Abstract
In this study, the contribution of efavirenz N-glucuronidation to efavirenz elimination in vivo was assessed. In a two-period placebo-controlled crossover trial design, a single 600-mg oral dose of efavirenz was administered to healthy volunteers (n = 10) pretreated with placebo pills or 600 mg/day rifampin orally for 10 days. Urine and plasma concentrations of efavirenz and 8-hydroxyefavirenz were measured by the liquid chromatography-tandem mass spectrometry method after enzymatic hydrolysis with β-glucuronidase (conjugated and unconjugated) and without enzymatic hydrolysis (unconjugated). Pharmacokinetic parameters of efavirenz within the placebo- or rifampin-treated group obtained after enzymatic hydrolysis did not show any statistically significant difference compared with those obtained without enzymatic hydrolysis (P > 0.05; paired t test, two-tailed). The amount of efavirenz excreted over 12 h was significantly larger after enzymatic hydrolysis in both the placebo (P = 0.007) and rifampin (P = 0.0001) treatment groups, supporting the occurrence of direct N-glucuronidation of efavirenz, but the relevance of this finding is limited because the amount of efavirenz excreted as unchanged or conjugated in urine is less than 1% of the dose administered. In both the placebo- and rifampin-treated groups, plasma concentrations of 8-hydroxyefavirenz and the amount excreted over 12 h were significantly larger (P < 0.00001) after enzymatic hydrolysis than without enzymatic hydrolysis. These findings suggest that although the occurrence of direct efavirenz N-glucuronidation is supported by the urine data, the abundance of efavirenz N-glucuronide in plasma is negligible and that the contribution of the N-glucuronidation pathway to the overall clearance of efavirenz seems minimal.
Efavirenz (EFV) is a potent nonnucleoside reverse transcriptase inhibitor (NNRTI) used for the treatment of human immunodeficiency virus (HIV) type 1 infection. An efavirenz-based combination antiretroviral regimen is the first-line initial treatment option in naïve patients (7). However, a large interindividual variability in efavirenz pharmacokinetics has been reported, and these pharmacokinetic differences have been linked to an increased risk of central nervous system (CNS) adverse effects or virological failure (4, 20, 28). Efavirenz is mainly cleared by oxidation via the cytochrome P450s (CYPs) (21). In vitro studies have shown that CYP2B6 is the principal catalyst of EFV 8-hydroxylation (5, 25, 29), the major clearance mechanism of efavirenz in humans (21). Clinical studies with HIV-infected patients have repeatedly shown that CYP2B6 polymorphisms (e.g., CYP2B6*6/*6) decrease efavirenz clearance and may be associated with CNS adverse effects (for a review, see reference 30). More recently, CYP2A6 has been implicated in efavirenz 7-hydroxylation in vitro (5, 25) and in vivo (8), and evidence exists that CYP2A6 genetic variation is associated with efavirenz exposure (1, 16, 17).
However, considerable variability in EFV exposure remains even after CYP2A6 and CYP2B6 genetic variation is accounted for (1, 27). In preclinical and clinical studies (8, 21, 22), efavirenz N-glucuronide has been detected in urine, plasma, and bile samples. Indeed, a recent in vitro study has reported that efavirenz can undergo direct N-glucuronidation and that this reaction is catalyzed by the human UDP-glucuronsyltransferase 2B7 (UGT2B7) (3). The UGT2B7 gene is highly polymorphic, with some variants having functional consequences (11). It is therefore possible that the intersubject variability in UGT2B7 activity brought about genetic variations or drug interactions may contribute to the variable efavirenz exposure seen in patients. However, the actual contribution of N-glucuronidation to the overall in vivo metabolism of efavirenz has not been established.
Rifampin is a cornerstone for the treatment of tuberculosis, an infection that is closely associated with HIV infection/AIDS. The therapy of choice for patients who are coinfected with HIV and Mycobacterium tuberculosis and who have had no previous antiretroviral experience includes an efavirenz-based antiretroviral regimen and an antituberculosis therapeutic regimen that contains rifampin (6). However, a major concern of such a combination has been rifampin's ability to induce multiple drug-metabolizing enzymes and drug transporters, which results in the altered disposition of many coadministered drugs (24). Evidence exists that rifampin enhances the metabolism of UGT substrates, such as mycophenolic acid (23) and zidovudine (2, 10). However, the effect of rifampin on the rate of N-glucuronidation of efavirenz remains unknown.
