Abstract
The MICs of echinocandins against Candida isolates with fks mutations are higher than those for wild-type (WT) isolates. However, the MIC ranges for susceptible and mutant populations overlap or are poorly separated. It was recently reported that a greater separation could be achieved in the presence of serum. To more fully explore this possibility, we compared the performances of the reference microdilution methods by using standard and bovine serum albumin (BSA)-supplemented growth medium. Anidulafungin, caspofungin, and micafungin MICs were determined according to EUCAST and CLSI methods and with 50% BSA in the medium for 93 clinical isolates, including Candida albicans (20/10 [number of isolates/number of mutants]), C. glabrata (19/10), C. dubliniensis (2/1), C. krusei (16/3), C. parapsilosis (19), and C. tropicalis (19/4) isolates. Stability of the plates was tested after storage at −80°C for 2 and 6 months, and the performance of two different lots of caspofungin was investigated. The addition of BSA to the medium resulted in higher MICs (1 to 9 2-fold dilution steps) for all isolates and compounds. The increases were greatest for anidulafungin and micafungin and, among WT isolates, for C. parapsilosis. The number of very major errors (VMEs) was reduced (24% [20/84 isolates] versus ≤7% [6/84 isolates]) using BSA-supplemented EUCAST medium but not using BSA-supplemented CLSI medium (6% versus 9%). MIC results were unchanged after 6 months of storage of test plates. The two lots of caspofungin yielded identical results. Addition of BSA to the EUCAST medium increases the ability to differentiate between WT isolates and isolates harboring resistance mutations.
Three echinocandin class drugs, anidulafungin, caspofungin, and micafungin, are licensed for the treatment of invasive candidiasis. Following increased use, sporadic cases of failures associated with elevated MICs have been reported. In the majority of cases, these failures have been associated with mutations in two hot spot regions of the FKS genes, which encode the target and major subunit of the 1,3-β-d-glucan synthase complex (4, 6, 18, 21, 22, 28, 29). Consequently, close monitoring and robust susceptibility testing methods have become increasingly important. For both CLSI and EUCAST reference methods, the MICs of the three echinocandins against isolates with fks mutations are higher than those for wild-type (WT) isolates, but the ranges for these susceptible and mutant populations either overlap one another or are separated by only 1 or 2 dilution steps, making correct identification of hot spot mutant isolates challenging (5). Since reproducibility as well as appropriate classification of susceptibility end points into susceptible (S), intermediate (I), and resistant (R) categories is highly correlated with the distance between MIC ranges for WT and mutant isolates, a modification of the reference methods achieving more separation would be a major step forward.
Earlier studies demonstrated that the addition of human serum to MIC assay media neutralizes differences between the in vitro properties of the echinocandin drugs (24, 26, 38). A recent preliminary study (14) reported that the addition of 50 mg/ml bovine serum albumin (BSA) to the growth medium leads to more separation between WT and fks mutant isolates. We therefore undertook the present study to investigate in a systematic manner if the addition of 50 mg/ml BSA to the growth medium would alter the discriminatory potential of the CLSI and EUCAST microdilution methods. Furthermore, we evaluated the robustness of the EUCAST assay by examining the stability of EUCAST susceptibility plates stored for up to half a year at −80°C.
We previously reported that while microdilution testing for anidulafungin and caspofungin performed equally well with respect to separation between WT and fks mutant isolates, caspofungin MIC distributions appear to be variable due to unknown factors (4). For this reason, we included two different lots of pure caspofungin in the study and retrospectively retrieved repeated MIC values for eight control strains representing different species.
MATERIALS AND METHODS
Isolates.
