddI to an antiretroviral regimen containing the other drug (Fig. 1). In one arm, 250 mg ddI taken once daily was added to a stable regimen containing 300 mg TDF once daily (ddI add-on arm). In the second arm, the dose of 400 mg ddI taken once daily was reduced to 250 mg upon addition of 300 mg TDF to a stable regimen containing ddI (TDF add-on arm). Due to the exploratory nature of this study, convenience sampling was used and no formal power or sample size calculations were done in order to determine the optimal cohort size for each arm of the study. Inclusion criteria were being on a stable ddI- or TDF-containing regimen for at least 12 weeks and having an HIV load of <400 copies/ml, CD4 count of >100 cells/ml, age of 18 to 65 years, weight of >60 kg, and creatinine clearance of >60 ml/min. Patients were excluded from the study if they had signs of acute hepatitis A, B, or C, evidence of decompensated liver disease, or a history of or current evidence of peripheral neuropathy or pancreatitis or were pregnant or lactating. Upon completion of the blood sampling performed on day 28, either TDF or ddI was discontinued from each subject's drug regimen. To follow up on observations made in patients on long-term ddI-containing regimens, the study was extended to include patients on long-term ddI therapy. The study was approved by the Institutional Review Board at St. Vincent's Hospital in Santa Fe, NM.
FIG. 1.
Study design. Plasma and PBMC samples were taken at 0, 2, 12, and 24 h on each indicated study week.
Patient population.
HIV-positive patients enrolled in the study were receiving treatment at the Southwest CARE Center, Santa Fe, NM. Ten patients on stable regimens containing TDF in the absence of ddI were enrolled. Only two patients who met the study inclusion criteria and who were receiving regimens including ddI but not TDF were identified during study enrollment. An additional four patients who had been on stable long-term ddI therapy were subsequently enrolled. These patients met all acceptance criteria for the add-on arms, except that two of the patients were on regimens containing both ddI and TDF. HIV RNA, CD4 count, and safety lab studies (including complete blood count, complete metabolic panel, amylase/lipase, and physical exams) were conducted at each visit. Written informed consent was obtained from all patients.
Sample collection.
On days that samples were collected, medications were administered with breakfast and blood samples were taken at 0, 2, 12, and 24 h at weeks −2, 0, 1, 2, and 4 for the TDF and ddI add-on arms and 2 weeks apart for the long-term ddI study extension. For each sample collection, peripheral blood mononuclear cells (PBMCs) were isolated from 8 ml of whole blood immediately following collection using cell preparation tubes (BD Biosciences, San Jose, CA). Cells were washed using 0.9% NaCl and subjected to a red blood cell lysis step (as previously described [10]). Cell pellets were flash frozen and stored at −80°C until cell counting and bioanalytical analysis (described below). Plasma was isolated from each blood sample taken for PBMC separation and analyzed to verify administration of the appropriate drugs.
Bioanalytical measurements.
The internal standard, Cl-ATP, was added to frozen PBMC pellets, and PBMCs were lysed by vortexing in 1 ml of ice-cold 70% methanol. Cell extracts were centrifuged, the supernatant was taken for analysis of nucleotides, and the cell pellets were used to evaluate cell number by a previously described DNA-based biochemical assay (6). Plasma TFV and PBMC dATP, dGTP, and TFV diphosphate (TFV-DP) were quantified using liquid chromatography (LC) coupled to positive-ion-mode tandem mass spectrometry (MS/MS) methods essentially as described previously (11). Plasma ddI and PBMC ddATP were quantified simultaneously using the respective LC methods described above for plasma and PBMCs. Positive-ion and multiple-reaction-monitoring-mode MS/MS was done using the parent mass-to-charge ratio (m/z) of 237 and monitoring hypoxanthine (m/z of 137) as the daughter ion for ddI and using the parent m/z of 476 and monitoring adenine (m/z of 136) as the daughter ion for ddATP. Adefovir and 2′3′-dideoxycytosine were used as internal standards for plasma tenofovir and ddI, respectively. Six-point standard curves prepared in blank plasma or PBMC extract covered concentrations of at least 3 orders of magnitude and showed linearity for each analyte in excess of an r2 value of 0.98. The lower limits of quantification (LLOQs) were defined as the nominal concentration of the lowest standard having a peak area greater than 3-fold the background and showing accuracy and precision within 20% between standard curve samples analyzed before and after each sample set. The LLOQs for detection of ddI and TFV in plasma were each 2.6 nM. The LLOQs for concentrations in PBMCs were 3.9, 2.5, 14.8, and 13.9 fmol/106 cells for TFV-DP, ddATP, dGTP, and dATP, respectively. Calculated LLOQs for concentrations in PBMCs in study samples varied with the cell count from each sample. Three independently prepared sets of quality control samples were analyzed at the beginning and end of analysis of each plasma (10, 80, and 4,000 nM) and PBMC (10 and 3,000 fmol/106 cells) sample set. On the basis of the results for the quality controls samples, intra- and interassay accuracy and precision within 20% were observed for all analytes in all sample sets.
