Abstract
NAI-107 is a novel lantibiotic active against Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA), glycopeptide-intermediate S. aureus (GISA), and vancomycin-resistant enterococci (VRE). The aim of this study was to evaluate the in vivo efficacy of NAI-107 in animal models of severe infection. In acute lethal infections induced with a penicillin-intermediate Streptococcus pneumoniae strain in immunocompetent mice, or with MRSA, GISA, and VRE strains in neutropenic mice, the 50% effective dose (ED50) values of NAI-107 were comparable or lower than those of reference compounds, irrespective of the strain and immune status (0.51 to 14.2 mg/kg of body weight for intravenous [i.v.] NAI-107, 5.1 to 22.4 for oral linezolid, and 22.4 for subcutaneous [s.c.] vancomycin). In the granuloma pouch model induced in rats with a MRSA strain, intravenous NAI-107 showed a dose-proportional bactericidal activity that, at a single 40-mg/kg dose, compared with 2 20-mg/kg doses at a 12-h or 24-h interval, caused a 3-log10-CFU/ml reduction of viable MRSA in exudates that persisted for more than 72 h. Rat endocarditis was induced with a MRSA strain and treated for five consecutive days. In a first experiment, using 5, 10, or 20 mg/kg/day, and in a second experiment, when 10 mg/kg at 12-h intervals was compared to 20 mg/kg/day, intravenous NAI-107 was effective in reducing the bacterial load in heart vegetations in a dose-proportional manner. Trough plasma levels, as determined on days 2 and 5, were several times higher than the NAI-107 minimal bactericidal concentration (MBC). NAI-107 binding to rat and human serum ranges between 93% and 98.6%. The rapid bactericidal activity of NAI-107 observed in vitro was thus confirmed by the efficacy in several models of experimental infection induced by Gram-positive pathogens, supporting further investigation of the compound.
The emergence of antibiotic-resistant organisms is a growing concern in both hospital and community settings (13). About 50% of pneumococci express some level of penicillin resistance, 50% of hospital-associated Staphylococcus aureus strains are methicillin resistant (MRSA), and about 30% of enterococci are vancomycin resistant (VRE). MRSA alone infects more than 94,000 people and kills nearly 19,000 in the United States every year, more deaths than are caused by HIV/AIDS, Parkinson's disease, emphysema, and homicide combined (2). The prevalence of MRSA changes geographically but is constantly increasing and is often associated with resistance to other antibiotics (22). VRE, traditionally regarded as low-grade pathogens, have emerged as an increasingly important cause of nosocomial infections in the last decade and are now considered a common problem among patients receiving hematopoietic stem cell transplants (5) and in VRE-colonized febrile neutropenic cancer patients, where the incidence of VRE infection can be as high as 38% (21). The continuous increase of drug-resistant infections is a global concern, and the World Health Organization has identified antimicrobial resistance as one of the three greatest threats to human health. The paucity of new therapeutic approaches to fight antibiotic resistance must be attacked by joined resources, as recently stated by the Infectious Diseases Society of America (IDSA) with the commitment to develop 10 new antibacterial drugs by 2020 (15).
Lantibiotics, which are ribosomally synthesized peptides that undergo posttranslational modifications to yield the active structures containing the typical thioether-linked (methyl)lanthionine residues, are produced mostly by strains belonging to the Firmicutes and, to a lesser extent, to the Actinobacteria. Because lantibiotics bind to lipid II at a site different from that affected by vancomycin and related glycopeptides, they are active against drug-resistant Gram-positive pathogens and have attracted attention as potential drug candidates (4, 10).
NAI-107 is a recently described lantibiotic (7) active against multidrug-resistant (MDR) Gram-positive pathogens, including MRSA, VRE, and penicillin-resistant Streptococcus pneumoniae. In addition, it shows activity against some fastidious Gram-negative bacteria (17). In the present work, we investigated the in vivo efficacy of NAI-107 in several experimental-infection models induced by clinically relevant pathogens.
(Data from this study were presented in part at the 49th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, 12 to 15 September 2009 [abstr. F1-1503; oral presentation F1-1221].)
MATERIALS AND METHODS
Bacterial strains and antimicrobials.
The bacterial strains used in this work are listed in Table 1. Acute lethal infections in mice were induced by S. aureus 4061, a glycopeptide-intermediate-resistant S. aureus (GISA) strain; S. pneumoniae 2868 (penicillin intermediate); Enterococcus faecium 569 (VanA); and Enterococcus faecalis A533 (VanA). The rat pouch granuloma and rat endocarditis were induced by S. aureus 1400 (MRSA) and 1524 (MRSA), respectively. NAI-107 was prepared in a solution containing 2.5% N-methyl pyrrolidone (NMP), 2.5% ethanol, 0.4% 1 N HCl, 20% polyethylene glycol (PEG) 400, and 3.92% glucose in demineralized water. Linezolid, vancomycin, and penicillin G were from commercial sources and prepared according to the manufacturers' instructions.
TABLE 1.