A well-characterized set of WT and fks hot spot mutant isolates was used (5), including 93 clinical isolates and two reference strains (Candida parapsilosis ATCC 22019 and C. krusei ATCC 6258). Clinical isolates included 10 FKS WT and 10 fks hot spot mutant C. albicans isolates, 9 FKS WT and 10 fks hot spot mutant C. glabrata isolates, 1 FKS WT and 1 fks hot spot mutant C. dubliniensis isolate, 13 FKS hot spot WT and 3 fks hot spot mutant C. krusei isolates, 19 FKS WT C. parapsilosis isolates, and 15 FKS hot spot WT and 4 fks hot spot mutant C. tropicalis isolates. Three isolates were found to harbor mutations outside the resistance hot spots and were regarded as WT concerning echinocandin susceptibility because of their normal kinetic inhibition properties (D. S. Perlin, unpublished data). Thus, a total of 28 isolates with characteristic echinocandin resistance mutations in the FKS hot spot regions were included. All isolates were coded, and tests were performed blinded for susceptibility patterns.
Compounds.
Pure substances were provided by the manufacturers (one lot of anidulafungin by Pfizer, two lots of caspofungin by Merck [TEK0010 and VEK0090], and one lot of micafungin by Astellas). Stock solutions were prepared in water (for CLSI testing) or in dimethyl sulfoxide (DMSO; Sigma) (for EUCAST testing), taking into account the potencies of the powders.
Retrospective comparison of pure caspofungin batches.
Caspofungin MIC results were retrieved retrospectively for four different caspofungin lots (NEK0040, TEK0010, LEK0030, and an unnamed batch received from Merck) and the following reference strains used routinely as quality controls: C. krusei ATCC 6258, C. parapsilosis ATCC 22019, C. albicans ATCC 64548, C. tropicalis CL-3412REX, C. glabrata ATCC 90030, Saccharomyces cerevisiae ATCC 9763, and C. lusitaniae CL-3408REX. The number of repetitions performed with these strains ranged from 7 to 21 times per caspofungin lot.
EUCAST microdilution.
EUCAST microdilution was performed strictly according to the standard (EDef 7.1) (35) and additionally using medium supplemented with 50 mg/ml BSA (BAH66-0500; Equitech Bio, Inc., Kerrville, TX). Three hundred fifty plates were prepared in one batch, sealed in aluminum foil, and stored at −80°C for 2 weeks, 2 months, and 6 months before susceptibility testing was performed. Microtiter plates were read spectrophotometrically at 490 nm after 24 h, and MICs were determined using 50% growth inhibition. C. krusei ATCC 6258 and C. parapsilosis ATCC 22019 were used as quality control strains throughout all experiments. In addition to the wild-type upper limit (WT-UL) values generated in the current study (see below), the following tentative EUCAST anidulafungin breakpoints were applied: for C. albicans, S breakpoint of ≤0.03 μg/ml; and for C. glabrata, C. krusei, and C. tropicalis, S breakpoint of ≤0.06 μg/ml.
CLSI microdilution.
CLSI microdilution was performed strictly according to the CLSI M27-A3 standard (9) and additionally using medium supplemented with 50 mg/ml BSA. Plates were stored at −86°C for a maximum of 15 days before use. Microtiter plates were read visually, and the MICs were determined using prominent inhibition (corresponding to 50%) as the end point. C. krusei ATCC 6258 and C. parapsilosis ATCC 22019 were used as quality control strains in all experiments. In addition to the upper limit values generated in the current study, the following revised echinocandin breakpoints for C. albicans, C. glabrata, C. krusei, and C. tropicalis were applied: S breakpoint of ≤0.25 μg/ml, except for micafungin and C. glabrata, in which case the S breakpoint was ≤0.06 μg/ml (33a).
FKS gene sequence analysis.
FKS gene sequence analysis was performed previously for all isolates (5).
Evaluation of test performance.
For each of the drug-bug combinations, the following parameters were used to evaluate and compare the test performances. The distance between end-point ranges (MICs) for fks hot spot mutant isolates and WT isolates was calculated as the number of 2-fold dilution steps. Negative values indicate the degree of overlap, expressed as the number of dilution steps involved in the overlap. Overlap was defined as the number of end points for the fks hot spot mutant isolates that overlapped with the end-point range for the WT populations. The WT-UL was defined as two 2-fold dilution steps higher than the MIC50. If the population was truncated, with all MIC values at or below the lowest concentration tested, the WT-UL was defined as two times the lowest dilution tested. The number of very major errors (VMEs) was the number of fks hot spot mutant isolates with MICs lower than or equal to the WT-UL. Finally, the number of very major errors was evaluated by applying the tentative EUCAST and revised CLSI breakpoints for susceptibility as mentioned above.