Data analysis.
On the basis of prior reports suggesting stable intracellular concentrations of dATP and dGTP, the rapid formation of ddATP and TFV-DP, and the long intracellular half-lives of ddATP and TFV-DP, the assumption was made that the intracellular concentrations of nucleotides were constant over each study day and the median of the four values measured each day in each patient was taken as the concentration in that patient for that day. The statistical significance of the results was assessed with the nonparametric Mann-Whitney U test using Prism (version 4) software (GraphPad Prism, Inc., La Jolla, CA). P values of less than or equal to 0.05 were considered significant.
RESULTS
Patient demographics.
A total of 16 patients were enrolled in the study: 10 patients in the ddI add-on arm, 2 patients in the TDF add-on arm, and an additional 4 patients in the long-term ddI therapy study extension phase. Patient characteristics and treatment regimens at study entry are summarized in Table 1. In the ddI add-on arm, patients had been on TDF for a median of 2.75 years (range, 1.25 to 5.25 years). The two patients in the TDF add-on arm had been on ddI therapy for 5.5 and 6.5 years, respectively. The additional four patients enrolled in the long-term ddI study extension had been on ddI for a median of 5 years (range, 4 to 6.5 years). No adverse events considered to be related to study drug were observed. There were a total of two adverse events reported during the study, both in the TDF add-on arm. One participant had a concussion, and another had leg cramps. All patients in the add-on arms discontinued ddI and continued on a TDF-containing regimen following the study, except for one of the patients in the TDF add-on arm, who discontinued TDF and for whom the ddI dose was increased from 250 mg to 400 mg.
TABLE 1.
Patient characteristicsa
| Arm | Subject | Age (yr) | Sex | Ethnicity | Regimen at entry |
|---|---|---|---|---|---|
| ddI add-on | 001 | 46 | M | C | TDF-ABC + ATV/r |
| 002 | 62 | M | C | TDF-FTC + NVP | |
| 003 | 42 | M | C | TDF-FTC + ATV/r | |
| 006 | 58 | M | C | TDF-FTC + ATV/r | |
| 007 | 63 | M | C | TDF + NVP-SQV/r | |
| 008 | 53 | M | H | TDF-3TC + NVP | |
| 009 | 51 | M | C | TDF-FTC + ATV/r | |
| 010 | 41 | M | H | TDF-FTC + FPV/r | |
| 011 | 47 | M | C | TDF-FTC + NVP | |
| 012 | 47 | M | H | TDF-3TC + NVP | |
| TDF add-on | 004 | 61 | M | H | ddI-ABC-3TC + ATV |
| 013 | 70 | M | C | ddI-AZT-FTC + ATV | |
| Long-term ddI | 901 | 36 | M | H | ddI-FTC + RAL |
| 902 | 41 | F | PI | ddI-FTC + ATV | |
| 903 | 39 | M | H | ddI-TDF + ATV/r | |
| 904 | 46 | M | B | ddI-TDF + ATV/r |
M, male; F, female; C, Caucasian; H, Hispanic; PI, Pacific Islander; B, black; ATV, atazanavir; r, ritonavir boosting; NVP, nevirapine; SQV, saquinavir; FPV, fosamprenavir; RAL, raltegravir.
Plasma pharmacokinetics of ddI and TFV.
Sparse plasma samples (plasma taken during PBMC isolation at 0, 2, 12, and 24 h) in the ddI and TDF add-on arms were analyzed at each study week to confirm appropriate administration of study drugs. Detectable concentrations of TFV and ddI in plasma samples from each patient were consistent with the arm and study week. While this study was designed primarily to measure intracellular nucleotide concentrations, the concentration-versus-time profiles of TFV and ddI were generally consistent with those observed in a more exhaustive drug-drug interaction study (24). The differences in the absolute AUC values estimated for TFV and ddI in patients stable on therapy with either drug in this study (median AUCs over 24 h during the course of the study, 12,800 and 7,540 nM·h, respectively) relative to the values reported by Kearney et al. (24) (9,200 and 14,200 nM·h, respectively) likely reflect the inability of the sparse sampling used in the present study to fully describe the plasma pharmacokinetics of TFV and ddI. In the add-on arms, there was no evidence for a marked change in the respective plasma AUC of TFV or ddI following addition of the second drug and ddI dose reduction (data not shown).