MICs and MBCs of NAI-107 and comparator agents against strains used in experimental infections
| Strain | Phenotype | Compound | MIC (μg/ml) | MBC (μg/ml) |
|---|---|---|---|---|
| S. aureus | ||||
| 4061a | GISA | NAI-107 | 0.5 | 1 |
| Linezolid | 1 | 4 | ||
| Vancomycin | 4 | 4 | ||
| 1400b | MRSA | NAI-107 | 0.125 | 0.125 |
| Linezolid | 2 | 16 | ||
| Vancomycin | 2 | 16 | ||
| 1524a | MRSA | NAI-107 | 0.06 | 0.25 |
| Vancomycin | 1 | 1 | ||
| S. pneumoniae 2868a | Penicillin intermediate | NAI-107 | 0.015 | 0.015 |
| Linezolid | 1 | 1 | ||
| Penicillin G | 1 | 1 | ||
| E. faecalis A533a | VanA | NAI-107 | 0.5 | 2 |
| Linezolid | 2 | 64 | ||
| E. faecium 569a | VanA | NAI-107 | 1 | 2 |
| Linezolid | 4 | 32 |
Clinical isolate.
Laboratory isolate.
Media and solutions.
Brain heart infusion (BHI) (Difco Laboratories, Detroit, MI) was used for inoculum preparation. Cation-adjusted Mueller-Hinton broth (CAMHB), used for MIC determination, was prepared by adding CaCl2 and MgCl2 at final concentrations of 20 and 10 mg/liter, respectively, to Mueller-Hinton broth (MHB) (Difco Laboratories, Detroit, MI). Lysed horse blood was added to CAMHB to 2% (vol/vol) when S. pneumoniae was grown. Todd-Hewitt agar (THA) or Todd-Hewitt soft agar (THSA (0.7% agar in Todd-Hewitt broth) were used for bacterial counts, Antibiotic medium 2 (AM2) (Difco Laboratories, Detroit, MI) was used for protein binding determination; peptonate saline solution (PSS) (0.9% NaCl supplemented with 1% peptone; Difco Laboratories, Detroit, MI) was used as a diluent for heart homogenates in the endocarditis model.
Animals.
Female ICR mice (Harlan Italy, S. Pietro al Natisone, Italy) weighing 23 to 25 g were used in the studies of systemic infections in immunocompetent or neutropenic animals. Sprague-Dawley rats (Harlan Italy, S. Pietro al Natisone, Italy) of both sexes, weighing 100 to 150 g, were used in the granuloma pouch model and in the endocarditis model. Procedures were approved by the Animal Care and Use Committee at our institution. Animals were euthanized by inhalation of 70% CO2 plus 30% O2.
In vitro methods.
MICs and minimal bactericidal concentrations (MBCs) were determined by the broth microdilution method according to CLSI (8). Bacterial counts from in vivo experiments were determined as follows: after 10-fold serial dilutions of the withdrawn sample, duplicate 0.025-ml aliquots were included in 3 ml of THSA and spread onto THA plates. Colonies were counted after 48 to 72 h at 35°C.
Acute lethal infection model in mice.
Cultures from frozen vials were incubated for 24 h at 35°C and then appropriately diluted with 5% bacteriological mucin (Difco Laboratories, Detroit, MI). Infection in immunocompetent mice was induced by intraperitoneal (i.p.) injection of 104 CFU of S. pneumoniae 2868 in a final volume of 0.5 ml. Neutropenia was induced by administering i.p. cyclophosphamide (Endoxan Baxter) on days −4 (at 200 mg/kg of body weight) and −1 (at 100 mg/kg) before infection. Animals were infected i.p. with 5 × 103 CFU of S. aureus 4061, 5.2 × 105 CFU of E. faecium 569, or 2.6 × 106 CFU of E. faecalis A533 in a final volume of 0.5 ml. In all experiments, these inocula resulted in 100% mortality in untreated controls within 48 to 72 h after infection. Within 10 to 15 min after infection, eight mice per group were treated at each dose level with 0.25 ml of a single-dose regimen of test antibiotic. Depending on the microorganism under study, NAI-107 was compared to linezolid, vancomycin, or penicillin G. The routes of administration were intravenous (i.v.) or subcutaneous (s.c.) for NAI-107 and s.c. for vancomycin and penicillin G. Linezolid was dosed orally, because of its complete bioavailability following this route of administration (26). Mortality was recorded daily. Mice surviving after 7 days of treatment were euthanized. The 50% effective doses (ED50) and 95% confidence limits were calculated by the Spearman-Kärber method from the animals surviving to 7 days at each dose. This method was also used to calculate the 50% lethal dose (LD50) of each infecting organism (11).
Rat granuloma pouch.
The granuloma pouch model was based on the model described by Dalhoff (9). Briefly, pouches were produced in 5 or 6 rats per group by injecting 30 ml of sterile filtered air followed by 0.5 ml of 0.5% croton oil in sesame seed oil deep into the loose connective dorsal tissue. Two days later, approximately 10 ml of air was withdrawn, and after 2 to 3 more days, 2 ml of 1% peptone and 0.35% agar in saline solution were injected into the pouch. After a further 5 to 8 days, bacterial suspensions of S. aureus 1400 were prepared from frozen stocks and diluted with 2.5% bacteriological gastric mucin, 0.5% peptone, and 0.35% agar to approximately 106 CFU/ml, and 1.0 to 1.5 ml was injected into the pouches. Treatment started 3 h after infection. NAI-107 was administered i.v. in a volume of 8 ml per kg of body weight.
