Abstract
Two-component signal transduction systems (TCSs) in prokaryotes often regulate gene clusters that induce pathogenicity, and thus they have frequently been proposed as potential drug targets for attenuating the virulence of pathogens. The pathogenic potential of Streptococcus mutans, the major etiological pathogen of dental caries, is also regulated by its TCSs. The object of this study was to evaluate the effect of a histidine kinase (HK) inhibitor against two major virulence factors of S. mutans: biofilm formation and acid tolerance. Walkmycin C (WKM C), an HK inhibitor isolated from the screening of inhibitors against WalK HK in Bacillus subtilis, inhibited the in vitro autophosphorylation activity of three purified S. mutans HKs, i.e., VicK, CiaH, and LiaS. Although S. mutans does not have any essential HK but only an essential response regulator, VicR, WKM C showed an MIC of 6.25 μg/ml. This inhibitory effect of WKM C suggests that blocking the autophosphorylation of multiple HKs may inhibit phosphotransfer to VicR from VicK and other HKs. When WKM C was added at sub-MIC levels, the cells formed abnormal biofilms and also showed a defect in competence. When the cells were pretreated with WKM C, an increase in acid sensitivity was observed. Our results show that WKM C represses two pathogenic phenotypes of S. mutans, indicating the possibility of developing histidine kinase inhibitors into antivirulence drugs.
Streptococcus mutans, the major etiological pathogen of dental caries, lives almost exclusively in densely populated biofilms that form on the tooth surface. Large quantities of organic acid are produced by the biofilm-embedded cells, creating an acidic environment that erodes the tooth surface and thus causes caries. Consequently, the virulence of S. mutans is caused by its abilities to form biofilms, to produce organic acids, and to tolerate environmental stresses, particularly under low-pH conditions. S. mutans has very few alternative sigma factors in its genome (2), and regulatory systems, such as the two-component signal transduction systems (TCSs), are considered to play a central role in stress tolerance (14). TCSs, which are present in bacteria, yeast, fungi, and plants but not in mammals, respond to the chemical and physical signals from the environment. The signals are first sensed by the sensor histidine kinase (HK), which autophosphorylates its conserved histidine residue and then transfers the phosphoryl group to the aspartic acid of the response regulator (RR). In most cases, phosphorylated RRs bind to the upstream regulatory region of genes and control their expression. Among the 14 TCS systems and one orphan regulator in S. mutans, 8 pairs of TCS and an orphan regulator, CovR (a.k.a. TarC or GcrR), have been found to play roles in stress tolerance (5, 10, 15). TCSs are often involved in the virulence of many pathogens, as well as S. mutans, making them an attractive target for antivirulence drug development.
To date, anti-TCS drugs that repress virulence have mostly targeted the sensory domains of their target HKs. Compared to the conserved catalytic domains of HKs, the sensory domains are unique to each HK, making sensory domain-targeting drugs effective only to a specific TCS (9). Moreover, the signals detected by sensors are still unclear in many of the TCSs involved in virulence, and there are various cases where signals from multiple TCSs are integrated to coordinate a cell response. In S. mutans, several TCSs are known to be involved in biofilm formation, competence, and acid tolerance. Additive attenuation of virulence and cariogenic potential are also seen by simultaneous inactivation of the ComDE and LiaSR TCSs (19). Therefore, broad-acting general anti-TCS drugs that simultaneously inhibit multiple TCSs are expected to efficiently repress the virulence of S. mutans. Such drugs should target the conserved catalytic domains of HKs so as to inhibit multiple HKs.
One of the target TCSs for HK inhibitors is the WalK/WalR (formerly YycG/YycF) system, ubiquitous within Firmicutes. The WalK/WalR TCSs play a key role in cell wall metabolism (8) and are essential for cell growth. Attempts have been made to discover inhibitory compounds against the WalK/WalR system (27, 34, 35, 38). Among these attempts is the differential growth assay using a temperature-sensitive walR mutant of Bacillus subtilis (23). Using this selective method, a number of inhibitors against the WalK HK have been isolated (24, 34). These inhibitors showed antibacterial activity against various Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus. The walkmycins are HK inhibitors recently isolated from our differential growth assay. Three active fractions were detected from methanol extracts of Streptomyces sp. strain MK632-100F11; namely, walkmycins A, B, and C. Walkmycin B was the major product among the three, and it showed inhibitory effects against autophosphorylation of the WalK cytoplasmic regions of B. subtilis and S. aureus (24). The VicK/VicR system in S. mutans is an orthologue of the WalK/WalR system in B. subtilis. Although the sensor VicK is not essential for cell growth, previous works have reported that this system is important for growth, adhesion, biofilm formation, oxidative stress tolerance, and the development of genetic competence in S. mutans (29, 30).
We attempted here to evaluate walkmycin C (WKM C) against the virulence factors of S. mutans. WKM C, which inhibited the in vitro autophosphorylation activity of three HKs (VicK, CiaH, and LiaS) of S. mutans, repressed at least two virulence factors of S. mutans, as well as genetic competence in in vivo analyses. Our results suggest the potential of broad-acting general inhibitors of histidine kinase for use in antivirulence drugs.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The strains and plasmids used in the present study are listed in Table 1. Escherichia coli strains were grown in an LB medium (1% polypeptone, 0.5% yeast extract, and 0.5% NaCl) or in a 2×YT medium (1.6% tryptone, 1% yeast extract, and 0.5% NaCl) for protein expression. S. mutans strains were grown in a BHI medium (Bacto brain heart infusion; BD Diagnostics, Sparks, MD) at 37°C in a 5% CO2 atmosphere created by a CO2 generator (AneroPack CO2; Mitsubishi Gas Chemical, Tokyo, Japan). For biofilm formation assays, S. mutans strains were grown in a semidefined BM medium (20) without MnCl2 and supplemented with glucose or sucrose at a final concentration of 20 mM. Ampicillin was used at 100 μg/ml for E. coli. Kanamycin, erythromycin, and spectinomycin were used at 1,000, 10, and 1,500 μg/ml, respectively, for S. mutans.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| BL21(DE3) | F−ompT hsdSB (rB− mB−) gal dcm (DE3) | Novagen |
| JM109 | ||
| S. mutans | ||
| UA159 | Wild type | ATCC |
| UA159vicK | ΔvicK::NPKmr | This study |
| UA159ciaH | ΔciaH::Emr | This study |
| UA159liaS | ΔliaS::NPKmr | This study |
| UA159comD | ΔcomD::Emr | This study |
| Plasmids | ||
| pSC-B | Cloning vector | Stratagene |
| pET22b(+) | His tag cloning vector | Novagen |
| pGEX-6P-2 | GST tag cloning vector | GE Healthcare |
| pSC-BSMvicRK | vicRK cloned into pSC-B | This study |
| pSC-BSMciaRH | ciaRH cloned into pSC-B | This study |
| pSC-BMliaSR | liaSR cloned into pSC-B | This study |
| pETSMVicK31-450 | vicK (31 to 450 aa) cloned into pET22b(+) | This study |
| pGEXSMCiaH200-435 | ciaH (200 to 435 aa) cloned into pGX-6P-2 | This study |
| pETSMLiaS86-334 | liaS (86 to 334 aa) cloned into pET22b(+) | This study |
| pETSMVicR | vicR cloned into pET22b(+) | This study |
| pETSMCiaR | ciaR cloned into pET22b(+) | This study |
| pETSMLiaR | liaR cloned into pET22b(+) | This study |
| pUC18Em | pUC18 harboring an Emr cassette | Y. Y. Chen, University of Florida |
| pALH124 | Vector harboring an NPKmr cassette | Y. Y. Chen, University of Florida |
| pDL278 | E. coli-Streptococcus shuttle vector; Spr | 13 |
Spr, spectinomycin resistance; Emr, erythromycin resistance.