The main purpose of this study is to assess the contribution of N-glucuronidation to efavirenz overall elimination in vivo. Thus, total (conjugated and unconjugated) and free (unconjugated) efavirenz and its main human metabolite, 8-hydroxyefavirenz (21), were measured in plasma and urine samples obtained from healthy subjects administered a single 600-mg oral dose of efavirenz after a 10-day pretreatment with placebo pills (basal) or 600 mg/day rifampin orally.
MATERIALS AND METHODS
Study design and subjects.
A total of 10 healthy volunteers participated in this study. The study protocol was approved by the Institutional Review Board (IRB) of Indiana University School of Medicine. Signed and dated written informed-consent forms were obtained from each subject. Subjects were ascertained to be healthy by physical examination, clinical laboratory tests, and medical history.
This study was a randomized, double-blinded, placebo-controlled crossover (two-period) trial. Eligible subjects were randomized to take either a daily 600-mg oral dose of rifampin or placebo from day 1 through day 10. Since rifampin discolors urine, riboflavin, which produces a similar urine color, was used as placebo. On day 11, after predose blood collection, subjects were given a single 600-mg oral dose of EFV on an empty stomach. Blood samples were collected at 0.5, 1, 2, 3, 4, 5, 6, 8, 10, 12, 24, 48, and 72 h after efavirenz dosing for pharmacokinetic analysis. Urine voided within the first 12 h (0 to 12 h) was collected. Two 10-ml urine aliquots were saved after the urine volume was recorded. Plasma samples were separated by centrifugation at 3,000 rpm for 20 min within an hour of blood collection. Plasma and urine samples were immediately stored at −80°C until analysis.
After a washout period of 11 days, subjects started taking rifampin or placebo in a crossover fashion for 10 consecutive days and underwent the same procedure as in the first phase of the study. Plasma and urine samples were processed as described above.
Assay of efavirenz and 8-hydroxyefavirenz.
Plasma concentrations of efavirenz and its metabolite were determined using a liquid chromatograph interfaced to an API 3200 triple-quadrupole mass spectrometer (Applied Biosystems, Foster City, CA) equipped with an electrospray probe in positive ionization mode as described elsewhere (25).
Enzymatic hydrolysis.
To assess the potential role of conjugation in the metabolism of efavirenz, total concentrations (unconjugated plus conjugated) of efavirenz and 8-hydroxyefavirenz were measured after incubation of plasma and urine samples (deconjugation) with β-glucuronidase (β-glucuronidase type H-5 from Helix pomata [Sigma-Aldrich, St. Louis, MO]). Plasma samples (200 μl) were incubated with 0.2 ml of 0.2 M sodium acetate buffer (pH 5.0), 10 μl of 0.6 M sodium azide, and 20 μl of β-glucuronidase (10 units/μl) at 37°C for 15 h. The β-glucuronidase type H-5 extracted from Helix pomata also contained sulfatase. Although it is likely that the conjugated metabolite of 8-hydroxyefavirenz represents O-glucuronide (21), we report the data as being those for conjugated 8-hydroxyefavirenz, as it was difficult to reliably distinguish between glucuronide and sulfate conjugates. For efavirenz, direct sulfation of efavirenz is unlikely, and any conjugated metabolite of it should represent an N-glucuronide.
To obtain the free (unconjugated) concentrations of efavirenz and its metabolite, plasma samples were incubated exactly as described above, except that β-glucuronidase was excluded from the incubation mixture (buffer was used instead). These samples were run in parallel with the corresponding samples treated with β-glucuronidase to avoid effects on efavirenz or metabolite stability. Thus, plasma samples (200 μl) were incubated with 0.2 ml of 0.2 M sodium acetate buffer (pH 5.0), 10 μl of 0.6 M sodium azide, and 20 μl of buffer at 37°C for 15 h. After the internal standard (30 μl of 0.5 μg/ml nevirapine) was added, the sample was extracted with 6 ml ethyl acetate under alkaline pH (1 M glycine, 1 M sodium hydroxide buffer, pH 11.3). The organic layer was evaporated to dryness, the residue was reconstituted in 50% formic acid (0.1% in water)-50% methanol (vol/vol), and 25 μl was injected onto the liquid chromatography-tandem mass spectrometry (LC-MS/MS) system equipped with an Agilent 1100 series high-performance liquid chromatography system.