Intracellular pharmacokinetics of continued NRTI.
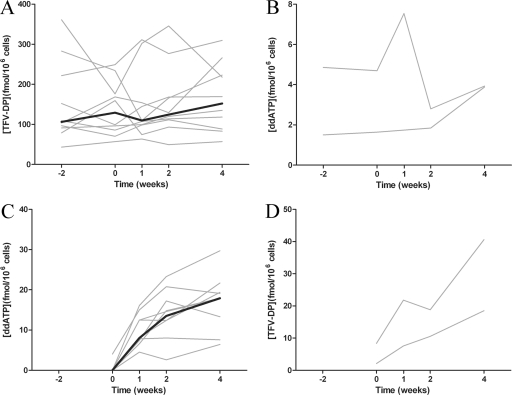
Addition of 250 mg ddI to 10 patients on 300 mg TDF did not cause an effect on PBMC TFV-DP concentrations (Fig. 2A). From screening to week 4, there was no statistically significant difference in TFV-DP concentrations (median values, 106 and 152 fmol/106 cells; P = 0.74, Mann-Whitney U test). The median TFV-DP concentration across all study weeks was 120 fmol/106 cells (range, 42.9 to 361 fmol/106 cells). A median intrapatient fluctuation in TFV-DP concentrations between visits of 1.6-fold was observed, and interpatient variability in the median concentration of TFV-DP was 5.3-fold. The intra- and interpatient variabilities in TFV-DP concentrations were similar to those reported in a previous study (17). While only two patients who met the acceptance criteria to be in the TDF add-on arm were identified, no consistent trends in PBMC ddATP concentrations were observed upon addition of 300 mg TDF and dose reduction from 400 mg to 250 mg ddI (Fig. 2B). In the two individuals, ddATP concentrations were 1.5 and 4.9 fmol/106 cells at screening and 3.9 and 3.9 fmol/106 cells at week 4.
FIG. 2.
Intracellular concentrations of TFV-DP (A; n = 10) and ddATP (B; n = 2) in patients stable on regimens containing TDF or ddI, respectively, before and after addition of the other NRTI. Intracellular concentrations of ddATP (C; n = 9; 1 of the 10 patients had undetectable concentrations in all samples) and TFV-DP (D; n = 2) in patients initiating ddI or TDF, respectively. Black lines represent the median for all patients, and values for individual patients are indicated by gray lines. No median was calculated for the two patients in the TDF add-on arm (B and D). In panel B, the LLOQ (2.8 fmol/106 cells) is indicated at week 2 for the patient on a stable ddI regimen with higher concentrations of ddATP because insufficient cells were collected to allow quantification of ddATP concentrations. Individual concentrations indicated at each study week are the medians of four measurements taken over 24 h for each patient.
Intracellular pharmacokinetics of added NRTI.
Intracellular concentrations of ddATP (Fig. 2C) and TFV-DP (Fig. 2D) increased over the 4 weeks following addition of ddI and TDF, respectively. Intracellular concentrations of ddATP increased from being quantifiable in only 1 of 10 patients on the first day of administration to being quantifiable in 9 of 10 patients at week 4. The concentration of ddATP appeared to be approaching steady state between weeks 2 and 4 on the basis of the lack of a significant difference in concentrations (median values, 13.5 versus 17.9 fmol/106 cells; P = 0.38, Mann-Whitney U test). The concentrations of ddATP obtained at week 4 in the ddI add-on arm were markedly higher than the concentrations observed in the two patients on stable ddI in the TDF add-on arm. In the two patients who had TDF added to an existing ddI regimen, the doubling in TFV-DP concentrations from weeks 2 to 4 suggests that steady state had not been attained.
2′-Deoxypurine nucleotide pools in add-on arms.