In vitro susceptibility. MICs were determined in accordance with Clinical and Laboratory Standards Institute (CLSI) methodology (CLSI M07-A7) by the agar dilution method (7). A Mueller-Hinton (MH; BD Diagnostics) medium supplemented with 5% sheep blood agar was used with 104 CFU/spot and 18 h of incubation at 37°C in 5% CO2 for Streptococcus pneumoniae and Streptococcus pyogenes. For the other bacteria, an MH medium agar was used with 104 CFU/spot and 18 h of incubation at 37°C in ambient air.
Construction of plasmids.
For the construction of expression plasmids of histidine kinases and response regulators of S. mutans, regions encoding vicRK, ciaRH, and liaSR were PCR amplified using specific primers, genome DNA of strain UA159 as the template, and PrimeSTAR polymerase (Takara Bio, Otsu, Japan). The amplified fragments were ligated into a pSC-B cloning vector (Stratagene, La Jolla, CA) to construct pSC-BSMvicRK, pSC-BSMciaRH, and pSC-BSMliaSR. Next, using these plasmids as templates, regions encoding the cytoplasmic regions of VicK (31 to 450 amino acids [aa]), CiaH (200 to 435 aa), LiaS (86 to 334 aa), VicR, CiaR, and LiaR were amplified by PCR with PrimeSTAR polymerase, digested with appropriate restriction enzymes, and ligated into a pET22b(+) (Novagen, Madison, WI) or pGEX-6P-2 (GE Healthcare Bio-Science, Piscataway, NJ) vector. DNA sequences of all of the constructs were confirmed with the ABI Prism 3100 genetic analyzer (ABI, Foster City, CA).
Purification of proteins.
Expression plasmids derived from pET22b(+) were transformed into E. coli BL21(DE3), and plasmids derived from pGEX-6P-2 were transformed into E. coli BL21. Transformants were grown in a 2×YT medium at 37°C with aeration to a cell optical density at 600 nm (OD600) of 0.5 to 0.6, followed by the addition of IPTG (isopropyl-β-d-thiogalactopyranoside) at a final concentration of 0.1 mM. After overnight induction at 18°C with aeration, cells were harvested and washed with lysis buffer (50 mM Tris-HCl [pH 8], 100 mM NaCl) and stored at −80°C until use. For purification of His-tagged proteins, frozen cells were resuspended in a lysis buffer with 1 mM phenylmethylsulfonyl fluoride, lysed by sonication, and centrifuged at 17,800 × g for 20 min at 4°C. The supernatant was affinity purified by Ni(II)-NTA agarose (Qiagen, Los Angeles, CA). For glutathione S-transferase (GST)-tagged proteins, frozen cells were resuspended in phosphate-buffered saline (PBS; pH 7.3) with 1 mM dithiothreitol and 1 mM phenylmethylsulfonyl fluoride, lysed by sonication, centrifuged, and affinity purified by glutathione-Sepharose 4B (GE Healthcare Bio-Science). Eluted protein samples were dialyzed against storage buffer (10 mM Tris-HCl [pH 7.6], 10 mM MgCl2, 0.1 mM EDTA, 200 mM KCl, and 50% [vol/vol] glycerol) and stored at −20°C. Protein concentration was determined by using the Bradford method (protein assay kit; Bio-Rad Laboratories, Hercules, CA) using bovine serum albumin as a standard, and the purity was checked by SDS-PAGE.
Autophosphorylation and phosphotransfer assays.
Purified histidine kinases (HKs) were autophosphorylated by incubation at 25°C in 50 mM Tris-HCl (pH 7.5 for VicK and LiaS and pH 8.5 for CiaH), 50 mM KCl, 10 mM MgCl2, 2.5 μM ATP, and 25 nCi of [γ-32P]ATP in a total volume of 10 μl. Furthermore, 50 mM NH4Cl was also added to the reaction mixtures for CiaH and LiaS. The reaction was terminated by adding an SDS-PAGE sample buffer. For phosphotransfer assays, cognate response regulators (RRs) of HKs were added to the autophosphorylated HKs and incubated at 25°C, and the reaction was terminated by the SDS-PAGE sample buffer. These samples were separated by SDS-PAGE. The gel was dried, exposed onto an imaging plate, and analyzed with FLA-7000, a laser scanner optimized for quantitative phosphorimaging (Fuji Film, Tokyo, Japan). Inhibition of the autophosphorylation activity of HKs was determined by adding 1 μl of drug to the autophosphorylation reaction mixture 5 min prior to addition of ATP, incubation at 25°C, and analysis as mentioned above. The signals detected by FLA-7000 were analyzed by MultiGauge (version 3.0; Fuji Film), and Prism 5 (GraphPad Software, La Jolla, CA) was used to calculate 50% inhibitory concentrations (IC50s).
Construction of deletion mutants.
The PCR ligation mutagenesis technique (12) was used for construction of deletion mutants. To delete ciaH and comD genes, specific P1 and P2 primers were used to amplify the 5′ region flanking the target gene, while specific P3 and P4 primers were used for the 3′ region flanking the target gene. Genome DNA of UA159 was used as the template, and PrimeSTAR polymerase was used for amplification. Each amplified fragment was digested with the enzyme AscI (5′ region) or FseI (3′ region) and then ligated to an erythromycin resistance (Emr) cassette, which was PCR amplified with a template pUC18erm and digested with AscI or FseI. Next, using specific P1 or P4 primers and a mixture of ligated 5′ region-Emr cassette and 3′ region-Emr cassette as templates, a second PCR was performed to amplify the 5′ region-Emr cassette-3′ region's fragment. This fragment was transformed into strain UA159, and the recombinants were selected by erythromycin. Nonpolar deletion of vicK and liaS genes was made by basically the same method as in the ciaH and comD mutants, but the 5′ and 3′ regions were digested with BamHI and ligated to a BamHI-digested NPKm cassette from pALH124. Recombinants were selected by kanamycin for these mutants. All of the deletions were confirmed by PCR with P1 and P4 primers, and the loss of expression was determined by quantitative real-time PCR (qRT-PCR).
Extraction of total RNA.
Total RNA was extracted from mid-exponential-phase cells (OD600 = 0.35 to 0.45) grown in a BHI or BM medium. RNA later (Ambion, Austin, TX) was added to the cell pellets immediately after harvest to prevent RNA degradation. The cells were suspended in an SV lysis buffer (SV total RNA isolation system; Promega, Madison, WI) with 0.1-mm glass beads and disrupted by the Multi-Beads Shocker (Yasui Kikai, Osaka, Japan). Total RNA was extracted with the SV total RNA isolation system, followed by DNase I digestion with Turbo DNA-free (Ambion) in order to eliminate the residual contaminating genomic DNA. The purity and concentration of the RNA were determined by spectrophotometry and gel electrophoresis.
qRT-PCR.