Efavirenz, its metabolite, and the internal standard were separated using a Luna C18 column (2.0 by 100 mm; 3 μm; Phenomenex, Torrance, CA) with a gradient elution that consisted of an initial mobile phase of methanol-formic acid (0.1% in water) (1:99; vol/vol) and a secondary mobile phase of methanol-formic acid (0.1% in water) (99:1; vol/vol). The secondary mobile phase percentage was increased from 50 to 90% linearly from 0 to 16 min; the initial mobile phase conditions were resumed after 16 min and remained constant for an additional 4 min, allowing the column to equilibrate. The eluate was introduced, without splitting, at 0.800 ml/min to the electrospray ionization source. Efavirenz, 8-hydroxyefavirenz, and nevirapine (the internal standard) were detected using multiple-reaction monitoring at m/z values of 316/244, 332/248, and 267/226, respectively, in positive mode. The limit of quantification of efavirenz and its hydroxylated metabolite was 3 ng/ml. The standard curve was linear over the range of 3 to 10,000 ng/ml, and the day-to-day and within-day coefficients of variation of the LC-MS/MS assay were less than 15% across these concentrations and quality controls.
Acid deconjugation.
To rule out the possibility that enzymatic hydrolysis of N-glucuronide might be incomplete, as has been suggested for other drugs (12), additional pilot experiments were performed using acid hydrolysis. Because efavirenz N-glucuronide was reported to be abundant at earlier time points after administration of efavirenz to experimental animals (21), plasma samples collected at 3 h (about the time to the maximum plasma concentration [Tmax] of efavirenz) after efavirenz dosing were analyzed (n = 3 subjects). Plasma samples (200 μl) were incubated with 230 μl of 6 M HCl at 90°C for 90 min. After the pH was adjusted to 11.3 using 1 M sodium hydroxide buffer and addition of the internal standard (30 μl of 0.5 μg/ml nevirapine), the samples were extracted with 30 ml ethyl acetate. The organic layer was evaporated to dryness, and the residue was reconstituted and analyzed by use of the LC-MS/MS system as described above.
Pharmacokinetic and statistical analysis.
Pharmacokinetic parameters were estimated from plasma concentration data by noncompartmental analysis using WinNonlin professional software (version 5.01; Pharsight, Mountain View, CA). The area under the plasma concentration-time curve (AUC) from the time of dosing through the sampling time of the last quantifiable concentration (AUC0-t) was estimated using the linear and logarithmic trapezoidal rules for the up and down portions of the curve, respectively. The apparent terminal elimination rate constant (λz) was determined by regression analysis of the terminal portion of the log plasma concentration-time curve. To estimate total AUC, AUC was extrapolated to infinity (AUC0-∞) by adding Ct/λz to AUCt, where Ct is the last measured concentration. The terminal elimination half-life (t1/2) was calculated from the equation 0.693/λz. The maximum plasma concentration (Cmax) and Tmax were estimated directly from the observed plasma concentration-versus-time curves.
The pharmacokinetic variables for efavirenz were compared between those treated with β-glucuronidase (conjugated and unconjugated) and those not treated (unconjugated) for both the placebo and rifampin phases using a paired t test or Wilcoxon's signed-rank test, as appropriate. Comparison of plasma concentrations of free (unconjugated) and total (unconjugated plus conjugated) efavirenz was performed by the Kruskal-Wallis test. Data were presented as means ± standard deviations (SDs). All statistical analyses were performed by SPSS software (version 12.0; SPSS, Chicago, IL), and P values of <0.05 were considered statistically significant.
RESULTS
The mean age and body weight of the 10 subjects were 22.6 years (range, 19 to 44 years) and 71.1 kg (range, 57 to 88 kg). The subjects comprised eight Caucasians (80%; five were females) and two male African Americans (20%). EFV and rifampin were generally well tolerated, except for mild CNS symptoms after efavirenz dosing.