RESULTS
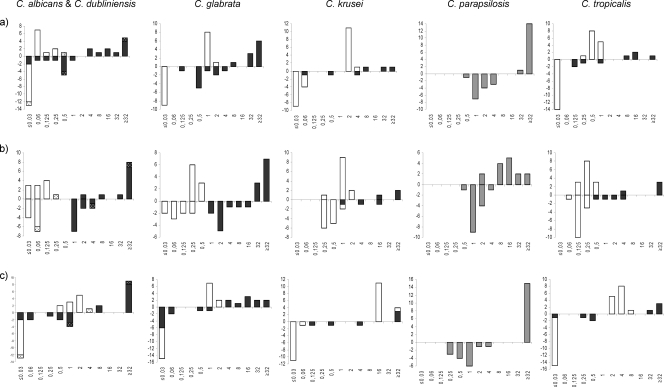
EUCAST microdilution testing performed according to the EUCAST Edef 7.1 method but using medium supplemented with 50 mg/ml BSA resulted in higher MIC values for all drug-bug combinations (Fig. 1). Overall, WT and fks hot spot mutant populations were separated by 1 to 8 dilution steps, and no overlap was observed between WT and fks hot spot mutant isolates for any of the drug-Candida sp. combinations (micafungin MICs for C. krusei extended above the tested range for WT as well as mutant populations and thus could not be evaluated for potential overlap). Using the WT-UL as a breakpoint for susceptibility, 1 (4%) VME was observed for anidulafungin (1/3 C. krusei mutants with a heterozygous F655F/C substitution in Fks1p), none were observed for caspofungin, and 2 (7%) VMEs were observed for micafungin (2/11 C. glabrata mutants, one with a D632G Fks1p substitution and one with a D666E Fks2p substitution) (Table 1 ).
FIG. 1.
MIC distributions obtained following EUCAST methodology but supplementing the medium with 50% BSA (above the x axis) and obtained by the standard procedure (below the x axis) for susceptible isolates (white bars) and FKS mutant isolates (black bars). (C. dubliniensis isolates are indicated with dotted bars.) (a) MICs of anidulafungin. (b) MICs of caspofungin. (c) MICs of micafungin.
TABLE 1.
WT-UL values and numbers of VMEs by antifungal compound and species for the EUCAST and CLSI methods performed with and without BSAa
| Compound and species | EUCAST results |
CLSI results |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WT-UL (μg/ml) |
No. of VMEs/no. of isolates |
WT-UL (μg/ml) |
No. of VMEs/no. of isolates |
|||||||
| Reference method | With BSA supplementation | Reference method | With BSA supplementation | Using EUCAST breakpointsb | Reference method | With BSA supplementation | Reference method | With BSA supplementation | Using revised CLSI breakpoints | |
| Anidulafungin | NA | NA | 4/28 | 1/28 | 3/28 | NA | NA | 1/27 | 2/27 | 6/27 |
| C. albicans | 0.06 | 0.25 | 3/10 | 0/10 | 2/10 | 0.03 | 0.25 | 0/10 | 0/10 | 2/10 |
| C. dubliniensis | 0.06 | 2 | 0/1 | 0/1 | NA | 0.03 | 0.5 | 0/1 | 0/1 | 0/1 |
| C. glabrata | 0.06 | 4 | 0/10 | 0/10 | 0/10 | 0.125 | 2 | 1/9 | 1/9 | 3/9 |
| C. krusei | 0.125 | 8 | 1/3 | 1/3 | 1/3 | 0.25 | 4 | 0/3 | 1/3 | 0/3 |
| C. parapsilosis | 4 | >64 | NA | NA | NA | 4 | 64 | NA | NA | NA |
