Abstract
The qnrS1 gene induces reduced susceptibility to fluoroquinolones in enterobacteria. We investigated the structure, antimicrobial susceptibility phenotype, and antimicrobial resistance gene characteristics of qnrS1 plasmids from hospitalized patients and community controls in southern Vietnam. We found that the antimicrobial susceptibilities, resistance gene characteristics, and plasmid structures of qnrS1 plasmids from the hospital differed from those from the community. Our data imply that the characteristics of the two plasmid groups are indicative of distinct selective pressures in the differing environments.
Quinolone resistance and reduced susceptibility to fluoroquinolones in members of the Enterobacteriaceae are related to mutations in the DNA gyrase and topoisomerase genes (2, 22). However, resistance can be increased by the activity of plasmid-mediated quinolone resistance (PMQR) genes, which can be transferred horizontally (18). qnrS1 is an example of a PMQR gene and encodes a protein which protects the DNA gyrase from quinolone activity (20). Transfer of a qnrS1 gene into a naive laboratory strain confers an increase in the MIC of some fluoroquinolones, including ciprofloxacin (13).
The majority of studies of PMQR plasmids have been performed on strains isolated from infection sites (11, 15, 16, 19, 21). Consequently, little is known about such plasmids in commensal Enterobacteriaceae species and the role they may play in disseminating other resistance genes. In Vietnam, fluoroquinolones are among the most commonly used antimicrobials and are available without prescription. Our previous work demonstrated that a high proportion of “healthy” members of the Vietnamese population carry Enterobacteriaceae species harboring qnrS1 in the gastrointestinal tract (12). We aimed to investigate the cotransfer of antimicrobial resistance genes with qnrS1 from plasmids that originated from hospitalized patients and from community controls in Ho Chi Minh City, Vietnam. We isolated plasmid DNA from 32 different qnrS1 PCR amplicon-positive strains from the hospital and the community, as previously described (12) (Table 1). These strains were selected from a larger collection (12) as a result of our inability to genetically define the region surrounding the qnrS1 gene, thus maximizing potential plasmid diversity. To ensure that we assessed only qnrS1 plasmids, an Escherichia coli strain was transformed with plasmid DNA and selected on media supplemented with ciprofloxacin. All organisms were subjected to qnrS1 PCR amplification and sized by a plasmid extraction method to ensure transformation of a single appropriate plasmid (5, 12). The plasmids were found to be of various sizes, ranging from 9 to 140 kbp, with a median of 84 kbp (Table 1). The plasmids carrying qnrS1 that originated from the hospital ranged from 48 to 105 kbp in size (median, 58 kbp), and those originating from the community ranged from 9 to 140 kbp in size (median, 103 kbp).
TABLE 1.
The origins and characteristics of 32 qnrS1-bearing plasmids
| Strain or plasmid identification no. | Plasmid name | Sourcea | Bacterial speciesb | Incompatibility group(s)c | Size (kbp) | Presence of ESBL | MIC (μg/ml) |
Resistance patternd | |
|---|---|---|---|---|---|---|---|---|---|
| NAL | CIP | ||||||||