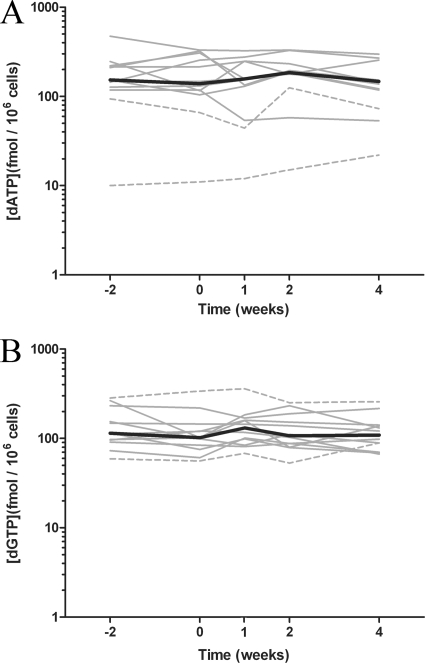
In order to determine if there is any effect of adding ddI or TDF to a stable regimen containing the other NRTI on endogenous purine nucleotides, dATP and dGTP concentrations were quantified in PBMC samples (n = 12; Fig. 3). Between screening and week 4, there were no significant differences in dATP concentrations (median values, 153 and 147 fmol/106 cells; P = 0.67, Mann-Whitney U test) or dGTP concentrations (median values, 114 and 109 fmol/106 cells; P = 0.47, Mann-Whitney U test). The median concentration across all study weeks for dATP was 154 fmol/106 cells (range, 10 to 474 fmol/106 cells), and that for dGTP was 111 fmol/106 cells (range, 53 to 359 fmol/106 cells). Concentrations of dATP and dGTP were relatively stable within a patient across visits (median intrapatient fluctuations in concentrations of dATP and dGTP, 2.2- and 1.7-fold, respectively). The interpatient variabilities in the median concentrations of dGTP and dATP in each patient were 4.8- and 28-fold, respectively. The lowest concentrations of dATP (less than 100 fmol/106 cells) were observed in the two patients in the TDF add-on arm who had been on long-term ddI therapy and one patient in the ddI add-on arm after initiation of ddI therapy.
FIG. 3.
Intracellular concentrations of the endogenous purine 2′-deoxynucleotides dATP (A) and dGTP (B) before and after coadministration of TDF and ddI (n = 12). The black lines represent the median for all patients, and the results for individual patients from the ddI (solid line) and TDF (broken line) add-on arms are indicated by gray lines. Individual concentrations at each study week that sampling took place are the medians of four measurements taken over 24 h for each patient.
Nucleotide concentrations in patients on long-term ddI therapy.
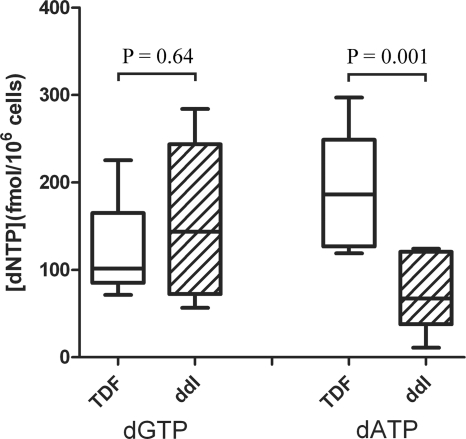
While there was no evidence for an intracellular drug interaction between TFV and ddI at the level of endogenous purine nucleotide or nucleotide analog concentrations, data from the two patients on long-term ddI suggested that they had markedly lower concentrations of dATP and ddATP than patients just starting ddI. To further assess the significance of this result, an additional four patients on long-term ddI (including the patients from the TDF add-on arm, to give a total of six patients) were enrolled and samples were collected 2 weeks apart to assess intracellular nucleotide concentrations. Median ddATP concentrations in the six patients on long-term ddI were statistically significantly less than those measured at week 4 in the nine patients with detectable concentrations of ddATP initiating ddI (median values, 3.30 and 17.9 fmol/106 cells; P = 0.0004, Mann-Whitney U test). Concentrations of ddATP in patients on stable regimens containing ddI were more similar to those previously reported in the literature (5.06 to 8.00 fmol/106 cells) (5, 35). While dGTP concentrations were similar, median dATP concentrations were also statistically significantly less than concentrations in patients on long-term TDF therapy (186 and 67.2 fmol/106 cells, respectively; Fig. 4). Similarly, dATP concentrations in patients stable on ddI were markedly lower than those reported in the literature in patients not on ddI (between 125 and 320 fmol/106 cells) (2, 18).
FIG. 4.
PBMC concentrations of the endogenous purine dNTPs dATP and dGTP in patients on stable regimens including TDF (n = 10) or ddI (n = 6). Values represent the median concentrations in samples from patients taken over 2 weeks (prior to initiation of the second NRTI in the add-on arms). The indicated statistical significance of differences in the respective dNTP concentrations between groups was determined using the Mann-Whitney U test.