A first experiment was used to establish effective doses of NAI-107. The compound was administered as a single dose at 10, 20, or 40 mg/kg. In a second experiment, two doses of 20 mg/kg each were used with a 12-h or 24-h interval. Vancomycin (100 mg/kg) was administered by intramuscular (i.m.) injection twice with a 12-h interval (16). Samples (0.2 ml) of infected pouch exudates were collected with a syringe just before treatment and at various time intervals after treatment to determine bacterial titers, which were expressed as the average log10 CFU/ml of exudates from five or six rats per treatment group. The detection limit of the method was 40 CFU/ml.
Rat endocarditis.
Endocarditis was induced in female SD rats, weighing 110 to 140 g, anesthetized by i.m. injection of fentanyl-droperidol (Leptofen; Farmitalia Carlo Erba). The right carotid artery was cannulated with a polyethylene catheter (PE 10; inside diameter, 0.28 mm; outside diameter, 0. 61 mm; Clay Adams). Two to 3 days after catheterization, the rats were injected in the tail vein with 0.5 ml of 4 × 104 to 5 × 104 CFU/ml S. aureus 1524 prepared from frozen cultures as described above. This inoculum produced aortic valve endocarditis in all rats with proper placement of the catheter. Seventeen hours after infection, before treatments were started, blood cultures were collected from the tail vein of each rat to determine bacterial counts, and animals that had positive cultures at that time and correct placement of the catheter across the aortic valve at necroscopy were included in the analysis of results. Mortality was recorded daily. At the end of the experiments, the surviving animals were euthanized.
Two separate experiments were conducted. In the first experiment, rats were treated at 12-h intervals with NAI-107 (at 5, 10, or 20 mg/kg i.v.) or with vancomycin (100 mg/kg i.m.). The 100-mg/kg i.m. vancomycin dosage was selected because it results in serum levels in the rat comparable to those obtained in humans at a therapeutic dose (6, 27). In the second experiment, rats received NAI-107 in two daily doses of 10 mg/kg i.v. administered at 12-h intervals or in a single 20-mg/kg i.v. daily dose, or 100 mg/kg i.m. vancomycin twice daily with a 12-h interval. In both experiments, the drugs were administered in a volume of 4 ml/kg, and treatment was continued for 5 days. Two untreated control groups were included to determine the incidence and severity of infection (start of therapy) and its evolution (end of therapy) by measuring CFU per gram of heart, as previously described (6). Animals in the end-of-therapy group were allowed to die. The start-of-therapy group was sacrificed 17 h after challenge, and the surviving treated rats were sacrificed after 5 days of antibiotic therapy (12 or 24 h after the last antibiotic dose). The hearts were weighed and homogenized in sterile filter bags (Bagpage-Interscience Bagsystem) with 3 ml of PSS. Duplicate aliquots (0.05 ml) were used for bacterial counts. By the plating method, any antibiotic in the heart material was diluted at least 1,000-fold. Bacterial titers were expressed as log10 CFU/g of heart. Heart vegetations were considered sterile when no colonies were seen in the two undiluted samples; for the purpose of calculating the mean number of CFU/mg of heart, the samples were considered to contain 1 CFU per 0.1 ml, which corresponds to 1.5 to 1.7 log10 CFU/g, depending on heart weights. Rats that died during treatment were autopsied, and their hearts were removed and stored at −20°C for microbiological evaluation; blood was not cultured.
Animals that were found dead during therapy but had received at least 80% of the planned doses were included in the statistical evaluation. Untreated animals (the end-of-therapy group) were all included in the statistical analysis irrespective of their time of death. The mean number of CFU per gram in the treatment group was compared by one-way analysis of variance. The Student-Newman-Keuls test was used to adjust for multiple comparisons. Frequencies of heart sterilization, blood cultures, and survival were compared by Fisher's exact test.
Pharmacokinetics.
Trough plasma levels of NAI-107 and vancomycin were determined on blood samples obtained 12 h after the last treatment on day 2 and on day 5 during the rat endocarditis experiment. Antibiotic concentrations were determined by a microbiological agar diffusion assay with Bacillus subtilis ATCC 6633 for vancomycin and Micrococcus luteus ATCC 9341 for NAI-107 as the indicator organisms. The detection limits were 0.8 and 0.4 μg/ml for NAI-107 and vancomycin, respectively.
Protein binding.
Triplicate samples of a 160-μg/ml solution of NAI-107 in methanol-water (80:20) were diluted with rat serum (collected from Sprague-Dawley rats) or human serum (Sigma Chemical Co., St. Louis, MO) to reach a 100- or 500-μg/ml final concentration. After a 30-min incubation at 33°C, samples were centrifuged twice (25 min; 1,500 × g) through a Centriprep-30 g-force cartridge (Millipore). Residual NAI-107 in the centrifuged solution was determined by agar diffusion with M. luteus ATCC 9341 as the indicator organism.
RESULTS
In vitro antibacterial activity.
The MICs and MBCs for NAI-107 and comparators against the strains used in the in vivo experiments are shown in Table 1. NAI-107 showed the lowest MICs and MBCs compared to reference compounds, consistent with previous data (7, 17).