| Strain | |||||||||
| Top10 | E. coli | − | 1.5 | 0.006 | |||||
| Plasmids | |||||||||
| LTMV1 | pEW62RMAN | Community | K. pneumoniae | A/C | 9 | − | 6 | 0.38 | CIPL |
| LTMV2 | p033CA22 | Community | E. coli | FIA; A/C | 101 | − | 4 | 0.25 | CHL, TET, SXT, CIPL |
| LTMV3 | p036CN2 | Community | E. coli | repF; HI1; FIA; A/C | 80 | − | 12 | 0.38 | CHL, GEN, CIPL |
| LTMV4 | pA003IaI | Community | K. pneumoniae | FIA; A/C; R | 132 | − | 4 | 0.25 | CHL, GEN, TET, SXT, CIPL |
| LTMV5 | pK261An | Hospital | K. pneumoniae | R | 70 | − | 4 | 0.25 | AMP, CHL, GEN, TET, SXT, CIPL |
| LTMV6 | p023CN2 | Community | E. coli | repF; FIB | 140 | − | 4 | 0.25 | AMP, CHL, CIPL |
| LTMV7 | p038CN2 | Community | K. pneumoniae | R | 64 | − | 4 | 0.25 | AMP, CHL, GEN, TET, SXT, CIPL |
| LTMV8 | p008CN1 | Community | E. coli | repF; FIB | 140 | − | 8 | 0.38 | CHL, GEN, CIPL |
| LTMV9 | p001CN2 | Community | E. coli | Unknown | 96 | − | 4 | 0.38 | AMP, CHL, GEN, TET, CIPL |
| LTMV11 | p024CAZZ | Community | E. coli | FIA | 44 | − | 6 | 0.38 | AMP, TET, SXT, CIPL |
| LTMV12 | p0022IbI | Community | K. pneumoniae | Unknown | 98 | − | 4 | 0.25 | AMP, TET, SXT, CIPL |
| LTMV13 | p045CN2 | Community | E. coli | repF | 107 | − | 6 | 0.25 | AMP, CHL, CIPL |
| LTMV14 | pK233Ca | Hospital | K. pneumoniae | Unknown | 51 | + | 4 | 0.12 | AMP, GEN, CRO, CIPL |
| LTMV15 | p025CN1 | Community | E. coli | Unknown | 135 | − | 6 | 0.38 | AMP, CHL, TET, SXT, CIPL |
| LTMV16 | p065CN2 | Community | K. pneumoniae | Unknown | 71 | − | 4 | 0.25 | AMP, CHL, GEN, TET, CIPL |
| LTMV17 | p048CN2 | Community | E. coli | R | 26 | − | 6 | 0.38 | CHL, CIPL |
| LTMV18 | pE18An | Hospital | E. coli | Unknown | 58 | − | 4 | 0.25 | AMP, CIPL |
| LTMV19 | pD025IaI | Community | E. coli | Unknown | 130 | − | 6 | 0.38 | AMP, CHL, GEN, TET, SXT, CIPL |
| LTMV20 | p051CN | Community | E. coli | R; ColE | 108 | − | 4 | 0.25 | AMP, CHL, GEN, TET, SXT, KAN, CIPL |
| LTMV21 | pK300N | Hospital | K. pneumoniae | R | 57 | + | 6 | 0.25 | AMP, FEP, GEN, TIC, CRO, CIPL |
| LTMV23 | p039CN2 | Community | E. coli | Unknown | 109 | − | 4 | 0.25 | AMP, CHL, GEN, TET, TIC, SXT, KAN, CIPL |
| LTMV24 | p008Na2 | Community | E. coli | Unknown | 43 | − | 6 | 0.12 | AMP, CIPL |
| LTMV25 | pA0001IaI | Community | E. coli | Unknown | 118 | − | 3 | 0.25 | AMP, CHL, GEN, SXT, CIPL |
| LTMV26 | pB011IaI | Community | E. coli | Y | 103 | − | 3 | 0.25 | AMP, CHL, GEN, CIPL |
| LTMV27 | pEW20NMAG | Community | K. pneumoniae | Unknown | 132 | − | 4 | 0.25 | AMP, GEN, TET, SXT, KAN, CIPL |
| LTMV28 | pK18An | Hospital | K. pneumoniae | N | 58 | − | 4 | 0.25 | GEN, CIPL |
| LTMV29 | pE66An | Hospital | E. coli | N | 87 | + | 4 | 0.25 | AMP, FEP, GEN, TET, SXT, CRO, CIPL |
| LTMV30 | pK218Ca | Hospital | K. pneumoniae | A/C | 48 | − | 4 | 0.12 | GEN, CIPL |
| LTMV31 | pK218Ca | Hospital | K. pneumoniae | N | 60 | + | 6 | 0.38 | AMP, FEP, GEN, CRO, CIPL |
| LTMV32 | pK79N | Hospital | K. pneumoniae | N | 74 | + | 6 | 0.38 | AMP, FEP, GEN, TET, SXT, CRO, CIPL |
| LTMV33 | pK263Ax | Hospital | K. pneumoniae | Unknown | 49 | − | 6 | 0.19 | GEN, CIPL |
| LTMV34 | pK279N | Hospital | K. pneumoniae | Unknown | 105 | + | 4 | 0.25 | AMP, FEP, GEN, CRO, CIPL |
Location of isolation of the original bacterial isolate containing qnrS1-carrying plasmid (12).