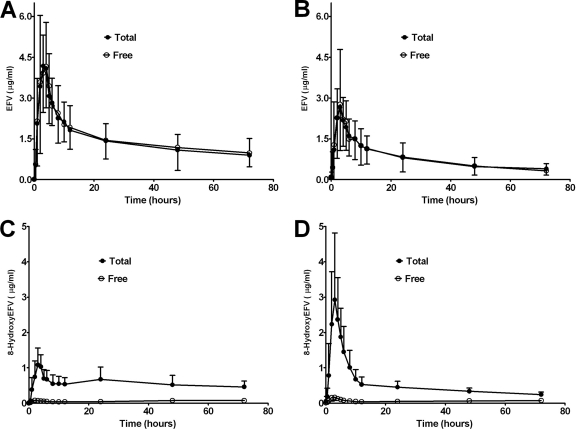
Efavirenz and 8-hydroxyefavirenz (the major oxidative metabolite) concentrations were determined in plasma and urine samples collected from healthy volunteers administered a single 600-mg oral dose of efavirenz after a 10-day treatment with placebo or rifampin (600 mg/day). The samples were analyzed after overnight incubation with β-glucuronidase (enzymatic hydrolysis) to reflect total (unconjugated plus conjugated) concentrations and without β-glucuronidase to reflect free (unconjugated) concentrations. Determination of total and free efavirenz concentrations for each subject was run simultaneously. Figure 1 shows the free (unconjugated) and total (conjugated plus unconjugated) plasma concentration-time profiles of a single 600-mg oral dose of efavirenz after a 10-day treatment with placebo pills (control) (Fig. 1A) and rifampin (600 mg/day) (Fig. 1B). The pharmacokinetic parameters derived from these data are listed in Table 1. No statistically significant difference between the efavirenz pharmacokinetic parameters derived after enzymatic hydrolysis (total) and those derived from no enzymatic hydrolysis (free) was observed. Since the concentrations obtained after pretreatment with β-glucuronidase (conjugated plus conjugated) and without β-glucuronidase (unconjugated) were obtained under the same assay conditions run in parallel, the stability of efavirenz is unlikely to be a factor.
FIG. 1.
Mean (±SD) plasma concentration-time profiles of a single 600-mg oral dose of EFV (A and B) and 8-hydroxyefavirenz (8-hydroxyEFV) (C and D) in healthy volunteers (n = 10) treated with placebo pills (control; left panel) or rifampin (600 mg/day) (treated; right panel) for 10 days. Total represents conjugated plus unconjugated (β-glucuronidase treatment), and free represents unconjugated (no β-glucuronidase treatment).
TABLE 1.
Pharmacokinetic parameters of a single 600-mg oral dose of efavirenz after a 10-day treatment with placebo pills (control) and rifampin (600 mg/day) for samples incubated overnight with β-glucuronidase (total) and without β-glucuronidase (free)a
| Compound and pharmacokinetic parameter | Placebo phase |
Rifampin phase |
||
|---|---|---|---|---|
| Total | Free | Total | Free | |
| Efavirenz | ||||
| Tmax (h) | 3 (2-4)b | 3 (2-5) | 3 (1-5) | 3 (1-5) |
| Cmax (μg/ml) | 5.00 ± 1.94 | 5.03 ± 1.74 | 3.14 ± 1.47 | 3.23 ± 1.71 |
| t1/2 (h) | 54.0 ± 14.9 | 55.4 ± 15.3 | 45.5 ± 16.6 | 43.4 ± 22.7 |
| AUC0-72 (μg·h/ml) | 101.34 ± 47.13 | 103.40 ± 37.91 | 54.86 ± 30.01 | 54.72 ± 25.35 |
| AUC0-∞ (μg·h/ml) | 176.16 ± 92.22 | 187.46 ± 81.69 | 80.28 ± 42.32 | 81.71 ± 41.15 |
| V/F (liters/kg) | 4.29 ± 1.30 | 4.08 ± 1.45 | 8.33 ± 4.38 | 7.16 ± 3.51 |
| CL/F (ml/min/kg) | 1.01 ± 0.52 | 0.89 ± 0.36 | 2.23 ± 1.32 | 2.17 ± 1.31 |
| 8-Hydroxyefavirenz | ||||
| Cmax (μg/ml) | 1.22 ± 0.40c | 0.12 ± 0.06 | 3.19 ± 1.63c | 0.21 ± 0.09 |
| AUC0-72 (μg·h/ml) | 39.53 ± 16.68c | 4.03 ± 1.73 | 37.37 ± 12.27c | 4.46 ± 1.68 |
Total represents conjugated plus unconjugated (β-glucuronidase treatment), and free represents unconjugated (no β-glucuronidase treatment). V/F, volume of distribution; CL/F, clearance. The AUC0-72 (total/free) values for the placebo and rifampin treatment phases were 0.96 ± 0.13 and 0.98 ± 0.14, respectively.
Values in parentheses are ranges.
P < 0.01 for total versus free.