| C. tropicalis | 0.06 | 2 | 0/4 | 0/4 | 0/4 | 0.03 | 0.5 | 0/4 | 0/4 | 1/4 |
| Caspofungin | NA | NA | 2/28 | 0/28 | NA | NA | NA | 0/27 | 0/27 | 3/27 |
| C. albicans | 0.25 | 0.25 | 0/10 | 0/10 | NA | 0.03 | 0.25 | 0/10 | 0/10 | 1/10 |
| C. dubliniensis | 0.25 | 1 | 0/1 | 0/1 | NA | 0.03 | 0.25 | 0/1 | 0/1 | 0/1 |
| C. glabrata | 0.25 | 1 | 0/10 | 0/11 | NA | 0.125 | 1 | 0/9 | 0/9 | 1/9 |
| C. krusei | 1 | 4 | 1/3 | 0/3 | NA | 0.5 | 4 | 0/3 | 0/3 | 0/3 |
| C. parapsilosis | 4 | ≥64 | NA | NA | NA | 1 | 8 | NA | NA | NA |
| C. tropicalis | 0.5 | 1 | 1/4 | 0/4 | NA | 0.06 | 0.5 | 0/4 | 0/4 | 1/4 |
| Micafungin | NA | NA | 14/28 | 2-5/28 | NA | NA | NA | 4/27 | ≥5/27 | 7/27 |
| C. albicans | 0.06 | 4 | 4/10 | 0/10 | NA | 0.03 | 2 | 0/10 | 0/10 | 2/10 |
| C. dubliniensis | 0.06 | 16 | 0/1 | 0/1 | NA | 0.25 | 2 | 0/1 | 0/1 | 0/1 |
| C. glabrata | 0.06 | 4 | 8/10 | 2/11 | NA | 0.06 | 4 | 4/9 | 3/9 | 4/9 |
| C. krusei | 0.125 | 64 | 1/3 | ≤3/3 | NA | 0.5 | 32 | 0/3 | ≥1/3 | 0/3 |
| C. parapsilosis | 4 | ≥64 | NA | NA | NA | 4 | >32 | NA | NA | NA |
| C. tropicalis | 0.06 | 16 | 1/4 | 0/4 | NA | 0.125 | 8 | 0/4 | 1/4 | 1/4 |
| Total | NA | NA | 24% | 4-7% | 11% | NA | NA | 6% | 9% | 20% |
NA, not applicable/available.
For C. albicans, S breakpoint of ≤0.03 μg/ml; for C. glabrata, C. krusei, and C. tropicalis, S breakpoint of ≤0.06 μg/ml.
In comparison, WT and fks hot spot mutant MIC populations were separated by −1 to 6 dilution steps with the reference EUCAST method, with the MIC range for C. krusei overlapping those for the WT population for anidulafungin and caspofungin (in both cases involving the isolate with the F655F/C substitution in Fks1p) (Fig. 1). For 4 drug-bug combinations, potential overlap could not be excluded, as MICs for the WT as well as fks mutant isolates were below the lowest dilution tested (anidulafungin with C. albicans and micafungin with C. albicans, C. glabrata, and C. tropicalis). Using the WT-UL as a breakpoint for susceptibility, four VMEs were observed for the reference method: these were for all three echinocandins and 1/3 C. krusei mutants with the F655F/C substitution in Fks1p and for caspofungin and 1/4 C. tropicalis mutants with an F76S substitution in Fks1p (Table 1). Finally, applying the tentative EUCAST breakpoints for anidulafungin, VMEs were observed for 3/28 (11%) mutants.
CLSI microdilution testing.