Original bacterial species from which the qnrS1-carrying plasmid was isolated.
Incompatibility group determined by PCR (4).
NAL, nalidixic acid; CIPL, reduced susceptibility to ciprofloxacin; CHL, chloramphenicol; TET, tetracycline; SXT, trimethoprim-sulfamethoxazole; GEN, gentamicin; AMP, ampicillin; CRO, ceftriaxone; KAN, kanamycin; FEP, cefepime; TIC, ticarcillin.
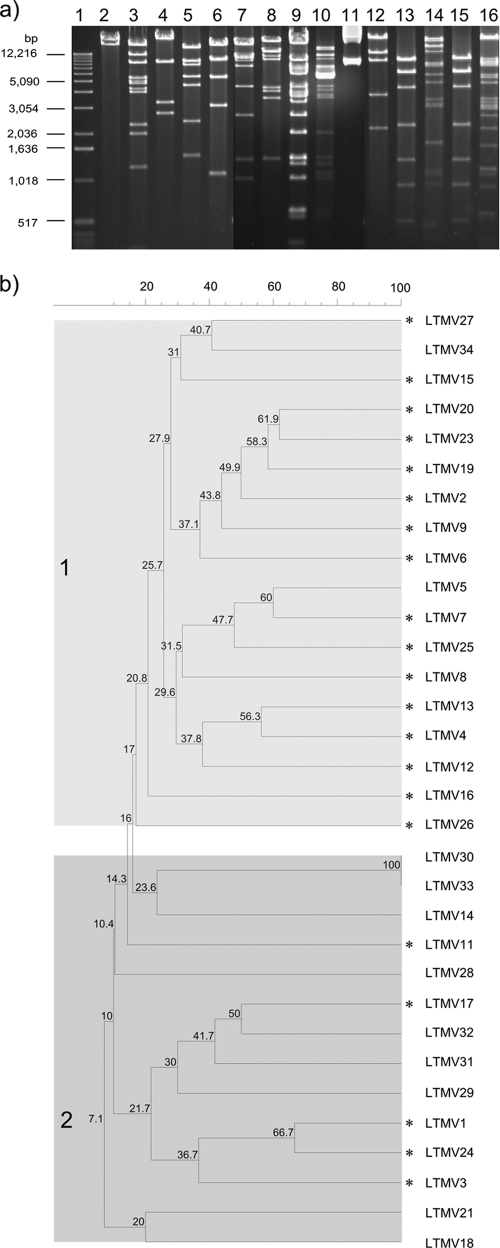
In order to compare plasmid structures, plasmid DNA was digested with EcoRI and plasmids were assigned to incompatibility groups by PCR-based replicon typing, as previously described (4, 7). We identified 11 different incompatibility groups, of which incN (n = 4) and incR (n = 4) were the most common members (Table 1). An association between qnr genes and incN plasmids was previously identified and is likely due to integration and dissemination of a qnr gene into this prevalent plasmid group (7). EcoRI restriction fragments were compared to identify common banding patterns (Fig. 1 ). Thirty strains demonstrated less than 67% identity, and the restriction patterns could be divided into groups 1 and 2. The digestion patterns of the qnrS1 plasmids originating in the community and the hospital were significantly associated with group 1 and 2, respectively (P = 0.0017; two-tailed Fisher's exact test).
FIG. 1.
EcoRI restriction digestions of plasmids carrying qnrS1. (a) An example of EcoRI digestion of 15 plasmids carrying qnrS1. Lanes: 1, 1-kbp ladder (Invitrogen); 2, plasmid LTMV21; 3, LTMV16; 4, LTMV28; 5, LTMV14; 6, LTMV11; 7, LTMV25; 8, LTMV5; 9, LTMV2; 10, LTMV20; 11, LTMV1; 12, LTMV18; 13, LTMV33; 14, LTMV6; 15, LTMV30; 16, LTMV19. (b) Neighbor-joining tree of 32 plasmids carrying qnrS1 derived from digestion with EcoRI. Plasmids that were isolated from strains originating in the community are indicated by asterisks. Numbers within the shaded boxes correspond to the percent relationship between the individual nodes. The shaded boxes labeled 1 and 2 correspond to the two major groups of digested plasmids.