First-strand cDNA templates were created from 100 ng of RNA by using a high-capacity cDNA RT kit (ABI) according to the recommended procedure. qRT-PCRs were performed with specific primers, cDNA templates (cDNA from 0.1 ng of RNA/reaction), and SYBR green Master Mix (ABI). PCR amplification, detection, and analysis were performed with the StepOne real-time PCR system (ABI). PCR conditions included an initial denaturation step at 95°C for 10 min, followed by a 40-cycle amplification (95°C for 15 s and 60°C for 60 s). Changes in the levels of gene expression were calculated by using the ΔΔCT method. The 16S rRNA gene was used as the housekeeping gene reference. Each assay was repeated using at least three independent RNA samples.
Biofilm assay.
Biofilm formation using microtiter plates was assessed as described by Ahn et al. (1) with modification. In brief, overnight cultures of S. mutans UA159 and its derivatives were transferred to prewarmed BHI medium and grown at 37°C in 5% CO2 to an OD600 of ∼0.5. The cultures were diluted 1:100 in BM medium supplemented with sucrose, and 200-μl aliquots of the cell suspension were placed into the wells of 96-well, flat-bottom polystyrene microtiter plates (Asahi Glass, Tokyo, Japan). When necessary, WKM C or diluent (50% methanol) was added to the wells at 2 μl/well and mixed thoroughly. Plates were incubated at 37°C in 5% CO2 for 24 h. The microtiter plates were then washed with water twice and air dried. The adherent bacteria were stained with 50 μl of 0.1% crystal violet for 15 min at room temperature, and then the plates were washed with water twice. The bound dye was extracted from the stained cells by adding 200 μl of ethanol-acetone (8:2). Biofilm formation was then quantified by measuring the absorbance of the solution at 595 nm. For biofilm formation in glass test tubes, overnight cultures of S. mutans UA159 and its derivatives were diluted at a 1:100 ratio in BM medium supplemented with 20 mM glucose or sucrose. When necessary, WKM C or diluent were added to the tubes at 30 μl per 3 ml of culture, mixed thoroughly, and grown at 37°C in 5% CO2 for 24 h. The tubes were stood up slanted so that biofilms could form on the sides of the tubes. After incubation, the liquid culture was gently decanted, and the residual biofilms were photographed.
Acid tolerance assay.
Acid tolerance was assessed as described by Biswas et al. (5) with modification. Briefly, 1 μl of WKM C or diluent were added to 5 μl of overnight culture in BHI medium (adjusted to OD600 = 1.0), and 4 μl of the BHI medium. After incubation at 37°C for 1 h, 40 μl of PBS was added to make a 10-fold dilution of the overnight culture. Serial 10-fold dilution of this cell suspension was made in PBS and spotted (5 μl/spot) onto a BHI agar medium adjusted to pH 5.3 or 7.0 with a 50 mM citrate-phosphate buffer. The plates were incubated at 37°C in 5% CO2 for 48 h.
Transformation assays.
The transformation efficiency was measured as described by Ahn et al. (1) with modification. In brief, overnight cultures of S. mutans UA159 and its derivatives in a BHI medium were adjusted to an OD600 of 1.0 and diluted to a 1:20 ratio in a prewarmed BHI medium containing 10% (vol/vol) horse serum. Cells were grown at 37°C in 5% CO2 until the early exponential phase. Then, 1-ml aliquots of the cultures were placed in glass tubes, and 2 μl of synthetic CSP solution (500 μg/ml) was added, and this was incubated at 37°C in 5% CO2 for 20 min to allow induction of competence. Next, 5 or 50 ng of plasmid pDL278 was added to the culture, further incubated at 37°C in 5% CO2 for 90 min; transformants were enumerated by plating cells on BHI agar plates with spectinomycin. Transformation efficiency was calculated as the number of transformants per ng of the added plasmid. For evaluation of WKM C, 200-μl aliquots of the OD600 = 0.2 cultures were placed in glass tubes, 2.5 μl of WKM C or diluent was added, and the tubes were incubated at 37°C for 10 min before the addition of CSP (2 μl of 100 μg/ml). The transformation efficiency was determined as described above, but only 1 ng of pDL278 was added in this case.
RESULTS AND DISCUSSION
Antimicrobial activity of WKM C.
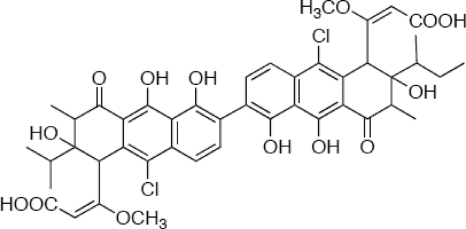
Walkmycin C is an HK inhibitor isolated from the screening of Streptomyces sp. by a differential growth assay (24, 34). From methanol extracts of Streptomyces sp. strain MK632-100F11, three active fractions, i.e., walkmycins A, B, and C, were detected (24). Although all three compounds possessed in vitro inhibitory activity against the purified cytoplasmic region of WalK HK of B. subtilis, we selected walkmycin C (WKM C, Fig. 1) for the present study due to its relatively good solubility in 50% methanol. Since the walkmycins are not stable in dimethyl sulfoxide and acetone, 50% methanol was used as the diluent throughout the present study.
FIG. 1.
Structure of WKM C.
WKM C showed antimicrobial activity (Table 2) against all of the Gram-positive bacteria tested with MIC values ranging from 0.0625 to 16 μg/ml. All of these strains except Micrococcus and Corynebacterium are known to possess the WalK/WalR TCS, which is essential for growth. On the other hand, WKM C did not show antimicrobial activity against any of the Gram-negative bacteria tested. These strains do not have any TCSs that are essential for growth. Thus, the antimicrobial activity of WKM C against Gram-positive strains may be attributed to the inhibition of the essential WalK/WalR. The antimicrobial activity of WKM C against Micrococcus and Corynebacterium suggest the presence of essential TCSs in these strains.
TABLE 2.
Antimicrobial activity of WKM C
| Test organism | MIC (μg/ml) |
|---|---|
| Gram-positive organisms | |
| Staphylococcus aureus FDA 209P | 0.06 |
| Staphylococcus aureus Smith | 0.25 |
| Staphylococcus aureus MS9610 | 0.06 |
| Staphylococcus aureus MRSA No. 5 | 0.13 |
| Staphylococcus aureus MRSA No. 17 | 0.25 |
| Micrococcus luteus FDA 16 | 0.06 |
| Micrococcus luteus IFO 3333 | 0.25 |
| Bacillus subtilis NRRL B-558 | 1 |
| Bacillus subtilis PCI 219 | 0.06 |
| Bacillus subtilis 168 | 1 |
| Bacillus cereus ATCC 10702 | 0.5 |
| Corynebacterium bovis 1810 | 8 |
| Enterococcus faecalis JCM 5803 | 8 |
| Enterococcus faecalis NCTC12201 | 2 |
| Enterococcus faecium JCM 5804 | 16 |
| Enterococcus faecium NCTC12202 | 8 |
| Streptococcus pneumoniae TY-5708 | 0.13 |
| Streptococcus pneumoniae TY-5745 | 0.13 |
| Streptococcus pneumoniae TY-5834 | 0.25 |
| Streptococcus pyogenes TY-5727 | 1 |
| Streptococcus pyogenes TY-5740 | 0.25 |
| Streptococcus pyogenes TY-5914 | 0.25 |
| Gram-negative organisms | |
| Escherichia coli NIHJ | >128 |
| Escherichia coli K-12 | >128 |
| Shigella dysenteriae J S11910 | >128 |
| Salmonella enterica serovar Enteritidis 1891 | >128 |
| Proteus vulgaris OX19 | >128 |
| Proteus mirabilis IFM OM-9 | >128 |
| Serratia marcescens | >128 |
| Pseudomonas aeruginosa A3 | >64 |
| Klebsiella pneumoniae PCI 602 | >128 |
Autophosphorylation and phosphotransfer activity of VicK, CiaH, and LiaS.