DISCUSSION
In this study assessing the effect of adding either ddI or TDF to a stable regimen including the other NRTI, we did not observe any evidence for an antagonistic interaction based on the lack of perturbations in PBMC concentrations of ddATP and TFV-DP upon addition of the other NRTI. A prior cross-sectional study of intracellular TFV-DP and ddATP concentrations in patients on TDF, ddI (400 mg), or TDF-ddI (250 mg) found no statistically significant difference between the groups. As part of the same study, three patients in the TDF-ddI (250 mg) group discontinued the combination and switched to ddI (400 mg), with no impact on intracellular ddATP concentrations being observed over 4 weeks (35). These findings are also consistent with the results of in vitro studies showing no reduction in ddATP and TFV-DP concentrations upon coincubation with quiescent or stimulated PBMCs (38) and no antagonism in antiviral combination studies (29). Taken together, these results suggest a lack of antagonism in the intracellular activation of TFV and ddI and that the ddI dose adjustment, implemented to normalize the plasma ddI AUC (Videx package insert) (24), does not adversely affect PBMC ddATP concentrations.
The likely mechanism for the increased ddI plasma AUC when it is coadministered with TDF is the inhibition of the PNP-dependent degradation of ddI by phosphorylated metabolites of TFV (36). Since PNP is involved in the regulation of endogenous purine nucleotide pools, some have speculated that TDF therapy may increase dATP and dGTP concentrations, antagonizing the antiviral activity of other NRTIs and resulting in CD4+ cell declines (22). In this study, addition of ddI or TDF to a stable regimen including the other NRTI did not have any effect on dATP or dGTP concentrations. Studies done in vitro have similarly shown that TFV alone or in combination with ddI was unable to affect intracellular 2′-deoxynucleoside triphosphate (dNTP) pools (42).
The ddI add-on arm provided the opportunity to study the intracellular accumulation of ddATP following initiation of therapy with ddI in 10 patients. We observed that it took 2 weeks for ddATP concentrations to approach steady state. Unfortunately, only two patients were identified for the TDF add-on arm. In these individuals, concentrations of TFV-DP still appeared to be increasing 4 weeks after initiation of TDF administration. The median intracellular half-lives of ddATP and TFV-DP have been reported to be 24 and 150 h, respectively (5, 17, 35). In our prior study, some patients had TFV-DP half-lives in excess of 175 h, with levels being detectable at up to 28 days after the last TDF dose (17). The extensive intracellular half-lives of ddATP and TFV-DP, equal to or greater than the dosing intervals of ddI and TDF, respectively, are consistent with the extensive accumulation observed in this study.
Due to the increased plasma AUC of ddI when non-dose-adjusted ddI is administered with TDF, reports of an increased incidence of ddI-associated adverse events, including pancreatitis, hepatic toxicity, and peripheral neuropathy, might be expected. However, reports of paradoxical CD4+ cell declines and renal adverse events are harder to reconcile because these side effects are not commonly associated with ddI therapy. However, it should be noted that leukopenia and renal tubular degeneration were observed in animals during preclinical studies exploring higher ddI plasma AUCs (7), and while they are rarely reported at typical ddI plasma AUCs, these adverse events have been reported in patients (20, 40).
Providing a possible clue to the mechanism for ddI's effects on CD4+ cells, this study observed an apparent effect of extended ddI therapy on PBMCs in patients on stable regimens including ddI. While this study is the first to associate ddI use as part of a normal HIV treatment regimen with reduced PBMC dATP concentrations, Bakshi et al. showed that the combination of ddI and hydroxyurea, but neither agent on its own, depleted PBMC dATP concentrations in 14 days (2). We also observed reduced PBMC concentrations of ddATP in patients on long-term ddI therapy relative to those in patients just initiating ddI therapy, while dGTP concentrations were unaffected. Upon initial inspection, the high ddATP concentrations observed in the ddI add-on arm appear to suggest a drug interaction increasing ddI activation. However, the lack of a difference in ddATP concentrations between patients stable on regimens including ddI and ddI-TDF in a previous study (35), the lower ddATP concentrations observed in the two patients on ddI therapy with a regimen including TDF in the long-term ddI study extension, and the lack of an effect of adding TDF to the regimens for the two patients stable on ddI in the TDF add-on arm suggest that concentrations of ddATP decrease over time with continued ddI therapy.
While the mechanism for reduced ddATP concentrations over time during ddI therapy is not clear, it is tempting to speculate that cell populations that produce less ddATP due to reduced activity of enzymes involved in 2′-deoxyadenosine anabolism are selected over time because they are less sensitive to ddI toxicity. Support for this hypothesis can be obtained from an in vitro study that generated cells resistant to the toxicity of the anti-hepatitis B virus agent adefovir and found a 2-fold reduction in whole-cell adenylate kinase activity (37). A reduction in ddATP formation could result in a reduction in the inhibition of mitochondrial DNA polymerase γ and the resulting mitochondrial toxicity. A recent study showed that therapy with ddI (in the absence of TDF) depletes mitochondrial DNA in CD4+ and CD8+ cells without observation of clinical symptoms (27). Indeed, intensification of ddI's effects by pharmacokinetic or pharmacodynamic drug-drug interactions may cause these normally subclinical effects to manifest themselves as observable adverse events. Reduced concentrations of ddATP may also have implications for the long-term efficacy of ddI-containing regimens. Reduced 3TC activation and a corresponding increase in the 2′-deoxycytidine triphosphate/3TC triphosphate ratio have been observed over 48 weeks and proposed to be a novel pharmacologic mechanism for reduced efficacy and resistance selection (18).