The 32 strains were subjected to antimicrobial susceptibility profiling and extended-spectrum β-lactamase (ESBL) testing (Table 1) (14). There was no significant disparity (apart from chloramphenicol) between the resistance profiles of plasmids originally isolated from E. coli and those isolated from Klebsiella pneumoniae. However, the profiles of the plasmids isolated in the community demonstrated several differences from the profiles of the plasmids isolated from the hospital. The qnrS1 plasmids isolated in the hospital were frequently more resistant to gentamicin (P = 0.0273; all compared by two-tailed Fisher's exact test), ceftriaxone (P = 0.0004), and cefepime (P = 0.0019), all of which are parenteral and not commonly used in the community. Only chloramphenicol resistance was associated with plasmids isolated from the community (P = 0.0118).
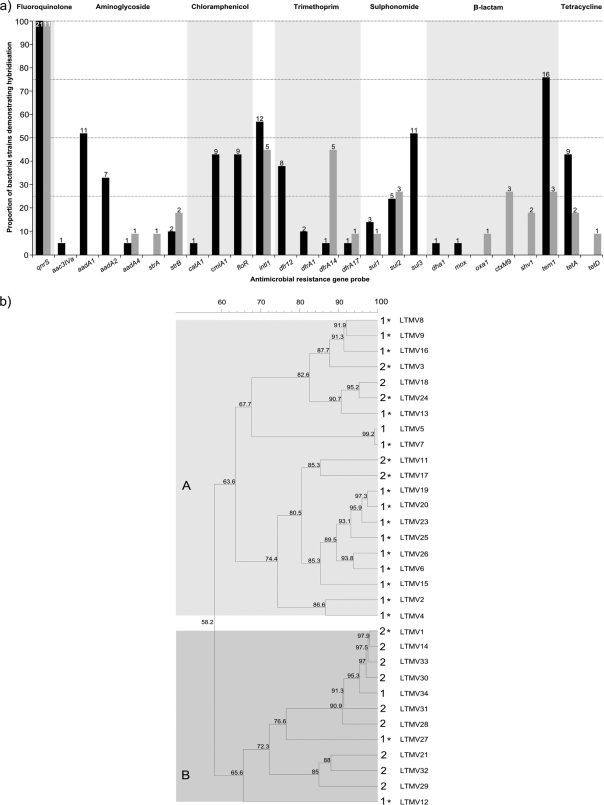
To assess the antimicrobial resistance gene characteristics, DNA from the 32 strains was hybridized to a miniaturized oligonucleotide antimicrobial resistance gene microarray containing probes for 54 antimicrobial resistance genes of clinical importance, and data were analyzed as previously described (1, 3). There were clear differences in hybridization patterns between plasmids that originated in the hospital and those that originated in the community (Fig. 2), thus partitioning the plasmids into groups A and B, corresponding to community and hospital plasmids (P < 0.001; Fisher's exact test). Grouping results also correlated with the plasmid digestion patterns, with identification of 14 and 2 community and hospital group 1A plasmids, respectively, and 8 and 1 hospital and community group 2B plasmids, respectively (P < 0.001; Fisher's exact test). The average number of genes detected by the assay in isolates from the community (6.3 per plasmid) differed significantly from the number detected in plasmids from the hospital (3.7 per plasmid) (P = 0.013; 2-tailed t test). Of the 32 plasmids, 25 demonstrated hybridization to probes for β-lactam resistance genes, with 19/32 (59.4%) and 4/32 (12.5%) producing a positive signal for tem1 and ctxM9, respectively. Hybridization to the oxa1, ctxM9, or shv1 gene was detected only in the hospital plasmids, and hybridization to tem1 was more common in community plasmids. Correspondingly, only the hospital plasmids exhibited an ESBL phenotype (Table 1).
FIG. 2.