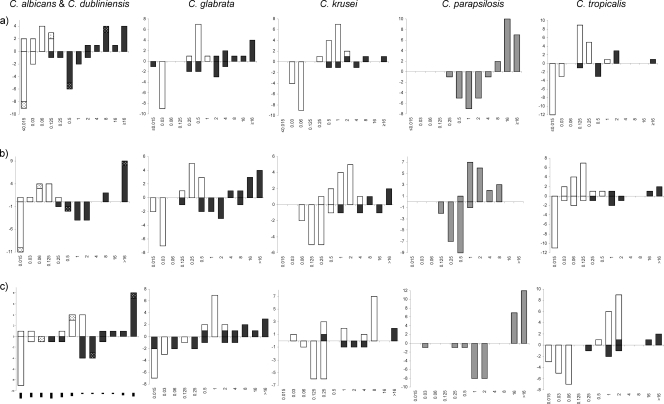
The influence of using BSA-supplemented growth medium was less uniform for the CLSI method. For C. albicans, no overlap or misclassifications from applying the WT-UL were observed, and WT and fks mutant populations were separated by four, four, and two 2-fold dilution steps for anidulafungin, caspofungin, and micafungin, respectively, in contrast to two, at least four, and two dilution steps for the reference method (Fig. 2). For the other species, overlap was observed for anidulafungin (1 C. krusei isolate with the F655F/C mutation) and micafungin (2 C. glabrata isolates, with Fks1p D632G and Fks2p F659V mutations, 1 C. krusei isolate with the F655F/C mutation, and 1 C. tropicalis isolate with the F76S mutation) with BSA-supplemented medium and for anidulafungin (1 C. glabrata isolate with the Fks2p P667T mutation) and micafungin (4 C. glabrata isolates, with Fks2p P667T and D632G, Fks2p F659V, and Fks2p D666G mutations) with the reference medium. Using the WT-UL as a breakpoint for susceptibility, seven VMEs were observed for the BSA-modified test, compared to five for the reference method (for two C. krusei isolates with MICs of >16 μg/ml, potential VMEs could not be evaluated) (Table 1). Most VMEs involved micafungin (5 VMEs) and C. glabrata (3 VMEs). Notably, no VMEs were observed for caspofungin and any of the species or CLSI-based methods (Table 1). Finally, applying the recently revised CLSI breakpoints for echinocandins, six VMEs were observed for anidulafungin (22%), three for caspofungin (11%), and eight for micafungin (30%) (Table 1).
FIG. 2.
MIC distributions obtained following CLSI methodology but supplementing the medium with 50% BSA (above the x axis) and obtained by the standard procedure (below the x axis) for susceptible isolates (white bars) and FKS mutant isolates (black bars). (C. dubliniensis isolates are indicated with dotted bars.) (a) MICs of anidulafungin. (b) MICs of caspofungin. (c) MICs of micafungin.
C. parapsilosis.
The EUCAST MIC50 (1 μg/ml) and WT-UL (4 μg/ml) for C. parapsilosis and all three echinocandins were considerably higher than those for the other species. Addition of 50 mg/ml BSA elevated the MIC ranges, particularly for anidulafungin and micafungin (MIC50 ≥ 32 μg/ml) (Fig. 1). The same was true for the CLSI-based methods, as MIC50 values for anidulafungin, caspofungin, and micafungin were 1, 0.25, and 1 μg/ml, respectively, for the standard method and 16, 2, and >32 μg/ml, respectively, for the BSA-modified method (Fig. 2).
Stability of plates stored at −80°C.
Storage of EUCAST plates with and without BSA for up to 6 months did not affect the performance of the susceptibility plates for any of the three echinocandins or either of the two media (Table 2). Overall, 95.4% of the results were either identical (69.2%) or within one dilution step after 2 months of storage, and the same was true for 94.6% (65.5%) of the results after 6 months, with equal distributions above and below the mean for the few isolates differing by two or more 2-fold dilutions for both time points (Table 2).
TABLE 2.