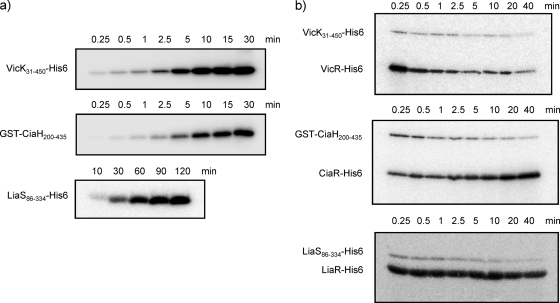
Of the 14 TCSs in S. mutans, the VicK/VicR (28-30), CiaH/CiaR (1, 26), LiaS/LiaR (HK11/RR11) (6, 17, 31), and ComD/ComE (4, 18) systems are among the TCSs related to pathogenicity and stress response (5, 15). In order to evaluate the inhibitory activity of WKM C against VicK, CiaH, and LiaS HKs of S. mutans, we first cloned vicRK, ciaRH, and liaSR from the genome DNA of strain UA159. Then, the cytoplasmic regions of vicK, ciaH, and liaS were fused to affinity tags (His tag for VicK and LiaS, GST tag for CiaH) and expressed, and the proteins were purified. We also constructed and expressed His-tagged CiaH, but only in an insoluble state. Attempts to express soluble His-tagged and GST-tagged ComD also failed. The three response regulators, VicR, CiaR, and LiaR, were fused to His tags, expressed, and purified. Figure 2 a shows the autophosphorylation of VicK31-450-His6, GST-CiaH200-435, and LiaS86-334-His6. Although 0.5 μM VicK and CiaH rapidly autophosphorylated, the concentration of LiaS had to be increased to 12.5 μM in order to obtain distinct autophosphorylated bands.
FIG. 2.
Autophosphorylation and phosphotransfer of VicK, CiaH, and LiaS. (a) Autophosphorylation of HKs. The purified cytoplasmic region of each HK was incubated with [γ-32P]ATP, subjected to SDS-PAGE, and analyzed by autoradiography. (b) Phosphotransfer from HK to its cognate RR. Each HK was autophosphorylated (VicK and CiaH for 10 min, LiaS for 60 min) prior to the addition of its cognate RR. After incubation, the reaction mixture was subjected to SDS-PAGE and analyzed by autoradiography.
Next, the cognate response regulators were added to the autophosphorylated HKs in order to confirm that the phosphorylated bands obtained in Fig. 2a were the HKs in concern. As shown in Fig. 2b, VicR, CiaR, and LiaR were all phosphorylated by their cognate HKs, confirming that the purified sensors were indeed VicK, CiaH, and LiaS. Phosphotransfer from the autophosphorylated HKs to noncognate RRs was also checked for all possible combinations, and no distinct phosphotransfer was observed (data not shown). Compared to autophosphorylation, the phosphotransfer reaction proceeded very rapidly, and the phosphorylated response regulator bands appeared as early as 15 s after the addition of the response regulators to the autophosphorylated HKs.
Inhibitory effect of WKM C against S. mutans HKs.
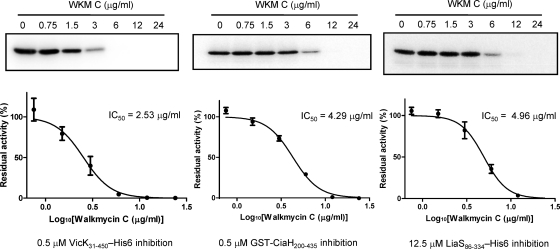
The inhibitory effect of WKM C against the autophosphorylation activity of the cytoplasmic regions of VicK, CiaH, and LiaS was analyzed. WKM C or an equivalent volume of 50% methanol as the diluent was incubated with the HKs for 5 min at 25°C before initiating the autophosphorylation reaction. As shown in Fig. 3, WKM C showed IC50s of 2.53, 4.29, and 4.96 μg/ml (2.87, 4.87, and 5.63 μM) against VicK, CiaH, and LiaS, respectively. WKM C also inhibited the autophosphorylation activity of both EnvZ and PhoQ HKs of E. coli with an IC50 of 1.1 μg/ml (data not shown). Since WKM C inhibited the activity of five HKs (VicK, CiaH, LiaS, EnvZ, and PhoQ) in addition to WalK HKs of B. subtilis and S. aureus (24), we suggest that this compound is a broad-acting general inhibitor of HK.
FIG. 3.
WKM C inhibits autophosphorylation activity of VicK, CiaH, and LiaS. Each HK was treated with WKM C for 5 min before the autophosphorylation reaction was initiated. The residual activity was calculated as the percentage of autophosphorylation activity after treatment with WKM C against the diluent-treated (50% methanol) control (WKM C, 0 μg/ml). Error bars represent the standard errors of the mean (SEM) of three independent assays.
Although the WalK of B. subtilis is an essential HK for cell growth, the VicKs of S. mutans, as well as that of Streptococcus pneumonia, are not essential (36), and the gene encoding vicK can thus be deleted. In fact, there is no essential HK in S. mutans, and each deletion mutant of every HK has been constructed and characterized (5, 15). However, VicR, the cognate response regulator of VicK, is essential for cell proliferation and thus cannot be deleted. The viability of the vicK mutant is explained by VicR being phosphorylated by other HKs, a serine-threonine kinase, or by small phospho-donors such as acetylphosphate (3, 22). When grown in a BM medium and a BHI medium, WKM C showed MICs of 2.5 and 6.5 μg/ml against S. mutans UA159, respectively. This antimicrobial activity may be due to the inhibition of VicK and other HKs, resulting in the inhibition of VicR phosphorylation.
Effect of WKM C against biofilm formation and cell morphology.