The antimicrobial resistance gene content of 32 plasmids carrying qnrS. (a) Graph showing the proportional relationships of transformants demonstrating hybridization to 25 specific antimicrobial resistance genes and one class one integrase gene (intl1). The graph is subdivided into those plasmids that were isolated in the community (black bars) (n = 21) and those that were isolated in the hospital (gray bars) (n = 11). The labels at the top of the diagram highlight the group to which the indicated antimicrobial resistance gene confers resistance. (b) Neighbor-joining tree representing DNA hybridization to the antimicrobial resistance gene microarray. The 32 strains fall into main clusters A and B, which are distinguished by light and dark gray shading. Asterisks identify those strains containing community plasmids; the numbers adjacent to the strain names correspond to the EcoRI restriction digestion results (Fig. 1). The numbers on each branch correspond to percent identity between individual branches.
There has been some previous characterization of plasmids which can carry and transfer PMQR genes (6, 7, 17). Work conducted in Asia investigated the genetic properties of two plasmids carrying qnrS1 and qnrB4 isolated from an infectious K. pneumoniae strain in China. Hu et al. found that the qnrB4 and qnrS1 genes were located on two plasmids that demonstrate significant genetic variations (8). A sentinel study concerning E. coli and K. pneumoniae in China found that 8% of the investigated ESBL-producing strains were also PCR amplicon positive for a qnr gene. The blaCTX-M gene was the most commonly identified in 27 of 29 qnr-positive isolates, and TEM-1-type β-lactamase was detected in 16 qnr-positive isolates (9).
Here, we demonstrate that the sizes, incompatibility groups, resistance gene characteristics, and antimicrobial resistance phenotypes of plasmids carrying qnrS1 are highly divergent. The concept that qnr genes circulate on unrelated plasmids is supported by recent work that characterized PMQR plasmids in Salmonella strains isolated from patients and poultry meat in the Netherlands (7). The spread of antimicrobial resistance is of global relevance and yet is particularly significant for many developing countries with improving economic prospects and unregulated use of antimicrobials. The recent report of a novel mechanism of resistance to carbapenems conferred by a metallo-β-lactamase in India highlights the origins and potential transcontinental spread of such determinants (10). Our data demonstrate that the qnrS1 gene is extremely widespread and capable of transfer into a number of variable replicons. Furthermore, we found different genotypic and phenotypic characteristics of plasmids carrying qnrS1 in isolates collected in the hospital and the community, which is indicative of the selection of such replicons by the use of specific antimicrobial agents in these differing settings.
Acknowledgments
This work was supported by The Wellcome Trust, London, United Kingdom. S.B. is supported by an OAK Foundation Fellowship through Oxford University.
Footnotes
Published ahead of print on 31 January 2011.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Anjum, M. F., et al. 2007. Pathotyping Escherichia coli by using miniaturized DNA microarrays. Appl. Environ. Microbiol. 73:5692-5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagel, S., V. Hullen, B. Wiedemann, and P. Heisig. 1999. Impact of gyrA and parC mutations on quinolone resistance, doubling time, and supercoiling degree of Escherichia coli. Antimicrob. Agents Chemother. 43:868-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batchelor, M., et al. 2008. Development of a miniaturised microarray-based assay for the rapid identification of antimicrobial resistance genes in Gram-negative bacteria. Int. J. Antimicrob. Agents 31:440-451. [DOI] [PubMed] [Google Scholar]
- 4.Carattoli, A., et al. 2005. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63:219-228. [DOI] [PubMed] [Google Scholar]