Influence of storage of prepared susceptibility plates on susceptibility results and comparison of two different lots of pure caspofungina
| Method and storage time or lots of drug | No. of isolates with MIC change (no. of 2-fold dilutions) |
||||||
|---|---|---|---|---|---|---|---|
| −3 | −2 | −1 | 0 | 1 | 2 | 3 | |
| Two months of storage | |||||||
| EUCAST Edef 7.1 | |||||||
| Anidulafungin | 7 | 69 | 12 | ||||
| Caspofungin TEK0010 | 4 | 20 | 51 | 18 | 1 | ||
| Caspofungin VEK0090 | 4 | 20 | 50 | 13 | 1 | ||
| Micafungin | 4 | 5 | 68 | 9 | 5 | ||
| BSA-modified EUCAST | |||||||
| Anidulafungin | 10 | 70 | 9 | ||||
| Caspofungin TEK0010 | 2 | 11 | 60 | 13 | 2 | 1 | |
| Caspofungin VEK0090 | 2 | 9 | 63 | 11 | 3 | 1 | |
| Micafungin | 1 | 1 | 11 | 66 | 10 | 1 | |
| Six months of storage | |||||||
| EUCAST Edef 7.1 | |||||||
| Anidulafungin | 11 | 68 | 8 | 1 | |||
| Caspofungin TEK0010 | 3 | 19 | 50 | 20 | 3 | ||
| Caspofungin VEK0090 | 5 | 16 | 40 | 26 | 2 | ||
| Micafungin | 1 | 8 | 66 | 12 | 2 | ||
| BSA-modified EUCAST | |||||||
| Anidulafungin | 2 | 6 | 62 | 16 | 2 | 1 | |
| Caspofungin TEK0010 | 3 | 2 | 18 | 57 | 8 | 1 | |
| Caspofungin VEK0090 | 2 | 4 | 10 | 61 | 10 | 2 | |
| Micafungin | 1 | 1 | 11 | 66 | 10 | 1 | |
| Two lots of caspofungin | |||||||
| EUCAST Edef 7.1 | 1 | 11 | 64 | 13 | |||
| BSA-modified EUCAST | 8 | 47 | 7 | ||||
Susceptibility plates were stored for 2 and 6 months, and MIC results were compared with the results obtained after less than 1 week of freezing.
Batch-to-batch variation of caspofungin.
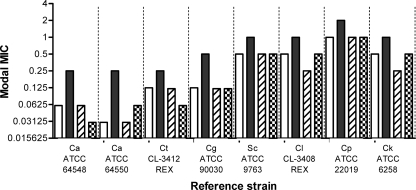
A retrospective compilation of MIC values for eight reference strains and four lots of caspofungin is shown in Fig. 3. One lot, TEK0010, consistently yielded higher modal MICs for all eight reference strains. The difference was less pronounced for C. parapsilosis ATCC 22019 and C. krusei ATCC 6258 than for the two C. albicans control strains. The 93 isolates were tested against the two available and most recently produced caspofungin batches, TEK0010 and VEK0090. The MICs were in 100% agreement, within 2 MIC steps, with identical MICs for the vast majority of the isolates (Table 2).
FIG. 3.
MIC variation among four different lots of caspofungin. Eight reference strains were tested according to the EUCAST methodology and using the following four different lots of caspofungin: NEK0040 (white bars; 7 repetitions), TEK0010 (black bars; 6 repetitions) (the batch also used in this study), LEK0030 (hatched bars; 18 repetitions), and unknown (checkerboard bars; 21 repetitions). Ca, C. albicans; Ct, C. tropicalis; Cg, C. glabrata; Sc, S. cerevisiae; Cl, C. lusitaniae; Cp, C. parapsilosis; Ck, C. krusei.
DISCUSSION
A direct comparison of MIC values obtained by EUCAST and CLSI methods with and without BSA supplementation was used to evaluate the discriminatory potential for differentiating fks hot spot mutants from WT isolates and then to compare the performances of the individual tests with respect to this parameter, expressed as distance between the populations, number of overlaps, and number of VMEs according to WT upper limits and proposed breakpoints.