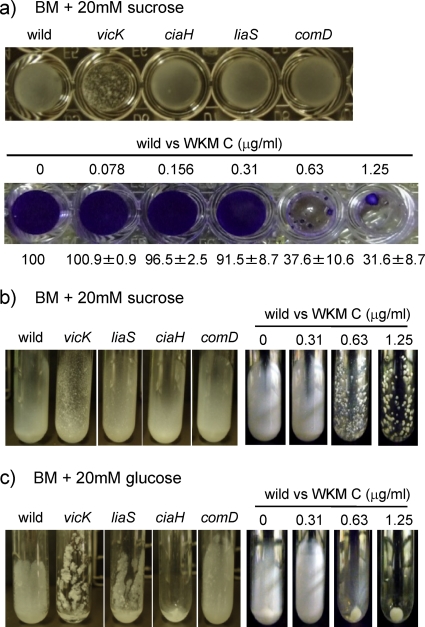
The VicK/VicR TCS is involved in regulation of sucrose-dependent biofilm formation (29), which is one of the major virulence factors in S. mutans. VicK and VicR are orthologues of the B. subtilis WalK and WalR with 45 and 71% identity and 67 and 81% similarity in amino acid sequence, respectively. Since WKM C was isolated as a WalK inhibitor, the inhibitory effect of sub-MIC levels of WKM C was analyzed against sucrose-dependent biofilm formation in a microtiter plate. As shown in Fig. 4 a, 0.63 and 1.25 μg of WKM C/ml caused aggregation of UA159 cells, inhibiting the formation of the smooth biofilms seen in the cells without WKM C treatment. This was accompanied with a reduction in biofilm quantity. When compared to the four HK mutants of UA159 (Fig. 4a), the WKM C-treated cells showed biofilm inhibition similar to that of the vicK mutant. The biofilms of three other mutants, liaS, ciaH, and comD, were as smooth as that of the wild-type strain. The same results were obtained when sucrose-dependent biofilms were formed on glass test tubes (Fig. 4b). Effect of WKM C on sucrose-independent biofilm formation was evaluated using glass test tubes because biofilms formed in the absence of sucrose easily detached from the wells of microtiter plates, and it was easier to compare the biofilms of WKM C-treated cells with the HK mutants in test tubes than microtiter plates. In the absence of sucrose, the addition of WKM C at 0.63 and 1.25 μg/ml caused sedimentation of cells, indicating loose attachment to the glass wall of the test tube (Fig. 4c). Among the HK mutants, this phenotype was observed in the ciaH mutant. In real-time monitoring of S. mutans biofilm formation by Tam et al. (32), deletion of ciaH affected bacterial attachment, and they proposed that ciaH had functions related to the initial and reversible attachment processes. From our results of the sucrose-independent biofilm formation, the CiaH HK seemed to be regulating the attachment of cells to the glass surface, and WKM C was inhibiting it.
FIG. 4.
Sucrose-dependent (a and b) and sucrose-independent (c) biofilm formation of HK mutants and WKM C-treated cells. (a) Cells were grown in microtiter plates in 5% CO2 atmosphere for 24 h. The upper panel shows biofilms of HK mutants after removal of BM medium. The lower panel shows crystal violet-stained biofilms of WKM C-treated cells. The numbers below the lower panel indicate biofilm quantity values (%), representing relative amount of biofilm calculated from the amount of crystal violet (averages ± the SEM, n = 5). (b and c) Cells were grown in slanted glass test tubes in 5% CO2 atmosphere for 24 h. Biofilms formed on the bottom side of the test tubes. Photos show the residual biofilms after gently decanting the liquid medium.
We next observed how WKM C affects the cell morphology of S. mutans UA159. When 2 μg of WKM C/ml was added to exponential-phase wild-type cells and incubated for an hour, long chains consisting of 20 to 30 cells were observed by confocal laser scanning microscopy (data not shown). In contrast, the control cells with only diluent addition formed short chains of about 5 to 10 cells per chain. The ciaH mutant formed short chains as in the wild-type strain, but the vicK mutant formed long chains as in the WKM C-treated cells. These results suggest that WKM C inhibited the VicK activity in vivo, judging from its effect on cell morphology.
Expression of VicK-regulated genes.
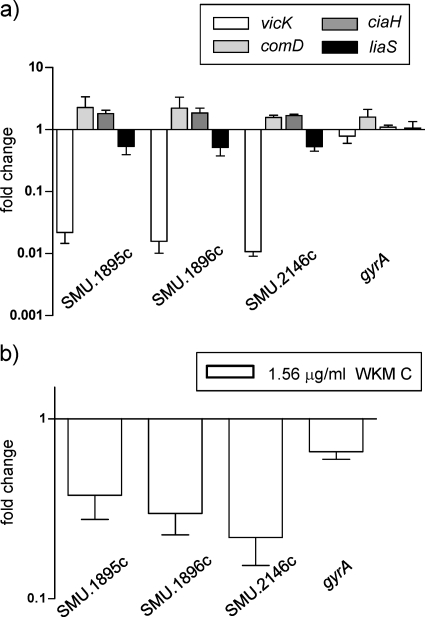
To further confirm the inhibition of VicK by WKM C in vivo, the expression of putative VicK-regulated genes was measured by using qRT-PCR (Fig. 5). A transcriptome analysis of a vicK mutant grown at pH 5.5 by Senadheera et al. (28) revealed that SMU.1895c, SMU.1896c, and SMU.2146c were among the most highly downregulated genes compared to the wild-type strain. SMU.1895c and SMU.1896c encode putative bacteriocins (33). SMU.2146c encodes an amino acid sequence that shows similarity to those of transglycosylases involved in cell wall remodeling (3). Expression of SMU.1895c, SMU.1896c, and SMU.2146c in our vicK mutant also decreased to 0.022-, 0.016-, and 0.011-fold of the wild-type strain (Fig. 5a). Expression of SMU.1895c and SMU.1896c in the comD mutant increased to 2.3- and 2.2-fold, but that of SMU.2146c did not change much. No changes >2-fold in the expression of the three genes were found in the ciaH and liaS mutants. When cells were treated with 1.56 μg of WKM C/ml (0.25× MIC), expression of SMU.1895c, SMU.1896c, and SMU.2146c decreased to 0.38-, 0.30-, and 0.22-fold of the diluents-treated cells (Fig. 5b), indicating the inhibitory effect of WKM C against VicK. Since the decrease of expression by WKM C was not as drastic as that observed in the vicK mutant, the WKM C level may not have been sufficient to completely inhibit the VicK activity.
FIG. 5.
Expression of VicK-regulated genes by qRT-PCR. (a) Expression in HK mutants relative to that in the wild-type strain (UA159). (b) Expression in WKM C (1.56 μg/ml)-treated cells relative to that in control cells (UA159). WKM C was added from the beginning of the culture, and cells were harvested in the mid-exponential phase. Error bars represent the SEM of at least three biological repeats.
Since SMU.2146c is predicted to be involved in cell wall remodeling, the decrease in the SMU.2146c level may have caused failure in cell separation and led to the formation of long chains in the vicK mutant and WKM C-treated cells. On the other hand, in addition to SMU.1895c and SMU.1896c, WKM C treatment also decreased expression of SMU.1342, encoding a putative bacitracin synthetase 1 (BacA) (data not shown). Decreased expression of these genes may cause reduced bacteriocin production, resulting in weaker competition against other oral microorganisms.
Expression of sucrose-dependent biofilm related genes.