This study has limitations. The infrequent use of ddI in the absence of TDF where this study was conducted made it difficult to recruit patients into the TDF add-on arm. While patients on regimens including ddI had consistently lower dATP and ddATP concentrations than patients who either were not on ddI or had been on ddI only for a short time, the contribution of the duration of ddI therapy could not be assessed. A larger longitudinal study following patients from initiation of therapy for longer than 4 weeks would be needed to better characterize the temporal effects of ddI therapy on nucleotide concentrations. This study was small, including only 16 patients, and was therefore subject to potential skewed conclusions on the basis of limited diversity in the study population and analysis of fewer samples. This concern was somewhat addressed by the longitudinal study design including 20 nucleotide concentration determinations in each patient over the course of the 6-week study. This study also used refined PBMC collection techniques and an analytical methodology that helped to reduce variability caused by the inherent difficulty in accurate quantification of nucleotides in cellular samples. Nevertheless, the conclusions made during this study were based on results for a small number of individuals and may not be representative for all patient populations.
In conclusion, no evidence for an antagonistic intracellular drug-drug interaction between TDF and ddI was observed. The lack of an effect on PBMC concentrations of ddATP of adding TDF combined with ddI dose reduction in patients on a stable regimen containing ddI serves to further validate ddI dose reduction. Compelling evidence for a perturbation in PBMC nucleotide concentrations caused by long-term ddI therapy was observed. This observation is deserving of further study, as it may provide insight into the pharmacology of ddI.
Acknowledgments
Technical and financial support for this study was provided by Gilead Sciences, Inc.
Footnotes
Published ahead of print on 31 January 2011.
REFERENCES
- 1.Annan, N. T., et al. 2009. The nucleoside backbone affects durability of efavirenz- or nevirapine-based highly active antiretroviral therapy in antiretroviral-naive individuals. J. Acquir. Immune Defic. Syndr. 51:140-146. [DOI] [PubMed] [Google Scholar]
- 2.Bakshi, R. P., et al. 2007. Effect of hydroxyurea and dideoxyinosine on intracellular 3′-deoxyadenosine-5′-triphosphate concentrations in HIV-infected patients. AIDS Res. Hum. Retroviruses 23:1360-1365. [DOI] [PubMed] [Google Scholar]
- 3.Barrios, A., et al. 2005. Simplification therapy with once-daily didanosine, tenofovir and efavirenz in HIV-1-infected adults with viral suppression receiving a more complex antiretroviral regimen: final results of the EFADITE trial. Antivir. Ther. 10:825-832. [PubMed] [Google Scholar]
- 4.Barrios, A., et al. 2005. Paradoxical CD4+ T-cell decline in HIV-infected patients with complete virus suppression taking tenofovir and didanosine. AIDS 19:569-575. [DOI] [PubMed] [Google Scholar]
- 5.Becher, F., et al. 2004. Monitoring of didanosine and stavudine intracellular trisphosphorylated anabolite concentrations in HIV-infected patients. AIDS 18:181-187. [DOI] [PubMed] [Google Scholar]
- 6.Benech, H., et al. 2004. Peripheral blood mononuclear cell counting using a DNA-detection-based method. Anal. Biochem. 330:172-174. [DOI] [PubMed] [Google Scholar]
- 7.Buroker, R. A., et al. 1996. A nonclinical safety assessment of didanosine, a nucleoside analogue with anti-human immunodeficiency virus activity. Curr. Ther. Res. 56:996-1008. [Google Scholar]
- 8.Costa, P., et al. 2009. Conserved T cell and natural killer cell function in treatment-experienced adults receiving tenofovir plus didanosine as nucleoside reverse transcription inhibitor backbone. Clin. Exp. Immunol. 158:55-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cote, H. C., et al. 2006. Exploring mitochondrial nephrotoxicity as a potential mechanism of kidney dysfunction among HIV-infected patients on highly active antiretroviral therapy. Antivir. Ther. 11:79-86. [PubMed] [Google Scholar]