BSA supplementation of the growth medium improved the discriminatory potential of the EUCAST test in terms of more separation of the WT and fks mutant populations leading to fewer overlaps and fewer fks mutants classified as susceptible by application of a WT upper limit as the breakpoint for susceptibility. The greater degree of separation was due to a more pronounced increase in MICs for fks mutants than for WT isolates. The distance between the WT population of each species and the individual mutants depended on the location of the mutation. Thus, the echinocandin MICs were consistently lower for one C. krusei mutant, leading to this isolate being misclassified by the majority of tests. This isolate had a heterozygous substitution in Fks1p at position F655 and may represent a less-resistant phenotype. For C. albicans, substitutions at Fks1p D648 and Fks1p P649 were associated with the lowest MICs, and similarly, C. glabrata isolates with amino acid substitutions at Fks1p D632 and Fks2p S663 and higher were the least-resistant mutants in vitro, with and without BSA in the medium. This is in agreement with differences in susceptibility based on the glucan synthase enzyme complex, which are dependent on the position of the amino acid substitution (13). It remains to be assessed if these in vitro observations translate into a differential susceptibility in vivo and thus if the challenges associated with the discrimination of these isolates from the wild-type isolates by in vitro susceptibility testing are less crucial.
The least separation between WT and fks hot spot mutant populations was observed for micafungin and C. glabrata, and although EUCAST testing with BSA eliminated overlap between the two populations, VMEs were still observed. Hypothetically, the more discrete micafungin MIC elevation may reflect better activity than that of the other two echinocandins against fks hot spot mutants of C. glabrata. However, C. glabrata breakthrough infections during micafungin therapy were recently described, involving isolates with mutations at Fks1p S629 or Fks2p S663 (34). In that study, the CLSI MICs for mutant isolates were 4 to 8 μg/ml and thus higher than those seen for similar fks1 and fks2 mutants in our study. Thus, the in vivo activity of micafungin in comparison with those of the other echinocandins in the treatment of C. glabrata fks hot spot mutants deserves further study.
CLSI testing with BSA-supplemented medium performed less well than expected for the species other than C. albicans. This is in contrast with the initial findings in the previous pilot study that prompted this investigation (14). The reason behind the apparent differential impact on the different methods and species is not understood, but it may partially reflect the fact that the tests were performed in different laboratories. One limitation associated with this study, however, is that the echinocandin concentration range in this study resulted in truncation of some of the distributions. Future studies with wider concentration ranges are needed to explore if the performance of CLSI testing with BSA-supplemented medium was underestimated in our study.
The MICs of all three echinocandins were higher for C. parapsilosis than for the other species, as previously described (10, 25, 31, 33). C. parapsilosis has a naturally occurring mutation at FKS1 hot spot 1, accounting for the elevated MIC levels (12). However, the overall clinical response for invasive C. parapsilosis infections treated with echinocandins is comparable to that for infections with the other Candida species (19, 23, 27, 33), which may be due in part to C. parapsilosis being less virulent (2, 3, 15-17, 37). Interestingly, the MIC increases for anidulafungin and micafungin tested in the presence of BSA were more pronounced than that for caspofungin, leading to MIC values that were notably higher than the peak concentrations obtained during treatment. This observation might suggest that these two compounds are less active against C. parapsilosis infections. So far, one study has compared the clinical outcomes for two echinocandins head to head, without demonstrating a difference in outcome between micafungin and caspofungin for C. parapsilosis (27). Thus, the potential implications of the observed differences in MICs in the presence of BSA remain uncertain.
We previously reported variability associated with caspofungin susceptibility testing, despite tests being performed in reference laboratories and correcting for potency, and showed that caspofungin values obtained following the EUCAST methodology are typically higher than those obtained following the CLSI standard (4, 5). Here we demonstrated that the variability was more pronounced for the C. albicans reference strains than for the most frequently used control strains, C. krusei ATCC 6258 and C. parapsilosis ATCC 22019. This observation is in line with the fact that variability between MIC ranges is most pronounced for species other than C. parapsilosis when comparing MIC ranges reported in different studies (1, 4, 7, 8, 20, 30-33) and indicates that variability may go unnoticed when C. krusei ATCC 6258 or C. parapsilosis ATCC 22019 is used as a quality control strain. One hypothesis has been that the higher glucose concentration in the EUCAST medium might affect potency, particularly if the plates are not used immediately. We found no change in MIC results after up to 6 months of storage, and a head-to-head comparison with 0.2 and 2% glucose in the growth medium was performed recently, without revealing any difference between the two (36). Another possibility is that potency may be affected by the choice of solvent for stock solutions of caspofungin. Water is recommended by both EUCAST and CLSI standards, but from a chemical point of view, the substance is more soluble in DMSO. In this study, we replaced water with DMSO for the stock preparation of caspofungin for the EUCAST plates and obtained systematically lower MIC values than those obtained by running the same strain collection in a previous study using water as the solvent (5).