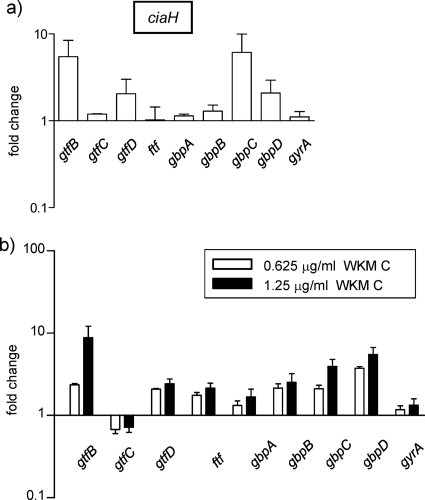
Glucosyltransferases (GtfB, C, and D), a fructosyltransferase (Ftf), and glucan-binding proteins (GbpA, B, C, and D) are known to be involved in the formation of sucrose-dependent biofilms (16, 21, 25). Glucosyltransferases and the fructosyltransferase synthesize glucan and fructan polymers from sucrose. Adhesive glucans mediate the attachment of bacteria to the tooth surface, as well as to other bacteria, and fructans act as binding sites for bacterial accumulation. Since the vicK mutant and WKM C-treated cells formed abnormal sucrose-dependent biofilms (Fig. 4a and b), expression of the related genes was investigated in mid-exponential-phase cells in a BM medium supplemented with 20 mM sucrose. Expression of gtfD, ftf, and gbpB in the vicK mutant was repressed to 0.40-, 0.28-, and 0.29-fold, and expression of gtfB and gtfC increased to 2.2- and 2.1-fold of the wild-type strain. The decrease in ftf and gbpB expression is consistent with the results of Senadheera et al. (29). Although the liaS and comD mutants did not show differences greater than 2-fold in expression, the ciaH mutant showed enhanced expression of gtfB, gtfD, gbpC, and gbpD, which was 5.5-, 2.1-, 5.5-, and 2.1-fold of the wild-type strain (Fig. 6a). Enhanced expression of gtfB and gbpC in a ciaH mutant has also been shown in a recent microarray study by Wu et al. (37). When 1.25 μg of WKM C/ml was added to mid-exponential-phase cells grown in a BM medium supplemented with 20 mM sucrose and incubated for 60 min before cell harvest, expression of gtfB, gbpC, and gbpD increased to 8.9-, 3.9-, and 5.5-fold of the control cells (Fig. 6b). Increased expression of gtfD, ftf, and gbpA was also seen but to a lower extent (2.4-, 2.1-, and 2.5-fold). The expression pattern of these genes in WKM C-treated cells is similar to the expression pattern found in the ciaH mutant, except for the increases in ftf and gbpA expressions. These increases may be due to HKs other than the four HKs studied here. An expression study of genes related to sucrose-dependent biofilm formation was also performed using a BM medium supplemented with 20 mM glucose, and results similar to those in Fig. 6 were obtained (data not shown). Together with the effect on biofilm formation in the 20 mM glucose-supplemented BM medium (Fig. 4c), it was suggested that CiaH, in addition to VicK, was inhibited by WKM C in vivo.
FIG. 6.
(a and b) Expression of genes related to sucrose-dependent biofilm in the ciaH mutant relative to that in the wild-type strain (UA159) (a) and in WKM C-treated cells relative to that in the control cells (b). WKM C was added to mid-exponential-phase cells in a BM medium supplemented with 20 mM sucrose and incubated at 37°C under 5% CO2 for 60 min before cell harvesting. Error bars represent the SEM of at least three biological repeats.
Effect of WKM C against acid tolerance.
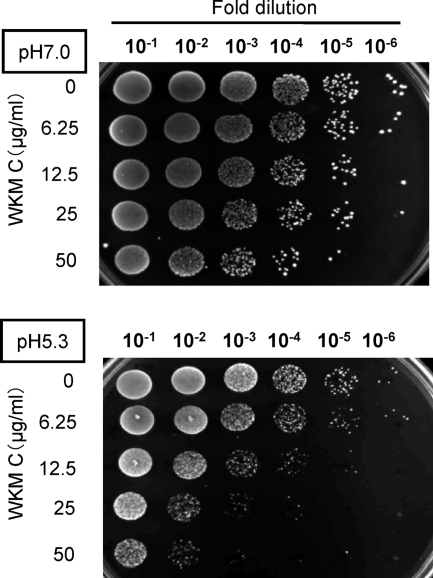
The CiaH/CiaR system is present in all sequenced streptococcal genomes but not in other Firmicutes (11). In S. mutans, CiaH/CiaR is involved in oxidative stress, acid stress, thermal stress, DNA damage, and mutacin production (5). In particular, the CiaH sensor was the only sensor involved in response to acid stress, when 14 sensor mutants were investigated by Biswas et al. (5). Since WKM C seemed to inhibit the activity of CiaH from our results of biofilm formation and expression studies, we investigated the effect of WKM C against acid tolerance in S. mutans. The wild-type strain and its four HK mutants were spotted onto BHI agar plates with pH adjusted to 7.0 and 5.3. Although the wild-type strain and vicK, liaS, and comD mutants did not show any difference in the number of colonies between pH 7.0 and 5.3, no colonies of the ciaH mutant appeared on the pH 5.3 plate, confirming that CiaH was involved in the response to acid stress (data not shown). When UA159 cells were treated with WKM C for 1 h prior to serial dilution by PBS and spotted onto pH 7.0 and 5.3 agar plates, the number of colonies on the pH 5.3 plate began to decrease from 12.5 μg/ml (equivalent to 2× MIC) WKM C (Fig. 7). To confirm that this inhibitory effect of WKM C was not found in conventional antibiotics, the same experiment was performed against kanamycin and erythromycin at concentrations up to 2.5 mg/ml and 16 μg/ml (equivalent to 10× MIC for kanamycin and 160× MIC for erythromycin). No decrease in acid tolerance was observed with these antibiotics (data not shown). Thus, WkmC repressed acid tolerance, another virulence factor of S. mutans, and this effect appeared to be caused by in vivo inhibition of CiaH.
FIG. 7.
Acid tolerance of WKM C-treated cells. WKM C or diluent was added to overnight-grown cultures of the wild-type cells, incubated at 37°C for 1 h, serially diluted, and spotted onto pH 5.3 and 7.0 agar plates. The plates were photographed after 48 h of incubation.
Effect of WKM C against transformation efficiency.
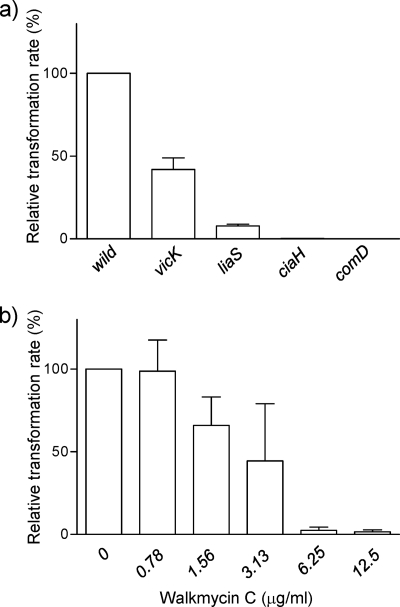
Another phenotype of the ciaH mutant is its defect in genetic competence. In S. mutans, ComD/ComE and CiaH/CiaR are the major TCSs related to the development of competence (1). When the competent stimulating peptide (CSP)-induced transformation efficiencies of the vicK, liaS, ciaH, and comD mutants were compared to that of the wild-type strain (Fig. 8 a), the relative transformation rates of the ciaH and comD mutants dropped to 0.12 and 0.03%. Although not as conspicuous as the ciaH and comD mutants, decreases in transformation efficiency were also observed in the liaS and vicK mutants, with relative transformation rates of 5.9 and 32.0%. This result showed that the LiaS/LiaR and VicK/VicR systems were also related to competence development, but to a lower extent compared to ComD/ComE and CiaH/CiaR. To evaluate the effect of WKM C against transformation efficiency, early-exponential-phase wild-type cells were incubated with WKM C at 37°C for 10 min prior to CSP addition and subjected to transformation with pDL278. Compared to the diluent treated cells, the transformation rate decreased in cells treated with WKM C at concentrations of 1.56 μg/ml and higher. Relative transformation rates were 2.4 and 1.6% for 6.25 and 12.5 μg of WKM C-treated cells/ml, which clearly indicated that WKM C inhibited the CSP-induced competence development (Fig. 8b). Since the MIC of WKM C against S. mutans in a BHI medium was 6.25 μg/ml, we investigated whether pretreatment with higher concentrations of WKM C inhibited cell growth. Cells were pretreated with WKM C, induced with CSP, and transformed with pDL278 as in the transformation assay, and then these cells were serially diluted and spotted onto BHI agar plates without antibiotics. No difference in cell viability was observed between diluent-treated and WKM C-treated cells (data not shown), indicating that WKM C was diluted during the serial dilution to a concentration that did not affect cell viability.