Acute lethal infection model.
The efficacy of intravenous NAI-107 was determined in neutropenic mice against the GISA strain 4061 (Table 1) in comparison to oral linezolid and subcutaneous vancomycin, and against two VRE strains (one E. faecalis and one E. faecium) in comparison to oral linezolid (Table 2). In all cases, NAI-107 was highly effective, with ED50 values ranging from 2.3 to 14.2 mg/kg, comparable to or lower than those of linezolid and vancomycin. Effective treatment of immunocompetent mice infected with a penicillin-intermediate S. pneumoniae isolate was obtained with doses of intravenous NAI-107 several times lower than those of oral linezolid (ED50, 0.51 versus 15.9 mg/kg, respectively). As expected, penicillin G, administered s.c. and included as a control, was moderately effective, with an ED50 of 31.6 mg/kg. NAI-107 was also tested subcutaneously, with ED50 values approximately 1.5 to 2.5 times higher than those obtained after i.v. administration, suggesting good bioavailability by this route (Table 2). The efficacy demonstrated by NAI-107 expands those from previously reported experiments with the reference strain S. aureus Smith 819 (7).
TABLE 2.
Efficacies of NAI-107 and comparator agents in murine lethal infection models induced by Gram-positive pathogens in immunocompetent or neutropenic animals
| Strain | Compound | Route | ED50 (mg/kg) (95% confidence limits) |
|---|---|---|---|
| S. aureus GISA 4061b | NAI-107 | i.v. | 14.2 (11.9-16.9) |
| s.c. | 25.1 (21.6-29.6) | ||
| Linezolid | p.o.c | 17.8 (15.3-20.8) | |
| Vancomycin | s.c. | 22.4 (18.0-27.9) | |
| E. faecium VanA 569 | NAI-107 | i.v. | 2.3 (1.7-3.0) |
| s.c. | 4.5 (3.5-5.6) | ||
| Linezolid | p.o. | 5.1a | |
| E. faecalis VanA A533 | NAI-107 | i.v. | 2.8 (2.4-3.3) |
| s.c. | 8.0 (6.1-10.5) | ||
| Linezolid | p.o. | 22.4 (18.6-27.0) | |
| S. pneumoniae 2868 | NAI-107 | i.v. | 0.51 (0.42-0.62) |
| s.c. | 1.1 (1.0-1.4) | ||
| Linezolid | p.o. | 15.9 (12.2-20.7) | |
| Penicillin G | s.c. | 31.6 (27.2-36.7) |
The confidence limits could not be calculated because survival in all treated groups was either 0 or 100%.
Infection was performed in neutropenic mice.
p.o., per os.
Rat granuloma pouch.
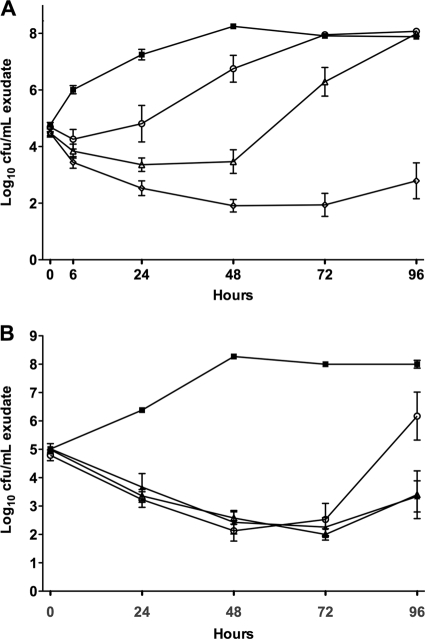
We were interested in determining whether NAI-107 caused a dose-dependent reduction in the bacterial load in a rat granuloma pouch induced by S. aureus 1400. A first experiment was designed to identify effective doses. A single 10-mg/kg dose of NAI-107 slowed bacterial growth compared with the untreated control group, whereas a progressive reduction in the number of viable bacteria was seen after single 20- and 40-mg/kg doses (Fig. 1A). Additionally, the single 40-mg/kg dose of NAI-107 prevented regrowth of the MRSA strain for at least 96 h after treatment (Fig. 1A). In a second experiment, NAI-107 was compared with vancomycin. Two 20-mg/kg NAI-107 doses, administered immediately after infection and 12 h or 24 h thereafter, reduced viable bacteria in pouch exudates by 3 log units within 72 h, with no relevant regrowth observed up to 96 h (Fig. 1B). While vancomycin, administered at 100 mg/kg i.m. immediately after infection and 12 h thereafter, produced a similar reduction in the bacterial load, regrowth was markedly faster than with NAI-107-treated animals and bacterial counts were close to those of controls 96 h after treatment (Fig. 1B). These results indicate that NAI-107 is effective at killing MRSA cells at a single 20-mg/kg dose and that 72 to 96 h is necessary for bacterial growth to reoccur (compare Fig. 1A and B). Bacterial colonies observed after treatment with 10 or 20 mg/kg NAI-107 (Fig. 1A) or with vancomycin (Fig. 1B) were not tested for resistance to NAI-107 and vancomycin, respectively. However, we did not observe any spontaneous NAI-107-resistant mutants of S. aureus 1400 by either plating at 10× or 100× the MIC or by serial passages at 0.5× the MIC (17).