In the same assay, the impact of enzymatic hydrolysis on 8-hydroxyefavirenz was studied. Free and total plasma concentration-time curves for placebo- and rifampin-treated subjects are shown in Fig. 1C and D, respectively. The corresponding pharmacokinetic parameters derived are listed in Table 1. The total Cmax and AUC0-72 of 8-hydroxyefavirenz after enzymatic hydrolysis were significantly higher in the placebo phase (10.3-fold [P = 0.005] and 9.8-fold [P = 0.005], respectively) and in the rifampin phase (by 15.2-fold [P = 0.005] and 8.8-fold [P = 0.005], respectively) than the Cmax and AUC0-72 of the free 8-hydroxyefavirenz (Table 1). It is likely that the conjugated metabolite mainly represents the O-glucuronide of 8-hydroxyefavirenz (21), but the enzymatic hydrolysis method used (β-glucuronidase treatment) contains sulfatase and did not allow us to distinguish a glucuronide from a sulfate conjugate with certainty.
To obtain additional information regarding the efficiency of deconjugation of efavirenz N-glucuronide, hydrolysis by HCl was compared with no hydrolysis (control) and enzymatic hydrolysis by β-glucuronidase. The means ± SDs of the total efavirenz concentrations were 1.49 ± 0.62, 1.45 ± 0.64, and 1.43 ± 0.79 μg/ml for the no hydrolysis, enzymatic hydrolysis, and acid hydrolysis groups, respectively (P = 0.96).
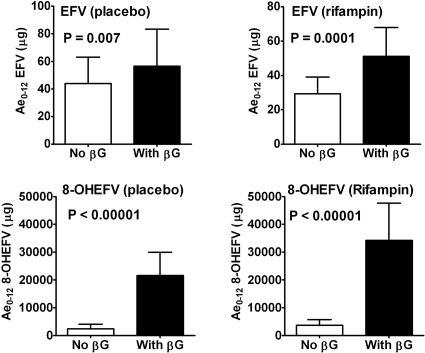
To determine the amount of total and free efavirenz and 8-hydroxyefavirenz excreted over 12 h in urine (Ae0-12; in hours), urine samples were treated with β-glucuronidase (total) or without β-glucuronidase (free). As shown in Fig. 2, the total amount of efavirenz excreted in urine over 12 h (after enzymatic hydrolysis) was significantly larger than the amount of free efavirenz excreted in urine for the placebo-treated (P = 0.007) and rifampin-treated (P = 0.0001) groups. The total amount of 8-hydroxyefavirenz in urine was much higher than the free amount (P < 0.00001 for both placebo- and rifampin-treated groups) (Fig. 2). The total amount of efavirenz and 8-hydroxyefavirenz excreted over 12 h represented less than 0.02% and 7% of the administered dose, respectively, in both treatment groups. Efavirenz N-glucuronide was reported to be abundant at early time points after administration of efavirenz to experimental animals (21) and thus to be abundant in plasma samples.
FIG. 2.
Amounts of EFV and 8-hydroxyefavirenz (8-OHEFV) excreted over 12 h (Ae0-12) after a single 600-mg oral dose of EFV in healthy volunteers (n = 10) treated with placebo pills (control) or rifampin (600 mg/day) for 10 days. Total and free represent conjugated plus unconjugated (β-glucuronidase [βG] treatment) and unconjugated (no β-glucuronidase treatment), respectively.
DISCUSSION
In the present study, we have shown that efavirenz can undergo N-glucuronidation, as revealed by the moderately but significantly larger amount of total efavirenz excreted over 12 h in urine compared to the amount recovered as free efavirenz; the abundance of circulating efavirenz N-glucuronide in the systemic circulation is negligible; rifampin enhances efavirenz metabolism probably via induction of CYP2B6-mediated 8-hydroxylation, and 8-hydroxyefavirenz exists primarily as a conjugate in plasma and urine samples. This information should contribute to improved understanding of the complex metabolism of efavirenz in humans and the mechanisms underlying drug interactions and pharmacogenomics of this clinically important drug.
The lack of any statistically significant difference between the free and total plasma efavirenz concentrations (or the associated pharmacokinetic parameters) in the present study indicates that the abundance of efavirenz N-glucuronide in the systemic circulation is negligible. As glucuronide metabolites can be rapidly excreted into bile and/or urine, the low circulating efavirenz N-glucuronide concentration by itself may not negate the possibility that direct glucuronidation is a significant metabolic pathway. Indeed, the amount of total efavirenz (conjugated plus unconjugated) excreted over 12 h in urine was moderately but significantly larger than the amount recovered as free (unconjugated) efavirenz, supporting the findings of previous studies that efavirenz can undergo direct N-glucuronidation in vivo and in vitro (3, 8, 21). However, the quantitative contribution of N-glucuronidation to efavirenz clearance seems small because the relative amount of unconjugated or conjugated efavirenz excreted via the urine represents a minor fraction of the dose administered (21). The possibility that biliary excretion may serve as the primary route of elimination of the N-glucuronide metabolite was not tested in the current study, but this unlikely impacts efavirenz exposure, probably due to the potential of enterohepatic circulation resulting from deconjugation of the glucuronide metabolite in the gut flora. Kwara et al. reported a significant association between the UGT2B7*1a allele and the efavirenz middose concentration in HIV-infected patients (16), but this association lacks validation. That study was conducted with HIV-infected patients taking multiple drugs, and only single point concentrations were considered. The functional relevance of the UGT2B7*1a allele is not fully understood.