- 10.Durand-Gasselin, L., D. Da Silva, H. Benech, A. Pruvost, and J. Grassi. 2007. Evidence and possible consequences of the phosphorylation of nucleoside reverse transcriptase inhibitors in human red blood cells. Antimicrob. Agents Chemother. 51:2105-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durand-Gasselin, L., et al. 2009. Nucleotide analogue prodrug tenofovir disoproxil enhances lymphoid cell loading following oral administration in monkeys. Mol. Pharm. 6:1145-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallant, J. E., et al. 2005. Early virologic nonresponse to tenofovir, abacavir, and lamivudine in HIV-infected antiretroviral-naive subjects. J. Infect. Dis. 192:1921-1930. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Benayas, T., et al. 2006. Higher risk of hyperglycemia in HIV-infected patients treated with didanosine plus tenofovir. AIDS Res. Hum. Retroviruses 22:333-337. [DOI] [PubMed] [Google Scholar]
- 14.Gerstoft, J., et al. 2003. Low efficacy and high frequency of adverse events in a randomized trial of the triple nucleoside regimen abacavir, stavudine and didanosine. AIDS 17:2045-2052. [DOI] [PubMed] [Google Scholar]
- 15.Gupta, S. K. 2008. Tenofovir-associated Fanconi syndrome: review of the FDA adverse event reporting system. AIDS Patient Care STDS 22:99-103. [DOI] [PubMed] [Google Scholar]
- 16.Hawkins, T., et al. 2009. Evaluation of potential intracellular drug interaction between tenofovir and didanosine in HIV infected patients, abstr. P26. Abstr. 10th Int. Workshop Clin. Pharmacol. HIV Ther.
- 17.Hawkins, T., et al. 2005. Intracellular pharmacokinetics of tenofovir diphosphate, carbovir triphosphate, and lamivudine triphosphate in patients receiving triple-nucleoside regimens. J. Acquir. Immune Defic. Syndr. 39:406-411. [DOI] [PubMed] [Google Scholar]
- 18.Hoggard, P. G., et al. 2002. Time-dependent changes in HIV nucleoside analogue phosphorylation and the effect of hydroxyurea. AIDS 16:2439-2446. [DOI] [PubMed] [Google Scholar]
- 19.Irizarry-Alvarado, J. M., J. P. Dwyer, L. M. Brumble, S. Alvarez, and J. C. Mendez. 2009. Proximal tubular dysfunction associated with tenofovir and didanosine causing Fanconi syndrome and diabetes insipidus: a report of 3 cases. AIDS Read. 19:114-121. [PubMed] [Google Scholar]
- 20.Izzedine, H., V. Launay-Vacher, and G. Deray. 2005. Fanconi syndrome associated with didanosine therapy. AIDS 19:844-845. [DOI] [PubMed] [Google Scholar]
- 21.Jemsek, J., P. Hutcherson, and E. Harper. 2004. Poor virologic response and early emergence of resistance in treatment naïve, HIV-infected patients receiving a once daily triple nucleoside regimen of didanosine, lamivudine, and tenofovir DF, abstr. 51. Abstr. 11th Conf. Retroviruses Opportunistic Infect.
- 22.Kakuda, T. N., P. L. Anderson, and S. L. Becker. 2004. CD4 cell decline with didanosine and tenofovir and failure of triple nucleoside/nucleotide regimens may be related. AIDS 18:2442-2444. [PubMed] [Google Scholar]
- 23.Karrer, U., et al. 2005. Dose-dependent influence of didanosine on immune recovery in HIV-infected patients treated with tenofovir. AIDS 19:1987-1994. [DOI] [PubMed] [Google Scholar]
- 24.Kearney, B. P., et al. 2005. Drug-drug and drug-food interactions between tenofovir disoproxil fumarate and didanosine. J. Clin. Pharmacol. 45:1360-1367. [DOI] [PubMed] [Google Scholar]
- 25.Leon, A., et al. 2005. Early virological failure in treatment-naive HIV-infected adults receiving didanosine and tenofovir plus efavirenz or nevirapine. AIDS 19:213-215. [DOI] [PubMed] [Google Scholar]
- 26.Lopez, S., et al. 2006. Longitudinal study on mitochondrial effects of didanosine-tenofovir combination. AIDS Res. Hum. Retroviruses 22:33-39. [DOI] [PubMed] [Google Scholar]
- 27.Maggiolo, F., et al. Mitochondrial changes during D-drug-containing once-daily therapy in HIV-positive treatment-naive patients. Antivir. Ther. 15:51-59. [DOI] [PubMed]
- 28.Martinez, E., et al. 2004. Pancreatic toxic effects associated with co-administration of didanosine and tenofovir in HIV-infected adults. Lancet 364:65-67. [DOI] [PubMed] [Google Scholar]