FIG. 1.
In vivo bactericidal activity of NAI-107 in the rat granuloma pouch model induced by S. aureus 1400. (A) Effects of single 10-mg/kg (circles), 20-mg/kg (triangles), and 40-mg/kg (diamonds) i.v. doses on bacterial counts (log10 CFU/ml) in pouch exudates over time. (B) Effects of 2 20-mg/kg doses i.v. every 24 h (q24) (filled triangles) and 2 20-mg/kg doses i.v. q12 (empty triangle) in comparison with two 100-mg/kg doses i.m. q12 (circles) of vancomycin on bacterial counts (log10 CFU/ml) in pouch exudates over time. Untreated controls are represented by filled squares. The error bars indicate standard deviation.
Rat endocarditis.
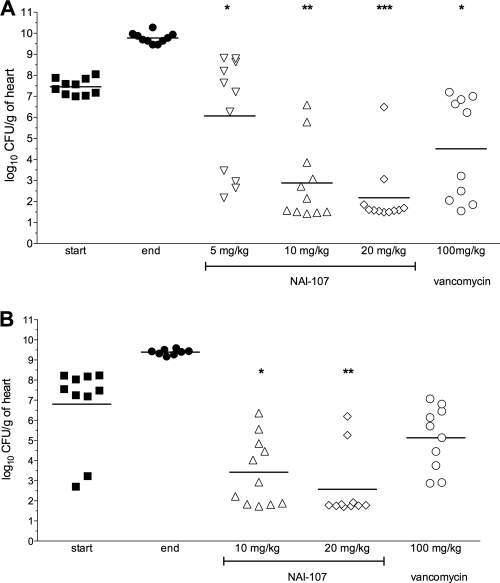
The bacterial load in heart vegetation was determined at the start and end of therapy to follow the severity and evolution of the infection induced by S. aureus strain 1524. In the first experiment, the mean bacterial loads for the untreated control rats (mean log10 CFU/g ± standard deviation [SD]) were 7.5 ± 0.4 when determined 17 h after bacterial challenge (start of therapy) and 9.8 ± 0.1 at the end of therapy, with all control animals showing infected heart vegetation (Fig. 2A). NAI-107 administered i.v. at 5, 10, and 20 mg/kg twice daily for five consecutive days resulted in a dose-proportional reduction in viable bacterial counts compared to untreated controls at the start of therapy. Only for the NAI-107 5-mg/kg dose was a statistically nonsignificant reduction in the heart vegetation load (−1.4 log10 CFU/g reduction) in comparison to the untreated controls at the start of therapy observed. The other two treatments were significantly effective, with NAI-107 at 10 and 20 mg/kg reducing heart vegetation counts to 2.9 ± 0.5 and 2.2 ± 0.4 log10 CFU/g, respectively, both significantly (P < 0.0001) lower that those determined in the untreated controls at the start of therapy. The 20-mg/kg dose was significantly more effective (P = 0.01) than 100 mg/kg vancomycin, with the latter leading to a bacterial titer of 4.5 ± 0.8 log10 CFU/g (Fig. 2A).
FIG. 2.
Efficacies of NAI-107 and vancomycin in reducing the bacterial load in heart vegetations in a model of rat endocarditis caused by S. aureus 1524. “Start” and “end” indicate bacterial loads at the start (17 h after infection) and end (5 days) of therapy, respectively. Each symbol represents the viable bacterial counts determined in each animal, while the horizontal lines represent the average value for each group. (A) NAI-107 at 5, 10, and 20 mg/kg i.v. q12 compared to 100 mg/kg i.m. q12 of vancomycin. Three animals treated with 5 mg/kg i.v. and two treated with 20 mg/kg i.v. of NAI-107 and two animals treated with 100 mg/kg i.m. of vancomycin were excluded from the statistical analysis due to incorrect placement of the catheter. *, P < 0.05 compared to start of therapy; **, P < 0.001 compared to start of therapy and NAI-107 at 5 mg/kg and P < 0.01 compared to vancomycin; ***, P < 0.001 compared to start of therapy, NAI-107 at 5 mg/kg, and vancomycin. (B) NAI-107 at 10 mg/kg q12 versus 20 mg/kg i.v. q24 compared to 100 mg/kg i.m. q12 of vancomycin. One animal treated with 10 mg/kg i.v. of NAI-107 was excluded from the statistical evaluation due to incorrect placement of the catether. *, P < 0.01 and P > 0.001 compared to control groups and vancomycin, respectively; **, P < 0.001 compared to start and end of therapy.
In the second rat endocarditis experiment (Fig. 2B), similar bacterial counts in heart vegetation were seen in untreated controls (7.8 ± 0.16 and 9.4 ± 0.1 log10 CFU/g at the start and end of therapy, respectively). The effect of NAI-107 given at 10 mg/kg twice daily was similar to that obtained after a single 20-mg/kg daily dose (3.4 ± 0.5 and 2.6 ± 0.5 log10 CFU/g, respectively); both dosages were significantly (P < 0.05 and P < 0.01, respectively) more effective than vancomycin administered at 100 mg/kg i.m. twice daily, which led to 5.1 ± 0.5 log10 CFU/g, similar to the value observed during the first endocarditis experiment.