FIG. 8.
Transformation efficiency of HK mutants and WKM C-treated cells. (a) CSP at a final concentration of 1 μg/ml was added to early-exponential-phase cultures of HK mutants and wild-type cells, followed by incubation for 20 min to allow induction of competence. Plasmid pDL278 was added to the culture, followed by incubation for 90 min, and transformants were selected by spectinomycin resistance. The relative transformation rate was calculated as the percentage of transformation efficiency of HK mutants against that of the wild-type strain. (b) WKM C or diluent was added to early-exponential-phase cultures of the wild-type strain, followed by incubation at 37°C for 10 min before the addition of CSP and transformation with pDL278. The relative transformation rate was calculated as the percent transformation efficiency of WKM C-treated cells against that of the diluents-treated control. Error bars represent the SEM of at least three biological repeats.
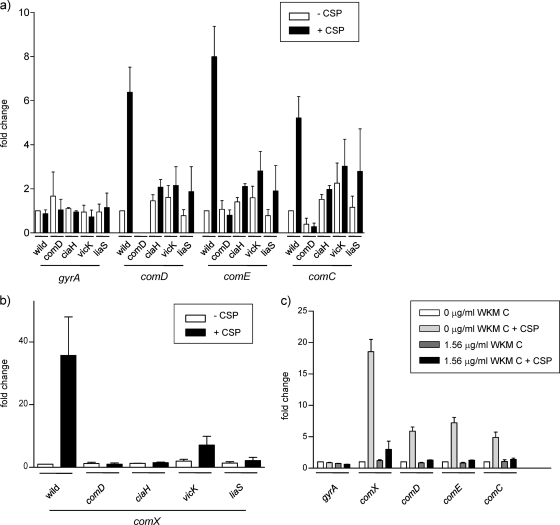
Expression of CSP-induced genes was also analyzed. When CSP was added to early-exponential-phase wild-type cells, and the cells were harvested at mid-exponential phase, expression of comD, comE, comC, and comX was induced to 6.4-, 8.0-, 5.2-, and 35.7-fold of cells without CSP addition (Fig. 9 a and b). However, the addition of CSP to the HK mutants did not cause significant induction of CSP-induced genes, except for the 3.6-fold increase of comX in the vicK mutant. Our results indicate that VicK and LiaS HKs are also involved in the regulation of CSP-induced genes, which agrees with the transformation results (Fig. 8a). Furthermore, wild-type cells were treated with WKM C, and the expression of their CSP-induced genes was analyzed. As shown in Fig. 9c, WKM C completely repressed the induction of comD, comE, and comC by CSP. Induction of comX by CSP was repressed to 2.5-fold of the WKM C-treated cells without CSP addition, whereas comX was induced 18.6-fold by CSP in cells without WKM C treatment. These results indicate that the decrease in CSP-induced transformation by WKM C is due to the repressed expression of the competent-related genes and that this repression is caused by the inhibition of HK activity by WKM C.
FIG. 9.
Expression of CSP-induced genes. (a and b) CSP was added at a final concentration of 1 μg/ml to wild-type cells and its derivatives in early-exponential-phase cells for induction of CSP-induced genes. Cells were harvested in the mid-exponential phase, and the expression of the genes was determined. Note that the ranges of the y axes of panels a and b are different. (c) Expression of CSP-induced genes in WKM C-treated cells relative to that in the control cells. WKM C or diluent was added from the beginning of the culture, and CSP was added in the early exponential phase. Cells were harvested in the mid-exponential phase. Error bars represent the SEM of at least three biological repeats.
Concluding remarks.
Previous studies on general HK inhibitors that target the cytoplasmic catalytic domains of HKs, rather than the sensory domain, have emphasized their antimicrobial potential. WKM C was also first discovered as an inhibitor of the WalK HKs of B. subtilis in an attempt to develop antimicrobial agents. In the present study, we evaluated WKM C against the virulence factors of S. mutans and found that sub-MIC levels of WKM C repressed biofilm formation, acid tolerance, and competence. From a comparison with HK mutants, our results suggest that treatment of S. mutans with WKM C repressed the activity of at least VicK and CiaH HKs. Whether other HKs of S. mutans were also repressed remains unclear and thus opens question for further investigation. In addition to S. mutans HKs, WKM C inhibits the autophosphorylation activity of HKs from B. subtilis, S. aureus, and E. coli. This opens the possibility of WKM C repressing the virulence of other pathogens. Although not as frequent as inducing virulence, TCSs of pathogens sometimes repress virulence. This raises concerns of a general HK inhibitor enhancing virulence in such cases. Furthermore, many beneficial commensal bacteria have these same TCSs, and these TCSs appear to play an equally important role in their growth and homeostasis. Therefore, HK inhibitors for certain diseases may have adverse consequences. Accordingly, general HK inhibitors should be carefully evaluated against various pathogens and also against beneficial bacteria, before their application as drugs that can attenuate the virulence of pathogens.
Acknowledgments
We thank Robert A. Burne and Sang-Joon Ahn for the generous gift of plasmids pUC18Em, pALH124, and pDL278. We also thank Kunio Inoue for determining the MICs and Noriko Kojima for technical support.
This study was supported by the Research and Development Program for Bio-Industry Initiatives (2006-2010) of the Bio-Oriented Technology Research Advancement Institution (BRAIN) and a Grant-in-Aid for Scientific Research (A, 20248012) of the Japan Society for the Promotion of Science.
Footnotes
Published ahead of print on 31 January 2011.
REFERENCES
- 1.Ahn, S. J., Z. T. Wen, and R. A. Burne. 2006. Multilevel control of competence development and stress tolerance in Streptococcus mutans UA159. Infect. Immun. 74:1631-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ajdic, D., et al. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. U. S. A. 99:14434-14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banu, L. D., et al. 2010. The Streptococcus mutans serine/threonine kinase, PknB, regulates competence development, bacteriocin production, and cell wall metabolism. Infect. Immun. 78:2209-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhagwat, S. P., J. Nary, and R. A. Burne. 2001. Effects of mutating putative two-component systems on biofilm formation by Streptococcus mutans UA159. FEMS Microbiol. Lett. 205:225-230. [DOI] [PubMed] [Google Scholar]
- 5.Biswas, I., L. Drake, D. Erkina, and S. Biswas. 2008. Involvement of sensor kinases in the stress tolerance response of Streptococcus mutans. J. Bacteriol. 190:68-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chong, P., L. Drake, and I. Biswas. 2008. LiaS regulates virulence factor expression in Streptococcus mutans. Infect. Immun. 76:3093-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 8th ed. CLSI document M07-A8, vol. 29, no. 2. CLSI, Wayne, PA.