We found no evidence that rifampin, a drug known to induce multiple drug transporters and drug-metabolizing enzymes (24, 26), including UGT2B7 (2, 10, 23), enhances efavirenz elimination through induction of N-glucuronidation. The plasma concentration-time curves of free and total efavirenz overlapped in the rifampin-treated group. The total/free ratios of efavirenz plasma concentrations (and the associated pharmacokinetic parameters) in the rifampin-treated group were not significantly different from those in the placebo-treated group. Although higher urine ratios of total/free amounts of efavirenz were observed in the rifampin-treated group (1.9-fold) than in the placebo-treated group (1.4-fold), the absolute amount of free efavirenz was lower in the rifampin than the placebo treatment group. On the other hand, it was found that rifampin substantially increases the total plasma concentrations of 8-hydroxyefavirenz and its amounts in urine, suggesting pronounced induction of the 8-hydroxylation pathway. It is also interesting to note that the amount of total (conjugated plus unconjugated) 8-hydroxyefavirenz was substantially larger than that of the free form (unconjugated) in both treatment groups, demonstrating that 8-hydroxyefavirenz is rapidly conjugated and exists primarily as a conjugate (21). Efavirenz 8-hydroxylation is the main clearance mechanism of efavirenz (21). Although multiple CYPs show activity toward this reaction in vitro, this pathway is predominantly catalyzed by CYP2B6 in vitro (5, 25, 29) and probably in vivo (15, 30). As rifampin is known to induce CYP2B6 in vitro (9, 19) and in vivo (14, 18), our data support the current knowledge that efavirenz is mainly cleared through CYP2B6-mediated hydroxylation and rifampin enhances its elimination via induction of CYP2B6.
The present study shows that conjugated 8-hydroxyefavirenz was highly susceptible to enzymatic hydrolysis and that the β-glucuronidase used in this study was able to deconjugate efavirenz N-glucuronide in urine, which should serve as a positive control. For certain N-glucuronide metabolites (e.g., olanzapine 10-N-glucuronide) that undergo incomplete or ineffective deconjugation by β-glucuronidase, acid hydrolysis may be effective (13). However, our data suggest that the yield of total efavirenz after acid hydrolysis was not different from that after enzymatic hydrolysis. In addition, findings from a previous study (21) and the urine data are consistent with the ability of β-glucuronidase to effectively hydrolyze efavirenz N-glucuronide. Therefore, the lack of a significant difference between free and total plasma efavirenz concentrations cannot be attributed to faulty β-glucuronidase or an incomplete (lack) of enzymatic hydrolysis.
One limitation of this study is the relatively short plasma and urine sampling time for a drug with a long half-life, which may not allow accurate estimation of efavirenz elimination parameters. However, the data presented are valid for the questions addressed in this report. It is likely that efavirenz undergoes first-pass N-glucuronidation by the hepatic UGT2B7 and that the N-glucuronide is more water soluble and thus more readily eliminated than the hydrophobic parent drug, efavirenz. Thus, the abundance of the N-glucuronide metabolite should be high at earlier times and its formation is rate limited at later times. This was observed with the pharmacokinetics of the hydroxylated metabolites of efavirenz. If there was a significant role for involvement of N-glucuronidation, a difference between total and free efavirenz should have been revealed to be a result of first-pass metabolism at early times rather than at later times (terminal elimination phase), where the pharmacokinetics of the N-glucuronide will be primarily controlled by the half-life of the efavirenz. Consistent with this suggestion, a previous study reported that the N-glucuronide was more prominent in urine and bile at early times of sample collection (0 to 4 h) than at later times after efavirenz administration (21). Since we did not find any meaningful difference in the plasma concentration of total and free efavirenz at earlier or later times, the lack of a significant difference likely reflects a low abundance of circulating efavirenz N-glucuronide. Another limitation of this study is that, due to the unavailability of standard efavirenz N-glucuronide, an indirect (subtractive) method which may be less sensitive than direct measurement was used to quantitate plasma efavirenz N-glucuronide concentrations. Despite these limitations, the role that N-glucuronidation plays in efavirenz elimination remains negligible, given that efavirenz is primarily cleared through oxidation by CYPs (21, 25, 29) and that the fraction of efavirenz dose metabolized through the N-glucuronidation route appears to be small, as shown in the present data and previous study (21).