- 29.Mulato, A. S., and J. M. Cherrington. 1997. Anti-HIV activity of adefovir (PMEA) and PMPA in combination with antiretroviral compounds: in vitro analyses. Antiviral Res. 36:91-97. [DOI] [PubMed] [Google Scholar]
- 30.Negredo, E., A. Bonjoch, R. Paredes, J. Puig, and B. Clotet. 2005. Compromised immunologic recovery in treatment-experienced patients with HIV infection receiving both tenofovir disoproxil fumarate and didanosine in the TORO studies. Clin. Infect. Dis. 41:901-905. [DOI] [PubMed] [Google Scholar]
- 31.Negredo, E., et al. 2008. Partial immunological and mitochondrial recovery after reducing didanosine doses in patients on didanosine and tenofovir-based regimens. Antivir. Ther. 13:231-240. [PubMed] [Google Scholar]
- 32.Negredo, E., et al. 2004. Unexpected CD4 cell count decline in patients receiving didanosine and tenofovir-based regimens despite undetectable viral load. AIDS 18:459-463. [DOI] [PubMed] [Google Scholar]
- 33.Negredo, E., et al. 2004. Safety and efficacy of once-daily didanosine, tenofovir and nevirapine as a simplification antiretroviral approach. Antivir. Ther. 9:335-342. [PubMed] [Google Scholar]
- 34.Podzamczer, D., et al. 2005. Early virological failure with a combination of tenofovir, didanosine and efavirenz. Antivir. Ther. 10:171-177. [PubMed] [Google Scholar]
- 35.Pruvost, A., et al. 2005. Measurement of intracellular didanosine and tenofovir phosphorylated metabolites and possible interaction of the two drugs in human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 49:1907-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ray, A. S., L. Olson, and A. Fridland. 2004. Role of purine nucleoside phosphorylase in interactions between 2′,3′-dideoxyinosine and allopurinol, ganciclovir, or tenofovir. Antimicrob. Agents Chemother. 48:1089-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robbins, B. L., M. C. Connelly, D. R. Marshall, R. V. Srinivas, and A. Fridland. 1995. A human T lymphoid cell variant resistant to the acyclic nucleoside phosphonate 9-(2-phosphonylmethoxyethyl)adenine shows a unique combination of a phosphorylation defect and increased efflux of the agent. Mol. Pharmacol. 47:391-397. [PubMed] [Google Scholar]
- 38.Robbins, B. L., C. K. Wilcox, A. Fridland, and J. H. Rodman. 2003. Metabolism of tenofovir and didanosine in quiescent or stimulated human peripheral blood mononuclear cells. Pharmacotherapy 23:695-701. [DOI] [PubMed] [Google Scholar]
- 39.Roge, B. T., et al. 2003. K65R with and without S68: a new resistance profile in vivo detected in most patients failing abacavir, didanosine and stavudine. Antivir. Ther. 8:173-182. [PubMed] [Google Scholar]
- 40.Schindzielorz, A., I. Pike, M. Daniels, L. Pacelli, and L. Smaldone. 1994. Rates and risk factors for adverse events associated with didanosine in the expanded access program. Clin. Infect. Dis. 19:1076-1083. [DOI] [PubMed] [Google Scholar]
- 41.Tung, M. Y., S. Mandalia, M. Bower, B. Gazzard, and M. Nelson. 2005. The durability of virological success of tenofovir and didanosine dosed at either 400 or 250 mg once daily. HIV Med. 6:151-154. [DOI] [PubMed] [Google Scholar]
- 42.Vela, J. E., M. D. Miller, G. R. Rhodes, and A. S. Ray. 2008. Effect of nucleoside and nucleotide reverse transcriptase inhibitors of HIV on endogenous nucleotide pools. Antivir. Ther. 13:789-797. [PubMed] [Google Scholar]
- 43.Vidal, F., et al. 2006. In vitro cytotoxicity and mitochondrial toxicity of tenofovir alone and in combination with other antiretrovirals in human renal proximal tubule cells. Antimicrob. Agents Chemother. 50:3824-3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Winston, A., et al. 2004. Which nucleoside and nucleotide backbone combinations select for the K65R mutation in HIV-1 reverse transcriptase. AIDS 18:949-951. [DOI] [PubMed] [Google Scholar]
- 45.Young, B., et al. 2006. Short-term safety and tolerability of didanosine combined with high- versus low-dose tenofovir disproxil fumarate in ambulatory HIV-1-infected persons. AIDS Patient Care STDS 20:238-244. [DOI] [PubMed] [Google Scholar]