Further details are reported in Table 3. While bacteria were detected in the heart vegetation of all rats treated with vancomycin or with 5 mg/kg NAI-107, a significant fraction of animals, up to 7/10 rats treated with 20 mg/kg NAI-107 in the second experiment, showed sterile heart vegetation, a result that was statistically significant versus vancomycin (P < 0.05).
TABLE 3.
Efficacies of NAI-107 and vancomycin following different treatment schedules in the model of rat endocarditis induced by S. aureus 1400
| Expt | Group | Dose (mg/kg) and route | No. of rats/group | No. of survivors (%) | No. of sterile heart vegetations (%)a |
|---|---|---|---|---|---|
| 1 | Start of therapy | 10 | 0 (0) | ||
| End of therapy | 10 | 0 (0) | 0 (0) | ||
| NAI-107 | 5 q12 i.v. | 11 | 5 (45.5) | 0 (0) | |
| 10 q12 i.v. | 11 | 11 (100) | 3 (27.3) | ||
| 20 q12 i.v. | 11 | 9 (82) | 5 (55.5)b | ||
| Vancomycin | 100 q12 i.m. | 10 | 8 (80) | 0 (0) | |
| 2 | Start of therapy | 8 | 0 (0) | ||
| End of therapy | 8 | 0 (0) | 0 (0) | ||
| NAI-107 | 10 q12 i.v. | 11 | 9 (82) | 4 (44.4)c | |
| 20 q24 i.v. | 10 | 10 (100) | 7 (70)d | ||
| Vancomycin | 100 q12 i.m. | 10 | 9 (90) | 0 (0) |
Determined in survivors.
P < 0.05 compared to control groups, NAI-107 at 5 mg/kg, and vancomycin (Fisher's exact test).
P < 0.05 compared to control groups and vancomycin (Fisher's exact test).
P < 0.001 compared to control groups and vancomycin (Fisher's exact test).
Pharmacokinetics and protein binding.
The rat endocarditis experiment was also used to measure some pharmacokinetic (PK) parameters. The trough plasma levels of NAI-107 and vancomycin after the last treatments on days 2 and 5 are reported in Table 4. In the first experiment (twice-daily doses), the trough concentrations after four doses of NAI-107 were 4.7, 13.5, and 23.7 μg/ml for 5, 10, and 20 mg/kg, respectively. The concentrations obtained at the time of sacrifice, 12 h after the 10th treatment, were 6.8, 16.6, and 30.4 μg/ml, respectively, suggesting some drug accumulation in the blood. Trough plasma levels obtained in the second experiment after 4 and 10 doses of NAI-107 administered at 10 mg/kg were 10.4 and 13.4 μg/ml, respectively, very similar to those determined on days 2 and 5 after single 20-mg/kg daily treatments (9.4 and 13.0 μg/ml, respectively). The trough levels of vancomycin (determined 12 h after the last treatment) were around 2 μg/ml, as previously reported (6). The MBCs against S. aureus 1524 determined in the presence of 50% rat plasma were 4 and 1 μg/ml for NAI-107 and vancomycin, respectively (19).
TABLE 4.
Trough plasma levels of NAI-107 and vancomycin after the last treatment on days 2 and 5 in the rat endocarditis model
| Expt | Group | Dose (mg/kg) and route | Trough plasma level (μg/ml) on day: |
|
|---|---|---|---|---|
| +2 | +5 | |||
| 1 | NAI-107 | 5 q12 i.v. | 4.7 ± 1.3 | 6.8 ± 2.2 |
| 10 q12 i.v. | 13.5 ± 2.3 | 16.6 ± 2.7 | ||
| 20 q12 i.v. | 23.7 ± 4.3 | 30.4 ± 3.6 | ||
| Vancomycin | 100 q12 i.m. | 2.4 ± 1.4 | 2.3 ± 1.4 | |
| 2 | NAI-107 | 10 q12 i.v. | 10.4 ± 2.2 | 13.4 ± 4.4 |
| 20 q24 i.v. | 9.4 ± 1.8 | 13.0 ± 3.6 | ||
| Vancomycin | 100 q12 i.m. | 2.1 ± 1.6 | 2.5 ± 1.1 | |
Binding of NAI-107 to rat serum was 93.2% and 96.3% at 100 and 500 μg/ml, respectively, while binding to human serum was 96.5% and 98.6% at 100 and 500 μg/ml, respectively.
DISCUSSION
Despite the global concern raised by the increasing incidence of infections due to MDR pathogens like MRSA and VRE, only one new compound (telavancin; Theravance, Inc.) has been approved by the FDA since 2007 for the treatment of infections caused by Gram-positive bacteria (1). Although an additional mechanism of action has been claimed for telavancin (14), this glycopeptide, obtained by chemical modification of vancomycin, cannot be considered a representative of a new chemical class, which leaves daptomycin as the last new-chemical-class antibiotc approved for nontopical applications. It is generally agreed that MDR bacteria can be best fought by new classes of antibiotics, i.e., compounds endowed with a novel mechanism of action and unlikely to be affected by existing resistance determinants. Despite this necessity, recent surveys indicated that most antibiotics under clinical development represent improved versions of approved antibiotics (10, 24).