In summary, analysis of urine data provides evidence that efavirenz can undergo direct conjugation (N-glucuronidation), while the exposure (abundance) of efavirenz N-glucuronide in plasma was negligible in placebo- or rifampin-treated subjects. Given that the excretion of efavirenz in the urine represents less than 1% of the dose (21, 29, 25), the present data and evidence in the literature (21) suggest that the quantitative contribution of N-glucuronidation to efavirenz metabolism is limited. These findings are consistent with the suggestion that efavirenz clearance is mainly controlled by CYP2B6-mediated 8-hydroxylation (and to some extent by CYP2A6-catalyzed 7- and 8-hydroxylation) (25, 29) and that rifampin enhances the elimination of a single oral dose of efavirenz through induction of CYP2B6 (and/or CYP2A6) (9, 19).
Acknowledgments
The project described was supported by award number R01GM078501 from the National Institute of General Medical Sciences and grant number M01-RR000042 from the National Center for Research Resources (NCRR), National Institutes of Health (Bethesda, MD).
The content is solely our responsibility and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
Footnotes
Published ahead of print on 31 January 2011.
REFERENCES
- 1.Arab-Alameddine, M. 2009. Pharmacogenetics-based population pharmacokinetic analysis of efavirenz in HIV-1-infected individuals. Clin. Pharmacol. Ther. 85:485-494. [DOI] [PubMed] [Google Scholar]
- 2.Barbier, O., et al. 2000. 3′-azido-3′-Deoxythimidine (AZT) is glucuronidated by human UDP-glucuronosyltransferase 2B7 (UGT2B7). Drug Metab. Dispos. 28:497-502. [PubMed] [Google Scholar]
- 3.Belanger, A. S., et al. 2009. Glucuronidation of the antiretroviral drug efavirenz (EFV) by UGT2B7 and an in vitro investigation of drug-drug interaction with zidovudine (AZT). Drug Metab. Dispos. 37:1793-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Csajka, C., et al. 2003. Population pharmacokinetics and effects of efavirenz in patients with human immunodeficiency virus infection. Clin. Pharmacol. Ther. 73:20-30. [DOI] [PubMed] [Google Scholar]
- 5.Desta, Z., et al. 2007. Impact of CYP2B6 polymorphism on hepatic efavirenz metabolism in vitro. Pharmacogenomics 8:547-558. [DOI] [PubMed] [Google Scholar]
- 6.Dheda, K., F. C. Lampe, M. A. Johnson, and M. C. Lipman. 2004. Outcome of HIV-associated tuberculosis in the era of highly active antiretroviral therapy. J. Infect. Dis. 190:1670-1676. [DOI] [PubMed] [Google Scholar]
- 7.DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. 2011. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. U.S. Department of Health and Human Services, Washington, DC. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed January 2011.
- 8.di Iulio, J., et al. 2009. In vivo analysis of efavirenz metabolism in individuals with impaired CYP2A6 function. Pharmacogenet. Genomics 19:300-309. [DOI] [PubMed] [Google Scholar]
- 9.Faucette, S. R., et al. 2004. Regulation of CYP2B6 in primary human hepatocytes by prototypical inducers. Drug Metab. Dispos. 32:348-358. [DOI] [PubMed] [Google Scholar]
- 10.Gallicano, K. D., et al. 1999. Induction of zidovudine glucuronidation and amination pathways by rifampicin in HIV-infected patients. Br. J. Clin. Pharmacol. 48:168-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Innocenti, F., et al. 2008. Single nucleotide polymorphism discovery and functional assessment of variation in the UDP-glucuronosyltransferase 2B7 gene. Pharmacogenet. Genomics 18:683-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kassahun, K., E. Mattiuz, R. Franklin, and T. Gillespie. 1998. Olanzapine 10-N-glucuronide. A tertiary N-glucuronide unique to humans. Drug Metab. Dispos. 26:848-855. [PubMed] [Google Scholar]
- 13.Kassahun, K., et al. 1997. Disposition and biotransformation of the antipsychotic agent olanzapine in humans. Drug Metab. Dispos. 25:81-93. [PubMed] [Google Scholar]