- 5.Cattoir, V., L. Poirel, V. Rotimi, C. J. Soussy, and P. Nordmann. 2007. Multiplex PCR for detection of plasmid-mediated quinolone resistance qnr genes in ESBL-producing enterobacterial isolates. J. Antimicrob. Chemother. 60:394-397. [DOI] [PubMed] [Google Scholar]
- 6.Chen, Y. T., et al. 2006. Complete nucleotide sequence of pK245, a 98-kilobase plasmid conferring quinolone resistance and extended-spectrum-beta-lactamase activity in a clinical Klebsiella pneumoniae isolate. Antimicrob. Agents Chemother. 50:3861-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.García-Fernández, A., D. Fortini, K. Veldman, D. Mevius, and A. Carattoli. 2009. Characterization of plasmids harbouring qnrS1, qnrB2 and qnrB19 genes in Salmonella. J. Antimicrob. Chemother. 63:274-281. [DOI] [PubMed] [Google Scholar]
- 8.Hu, F. P., X. G. Xu, D. M. Zhu, and M. G. Wang. 2008. Coexistence of qnrB4 and qnrS1 in a clinical strain of Klebsiella pneumoniae. Acta Pharmacol. Sin. 29:320-324. [DOI] [PubMed] [Google Scholar]
- 9.Jiang, Y., et al. 2008. Plasmid-mediated quinolone resistance determinants qnr and aac(6′)-Ib-cr in extended-spectrum B-lactamase-producing Escherichia coli and Klebsiella pneumoniae in China. J. Antimicrob. Chemother. 61:1003-1006. [DOI] [PubMed] [Google Scholar]
- 10.Kumarasamy, K. K., et al. 2010. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect. Dis. 10:597-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lavilla, S., et al. 2008. Prevalence of qnr genes among extended-spectrum beta-lactamase-producing enterobacterial isolates in Barcelona, Spain. J. Antimicrob. Chemother. 61:291-295. [DOI] [PubMed] [Google Scholar]
- 12.Le, T. M., et al. 2009. High prevalence of plasmid-mediated quinolone resistance determinants in commensal members of the Enterobacteriaceae in Ho Chi Minh City, Vietnam. J. Med. Microbiol. 58:1585-1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martínez-Martínez, L., A. Pascual, and G. A. Jacoby. 1998. Quinolone resistance from a transferable plasmid. Lancet 351:797-799. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen, N. T., et al. 2010. The sudden dominance of blaCTX-M harbouring plasmids in Shigella spp. circulating in Southern Vietnam. PLoS Negl. Trop. Dis. 4:e702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oktem, I. M., Z. Gulay, M. Bicmen, and D. Gur. 2008. qnrA prevalence in extended-spectrum beta-lactamase-positive Enterobacteriaceae isolates from Turkey. Jpn. J. Infect. Dis. 61:13-17. [PubMed] [Google Scholar]
- 16.Pitout, J. D., Y. Wei, D. L. Church, and D. B. Gregson. 2008. Surveillance for plasmid-mediated quinolone resistance determinants in Enterobacteriaceae within the Calgary Health Region, Canada: the emergence of aac(6′)-Ib-cr. J. Antimicrob. Chemother. 61:999-1002. [DOI] [PubMed] [Google Scholar]
- 17.Poirel, L., M. Van De Loo, H. Mammeri, and P. Nordmann. 2005. Association of plasmid-mediated quinolone resistance with extended-spectrum beta-lactamase VEB-1. Antimicrob. Agents Chemother. 49:3091-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strahilevitz, J., G. A. Jacoby, D. C. Hooper, and A. Robicsek. 2009. Plasmid-mediated quinolone resistance: a multifaceted threat. Clin. Microbiol. Rev. 22:664-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szabo, D., et al. 2008. First detection of plasmid-mediated, quinolone resistance determinants qnrA, qnrB, qnrS and aac(6′)-Ib-cr in extended-spectrum {beta}-lactamase (ESBL)-producing Enterobacteriaceae in Budapest, Hungary. J. Antimicrob. Chemother. 62:630-632. [DOI] [PubMed] [Google Scholar]
- 20.Tran, J. H., and G. A. Jacoby. 2002. Mechanism of plasmid-mediated quinolone resistance. Proc. Natl. Acad. Sci. U. S. A. 99:5638-5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang, A., et al. 2008. Occurrence of qnr-positive clinical isolates in Klebsiella pneumoniae producing ESBL or AmpC-type beta-lactamase from five pediatric hospitals in China. FEMS Microbiol. Lett. 283:112-116. [DOI] [PubMed] [Google Scholar]
- 22.Weigel, L. M., C. D. Steward, and F. C. Tenover. 1998. gyrA mutations associated with fluoroquinolone resistance in eight species of Enterobacteriaceae. Antimicrob. Agents Chemother. 42:2661-2667. [DOI] [PMC free article] [PubMed] [Google Scholar]