Lantibiotics, which are ribosomally synthesized peptides that undergo posttranslational modifications, are potent antimicrobial compounds with activities limited to Gram-positive bacteria. The prototype molecule is nisin, discovered in the 1920s and used as a food preservative for over 40 years (29). As lantibiotics exert their antimicrobial activities by binding to lipid II at a site different from that affected by glycopeptides, they are active against MDR Gram-positive pathogens and have attracted attention as potential drug candidates. NAI-107 emerged from a screening program designed to find new bacterial cell wall inhibitors (7, 20). It represents the first example of a class I lantibiotic produced by actinomycetes (29).
As previously described (7), NAI-107 possesses an interesting antibacterial profile, covering all MDR Gram-positive pathogens and, although less potently, some fastidious Gram-negative pathogens typically related to respiratory tract infections, as well as a rapid and potent bactericidal activity (17). These data have been substantiated by different models of experimental infections caused by these difficult pathogens, as documented by the present work.
In an acute murine lethal infection induced by MRSA, GISA, or penicillin-intermediate S. pneumoniae, the efficacy of intravenously administered NAI-107 was comparable or superior to those of reference compounds administered in doses and by routes that reflect the exposures attained in humans at effective doses. NAI-107 was more effective when administered i.v. than subcutaneously, suggesting limited bioavailability by the latter route.
The rapid dose-proportional effect observed for NAI-107 in the rat granuloma pouch model induced by MRSA is consistent with its high potency and suggests good tissue penetration. The results observed for vancomycin in this study are consistent with those previously reported (16). Interestingly, two 20-mg/kg doses of NAI-107 were sufficient to produce the same reduction of bacterial counts achieved by two 100-mg/kg i.m. doses of vancomycin. However, while in the vancomycin group bacterial regrowth was observed starting 72 h after treatment, a low bacterial titer was maintained for up to 96 h in the NAI-107 groups (Fig. 1B).
Infective endocardites can arise as a complication of various clinical conditions, such as prosthetic valve insertions or bloodstream infections, or can be favored by intravenous drug use. In any case, they always lead to extended hospitalization and an in-hospital mortality rate of ∼20% (3, 25). The presence of MRSA or VRE as an infective agent is an additional cause for concern (12). In the present paper, we have documented the efficacy of intravenous NAI-107 in an infective endocarditis model induced by MRSA. NAI-107 at a dose as low as 10 mg/kg every 12 h significantly reduced the number of viable bacteria in heart vegetation compared to two 100-mg/kg i.m. daily doses of vancomycin (this dosage is necessary to produce blood exposure comparable to that obtained in humans at therapeutic doses [27]). Moreover, NAI-107 at 10 or 20 mg/kg given twice daily or at 20 mg/kg/day was capable of reducing the bacterial load in heart vegetation below detection limits in a significant fraction of animals. The efficacy observed with NAI-107 may be due to its rapid bactericidal activity (17) and/or to its measured concentration in plasma, with trough plasma levels several times higher than the MBC determined in the presence of 50% rat plasma. Preliminary experiments performed in the rat, following a single 20-mg/kg i.v. administration of NAI-107, suggest a plasma half-life exceeding 14 h and an area under the curve at infinity (AUCinf) of 940 h·mg/liter (S. Riva, G. Candiani, and D. Jabes, unpublished results). This long persistence in plasma may be related to NAI-107's high protein binding and is reminiscent of the properties seen with the glycopeptide dalbavancin (25).
In conclusion, we have demonstrated the efficacy of the new antibiotic NAI-107 in several models of experimental infection caused by MDR Gram-positive pathogens. These findings, coupled with its in vitro antibacterial spectrum and potency (17), make NAI-107 a valuable candidate for further evaluation and one of the few examples of a novel-class antibiotic in preclinical development.
Footnotes
Published ahead of print on 10 January 2011.
REFERENCES
- 1.Anonymous. 2010. New antimicrobial agents approved by the U. S. Food and Drug Administration from 2007 to 2009 and new indications for previously approved agents. Antimicrob. Agents Chemother. 54:4033-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymous. 2009. Urgently needed: new antibiotics. Lancet 374:1868. [DOI] [PubMed] [Google Scholar]
- 3.Baddour, L. M., et al. 2005. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 111:394-434. [DOI] [PubMed] [Google Scholar]
- 4.Boakes, S., A. N. Appleyard, J. Cortés, and M. J. Dawson. 2010. Organization of the biosynthetic genes encoding deoxyactagardine B (DAB), a new lantibiotic produced by Actinoplanes liguriae NCIMB41362. J. Antibiot. (Tokyo). 63:351-358. [DOI] [PubMed] [Google Scholar]
- 5.Bossaer, J. B., P. D. Hall, and E. Garrett-Mayer. 2010. Incidence of vancomycin-resistant enterococci (VRE) infection in high-risk febrile neutropenic patients colonized with VRE. Support Care Cancer 19:231-237. [DOI] [PubMed] [Google Scholar]
- 6.Candiani, G. P., M. Abbondi, G. Romano, and F. Parenti. 1999. In vitro and in vivo antibacterial activity of BI397, a new semi-synthetic glycopeptide antibiotic. J. Antimicrob. Chemother. 44:179-192. [DOI] [PubMed] [Google Scholar]
- 7.Castiglione, F., et al. 2008. Determining the structure and mode of action of microbisporicin, a potent lantibiotic active against multiresistant pathogens. Chem. Biol. 15:22-31. [DOI] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 7th ed. CLSI document M7-A7. CLSI, Wayne, PA.