- 14.Kharasch, E. D., D. Mitchell, and R. Coles. 2008. Stereoselective bupropion hydroxylation as an in vivo phenotypic probe for cytochrome P4502B6 (CYP2B6) activity. J. Clin. Pharmacol. 48:464-474. [DOI] [PubMed] [Google Scholar]
- 15.King, J., and J. A. Aberg. 2008. Clinical impact of patient population differences and genomic variation in efavirenz therapy. AIDS 22:1709-1717. [DOI] [PubMed] [Google Scholar]
- 16.Kwara, A., M. Lartey, K. W. Sagoe, E. Kenu, and M. H. Court. 2009. CYP2B6, CYP2A6 and UGT2B7 genetic polymorphisms are predictors of efavirenz mid-dose concentration in HIV-infected patients. AIDS 23:2102-2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwara, A., M. Lartey, K. W. Sagoe, N. L. Rzek, and M. H. Court. 2009. CYP2B6 (c.516G→T) and CYP2A6 (*9B and/or *17) polymorphisms are independent predictors of efavirenz plasma concentrations in HIV-infected patients. Br. J. Clin. Pharmacol. 67:427-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loboz, K. K., et al. 2006. Cytochrome P450 2B6 activity as measured by bupropion hydroxylation: effect of induction by rifampin and ethnicity. Clin. Pharmacol. Ther. 80:75-84. [DOI] [PubMed] [Google Scholar]
- 19.Madan, A., et al. 2003. Effects of prototypical microsomal enzyme inducers on cytochrome P450 expression in cultured human hepatocytes. Drug Metab. Dispos. 31:421-431. [DOI] [PubMed] [Google Scholar]
- 20.Marzolini, C., et al. 2001. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS 15:71-75. [DOI] [PubMed] [Google Scholar]
- 21.Mutlib, A. E., et al. 1999. Identification and characterization of efavirenz metabolites by liquid chromatography/mass spectrometry and high field NMR: species differences in the metabolism of efavirenz. Drug Metab. Dispos. 27:1319-1333. [PubMed] [Google Scholar]
- 22.Mutlib, A. E., et al. 2000. The species-dependent metabolism of efavirenz produces a nephrotoxic glutathione conjugate in rats. Toxicol. Appl. Pharmacol. 169:102-113. [DOI] [PubMed] [Google Scholar]
- 23.Naesens, M., et al. 2006. Rifampin induces alterations in mycophenolic acid glucuronidation and elimination: implications for drug exposure in renal allograft recipients. Clin. Pharmacol. Ther. 80:509-521. [DOI] [PubMed] [Google Scholar]
- 24.Niemi, M., J. T. Backman, M. F. Fromm, P. J. Neuvonen, and K. T. Kivisto. 2003. Pharmacokinetic interactions with rifampicin: clinical relevance. Clin. Pharmacokinet. 42:819-850. [DOI] [PubMed] [Google Scholar]
- 25.Ogburn, E. T., et al. 2010. Efavirenz primary and secondary metabolism in vitro and in vivo: identification of novel metabolic pathways and cytochrome P450 (CYP) 2A6 as the principal catalyst of efavirenz 7-hydroxylation. Drug Metab. Dispos. 38:1218-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rae, J. M., M. D. Johnson, M. E. Lippman, and D. A. Flockhart. 2001. Rifampin is a selective, pleiotropic inducer of drug metabolism genes in human hepatocytes: studies with cDNA and oligonucleotide expression arrays. J. Pharmacol. Exp. Ther. 299:849-857. [PubMed] [Google Scholar]
- 27.Rotger, M., et al. 2007. Predictive value of known and novel alleles of CYP2B6 for efavirenz plasma concentrations in HIV-infected individuals. Clin. Pharmacol. Ther. 81:557-566. [DOI] [PubMed] [Google Scholar]
- 28.Stahle, L., L. Moberg, J. O. Svensson, and A. Sonnerborg. 2004. Efavirenz plasma concentrations in HIV-infected patients: inter- and intraindividual variability and clinical effects. Ther. Drug Monit. 26:267-270. [DOI] [PubMed] [Google Scholar]
- 29.Ward, B. A., et al. 2003. The cytochrome P4502B6 (CYP2B6) is the main catalyst of efavirenz primary and secondary metabolism: implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP2B6 catalytic activity. J. Pharmacol. Exp. Ther. 306:287-300. [DOI] [PubMed] [Google Scholar]
- 30.Zanger, U. M., et al. 2007. Polymorphic CYP2B6: molecular mechanisms and emerging clinical significance. Pharmacogenomics 8:743-759. [DOI] [PubMed] [Google Scholar]