- 8.Dubrac, S., I. G. Boneca, O. Poupel, and T. Msadek. 2007. New insights into the WalK/WalR (YycG/YycF) essential signal transduction pathway reveal a major role in controlling cell wall metabolism and biofilm formation in Staphylococcus aureus. J. Bacteriol. 189:8257-8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gotoh, Y., et al. 2010. Two-component signal transduction as potential drug targets in pathogenic bacteria. Curr. Opin. Microbiol. 13:232-239. [DOI] [PubMed] [Google Scholar]
- 10.Idone, V., et al. 2003. Effect of an orphan response regulator on Streptococcus mutans sucrose-dependent adherence and cariogenesis. Infect. Immun. 71:4351-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jordan, S., M. I. Hutchings, and T. Mascher. 2008. Cell envelope stress response in Gram-positive bacteria. FEMS Microbiol. Rev. 32:107-146. [DOI] [PubMed] [Google Scholar]
- 12.Lau, P. C., C. K. Sung, J. H. Lee, D. A. Morrison, and D. G. Cvitkovitch. 2002. PCR ligation mutagenesis in transformable streptococci: application and efficiency. J. Microbiol. Methods 49:193-205. [DOI] [PubMed] [Google Scholar]
- 13.LeBlanc, D. J., L. N. Lee, and A. Abu-Al-Jaibat. 1992. Molecular, genetic, and functional analysis of the basic replicon of pVA380-1, a plasmid of oral streptococcal origin. Plasmid 28:130-145. [DOI] [PubMed] [Google Scholar]
- 14.Lemos, J. A., and R. A. Burne. 2008. A model of efficiency: stress tolerance by Streptococcus mutans. Microbiology 154:3247-3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levesque, C. M., et al. 2007. Systemic inactivation and phenotypic characterization of two-component systems in expression of Streptococcus mutans virulence properties. Lett. Appl. Microbiol. 45:398-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li, Y., and R. A. Burne. 2001. Regulation of the gtfBC and ftf genes of Streptococcus mutans in biofilms in response to pH and carbohydrate. Microbiology 147:2841-2848. [DOI] [PubMed] [Google Scholar]
- 17.Li, Y. H., et al. 2002. Novel two-component regulatory system involved in biofilm formation and acid resistance in Streptococcus mutans. J. Bacteriol. 184:6333-6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, Y. H., et al. 2002. A quorum-sensing signaling system essential for genetic competence in Streptococcus mutans is involved in biofilm formation. J. Bacteriol. 184:2699-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, Y. H., X. L. Tian, G. Layton, C. Norgaard, and G. Sisson. 2008. Additive attenuation of virulence and cariogenic potential of Streptococcus mutans by simultaneous inactivation of the ComCDE quorum-sensing system and HK/RR11 two-component regulatory system. Microbiology 154:3256-3265. [DOI] [PubMed] [Google Scholar]
- 20.Loo, C. Y., D. A. Corliss, and N. Ganeshkumar. 2000. Streptococcus gordonii biofilm formation: identification of genes that code for biofilm phenotypes. J. Bacteriol. 182:1374-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsumoto-Nakano, M., K. Fujita, and T. Ooshima. 2007. Comparison of glucan-binding proteins in cariogenicity of Streptococcus mutans. Oral Microbiol. Immunol. 22:30-35. [DOI] [PubMed] [Google Scholar]
- 22.Ng, W. L., H. C. Tsui, and M. E. Winkler. 2005. Regulation of the pspA virulence factor and essential pcsB murein biosynthetic genes by the phosphorylated VicR (YycF) response regulator in Streptococcus pneumoniae. J. Bacteriol. 187:7444-7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okada, A., et al. 2007. Targeting two-component signal transduction: a novel drug discovery system. Methods Enzymol. 422:386-395. [DOI] [PubMed] [Google Scholar]
- 24.Okada, A., et al. 2010. Walkmycin B targets WalK (YycG), a histidine kinase essential for bacterial cell growth. J. Antibiot. (Tokyo) 63:89-94. [DOI] [PubMed] [Google Scholar]
- 25.Ooshima, T., et al. 2001. Contributions of three glycosyltransferases to sucrose-dependent adherence of Streptococcus mutans. J. Dent. Res. 80:1672-1677. [DOI] [PubMed] [Google Scholar]
- 26.Qi, F., J. Merritt, R. Lux, and W. Shi. 2004. Inactivation of the ciaH gene in Streptococcus mutans diminishes mutacin production and competence development, alters sucrose-dependent biofilm formation, and reduces stress tolerance. Infect. Immun. 72:4895-4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin, Z., et al. 2006. Structure-based discovery of inhibitors of the YycG histidine kinase: new chemical leads to combat Staphylococcus epidermidis infections. BMC Microbiol. 6:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Senadheera, D., et al. 2009. Inactivation of VicK affects acid production and acid survival of Streptococcus mutans. J. Bacteriol. 191:6415-6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Senadheera, M. D., et al. 2005. A VicRK signal transduction system in Streptococcus mutans affects gtfBCD, gbpB, and ftf expression, biofilm formation, and genetic competence development. J. Bacteriol. 187:4064-4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Senadheera, M. D., et al. 2007. The Streptococcus mutans vicX gene product modulates gtfB/C expression, biofilm formation, genetic competence, and oxidative stress tolerance. J. Bacteriol. 189:1451-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suntharalingam, P., M. D. Senadheera, R. W. Mair, C. M. Levesque, and D. G. Cvitkovitch. 2009. The LiaFSR system regulates the cell envelope stress response in Streptococcus mutans. J. Bacteriol. 191:2973-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tam, K., et al. 2007. Real-time monitoring of Streptococcus mutans biofilm formation using a quartz crystal microbalance. Caries Res. 41:474-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Ploeg, J. R. 2005. Regulation of bacteriocin production in Streptococcus mutans by the quorum-sensing system required for development of genetic competence. J. Bacteriol. 187:3980-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watanabe, T., et al. 2003. Isolation and characterization of inhibitors of the essential histidine kinase, YycG in Bacillus subtilis and Staphylococcus aureus. J. Antibiot. (Tokyo) 56:1045-1052. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe, T., A. Okada, Y. Gotoh, and R. Utsumi. 2008. Inhibitors targeting two-component signal transduction. Adv. Exp. Med. Biol. 631:229-236. [DOI] [PubMed] [Google Scholar]
- 36.Winkler, M. E., and J. A. Hoch. 2008. Essentiality, bypass, and targeting of the YycFG (VicRK) two-component regulatory system in gram-positive bacteria. J. Bacteriol. 190:2645-2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu, C., et al. 2010. Regulation of ciaXRH operon expression and identification of the CiaR regulon in Streptococcus mutans. J. Bacteriol. 192:4669-4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamamoto, K., et al. 2001. Antibacterial agents that inhibit histidine protein kinase YycG of Bacillus subtilis. Biosci. Biotechnol. Biochem. 65:2306-2310. [DOI] [PubMed] [Google Scholar]