- 9.Dalhoff, A. 1986. The granuloma pouch, p. 123-137. In O. Zak and M. A. Sande (ed.), Experimental models in antimicrobial chemotherapy. Academic Press, London, United Kingdom.
- 10.Donadio, S., S. Maffioli, P. Monciardini, M. Sosio, and D. Jabes. 2010. Antibiotic discovery in the 21st century: current trends and future perspectives. J. Antibiot. (Tokyo) 63:423-430. [DOI] [PubMed] [Google Scholar]
- 11.Finney, D. J. 1952. The Spearman-Kärber method. p. 524-530. In D. J. Finney (ed.) Statistical method in biological assay. Charles Griffin & Company Ltd., London, United Kingdom.
- 12.Fowler, V. G., Jr., et al. 2005. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 293:3012-3021. [DOI] [PubMed] [Google Scholar]
- 13.Furtado, G. H., and D. P. Nicolau. 2010. Overview perspective of bacterial resistance. Expert Opin. Ther. Pat. 20:1273-1276. [DOI] [PubMed] [Google Scholar]
- 14.Higgins, D. L., et al. 2005. Telavancin, a multifunctional lipoglycopeptide, disrupts both cell wall synthesis and cell membrane integrity in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 49:1127-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Infectious Diseases Society of America. 2010. The 10 x ′20 Initiative: pursuing a global commitment to develop 10 new antibacterial drugs by 2020. Clin. Infect. Dis. 50:1081-1083. [DOI] [PubMed] [Google Scholar]
- 16.Jabes, D., et al. 2004. Efficacy of dalbavancin against methicillin-resistant Staphylococcus aureus in the rat granuloma pouch infection model. Antimicrob. Agents Chemother. 48:1118-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jabes, D., C. Brunati, P. Guglierame, and S. Donadio. 2009. In vitro antibacterial profile of the new lantibiotic NAI-107, abstr. F1-1502. Progr. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother. [DOI] [PMC free article] [PubMed]
- 18.Jabes, D., G. Candiani, G. Romano, C. Brunati, and S. Riva. 2009. NAI-107: SUperior efficacy in animal models of MDR Gram-positive infection, abstr. F1-1503. Progr. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother.
- 19.Jabes, D., G. Candiani, G. Romano, C. Brunati, and S. Riva. 2009. NAI-107: superior efficacy in MRSA rat endocarditis, abstr. F1-1221. Progr. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother.
- 20.Jabes, D., and S. Donadio. 2010. Strategies for the isolation and characterization of antibacterial lantibiotics. Methods Mol. Biol. 618:31-45. [DOI] [PubMed] [Google Scholar]
- 21.Kamboj, M., et al. 2010. The changing epidemiology of vancomycin-resistant enterococcus (VRE) bacteremia in allogeneic hematopoietic stem cell transplant (HSCT) recipients. Biol. Blood Marrow Transplant. 16:1576-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klevens, R. M., et al. 2006. Changes in the epidemiology of methicillin-resistant Staphylococcus aureus in intensive care units in US hospitals, 1992-2003. Clin. Infect. Dis. 42:389-391. [DOI] [PubMed] [Google Scholar]
- 23.Olivier, C. N., R. K. Blake, L. L. Steed, and C. D. Salgado. 2008. Risk of vancomycin-resistant enterococcus (VRE) bloodstream infection among patients colonized with VRE. Infect. Control Hosp. Epidemiol. 29:404-409. [DOI] [PubMed] [Google Scholar]
- 24.Theuretzbacher, U. 2009. Future antibiotics scenarios: is the tide starting to turn? Int. J. Antimicrob. Agents 34:15-20. [DOI] [PubMed] [Google Scholar]
- 25.Sobel, J. 1992. Nosocomial infective endocarditis, p. 316. In D. Kaye (ed.), Infective endocarditis, 2nd ed. Raven Press Ltd., New York, NY.
- 26.Rubinstein, E., et al. 2003. Worldwide assessment of linezolid's clinical safety and tolerability: comparator-controlled phase III studies. Antimicrob. Agents Chemother. 47:1824-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voorn, G. P., J. Kuyvenhoven, W. H. Goessens, W. C. Schmalbauer, and P. H. Broeders. 1994. Role of tolerance in treatment and prophylaxis of experimental Staphylococcus aureus endocarditis, with vancomycin, teicoplanin, and daptomycin. Antimicrob. Agents Chemother. 38:487-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wenzel, R. P. 2004. The antibiotic pipeline: challenges, costs, and values. N. Engl. J. Med. 351:523-526. [DOI] [PubMed] [Google Scholar]
- 29.Willey, J. M., and W. A. van der Donk. 2007. Lantibiotics: peptides with diverse structure and function. Annu. Rev. Microbiol. 61:477-501. [DOI] [PubMed] [Google Scholar]