In conclusion, this study demonstrates for the first time that the addition of BSA to the test medium improves the ability of the EUCAST reference method to separate fks hot spot mutants from WT isolates, that susceptibility plates are stable for up to 6 months of storage, and that variability of caspofungin MIC values is observed by systematically comparing different lots of caspofungin. For the CLSI method, however, a benefit of adding BSA to the medium was seen only for C. albicans, and correct classification of fks hot spot mutants is still challenging, even after the revision of the CLSI echinocandin breakpoints. Therefore, mutational analysis is currently the most precise way to detect echinocandin resistance. However, this approach may not yet be applicable for every isolate and routine laboratory and has the limitation of not detecting resistance due to other resistance mechanisms, should they exist.
Acknowledgments
We thank Birgit Brandt for excellent technical assistance. We thank Francoise Dromer, Unité de Mycologie Moléculaire, Institut Pasteur, Paris, France, for providing one of the echinocandin fks hot spot mutant C. krusei isolates (11). We thank Astellas for providing pure micafungin, Merck for providing pure caspofungin, and Pfizer for providing pure anidulafungin.
This study was supported in part by research grants from the investigator-initiated study programs of Astellas Pharma, Merck & Co Inc., and Pfizer Aps. Additionally, it was supported by NIH grant AI069397 to D.S.P.
The opinions expressed in this paper are those of the authors and do not necessarily represent those of the pharmaceutical companies.
M.C.A. has been a consultant for Astellas, Merck, Pfizer, and SpePharm, has been an invited speaker for Astellas, Cephalon, Merck Sharp & Dohme, Pfizer, Schering-Plough, and Swedish Orphan, and received research funding for this particular study from Astellas, Merck, and Pfizer. D.S.P. is a shareholder in Merck, has acted as a consultant for Merck, Pfizer, and Astellas, is an advisory board member for Merck, Pfizer, Astellas, and Myconostica, holds the patent “Assays for resistance to echinocandin-class drugs” (patent application 07763-O69WO1), has received research funding (although not for this particular study) from Merck, Pfizer, Astellas, and Myconostica, and has been an invited speaker for Merck, Pfizer, Astellas, and Myconostica. In the past 5 years, J.-L.R.-T. has received grant support from Astellas Pharma, Gilead Sciences, Merck Sharp & Dohme, Pfizer, Schering Plough, Soria Melguizo SA, the European Union, the Spanish Agency for International Cooperation, the Spanish Ministry of Culture and Education, The Spanish Health Research Fund, The Instituto de Salud Carlos III, The Ramon Areces Foundation, and The Mutua Madrileña Foundation. He has been an advisor/consultant to the Panamerican Health Organization, Gilead Sciences, Merck Sharp & Dohme, Myconostica, Pfizer, and Schering Plough. He has been paid for talks on behalf of Gilead Sciences, Merck Sharp & Dohme, Pfizer, and Schering Plough. In the past 5 years, M.C.-E. has received grant support from Astellas Pharma, bioMérieux, Gilead Sciences, Merck Sharp & Dohme, Pfizer, Schering Plough, Soria Melguizo SA, the European Union, the ALBAN program, the Spanish Agency for International Cooperation, the Spanish Ministry of Culture and Education, The Spanish Health Research Fund, The Instituto de Salud Carlos III, The Ramon Areces Foundation, and The Mutua Madrileña Foundation. He has been an advisor/consultant to the Panamerican Health Organization, Gilead Sciences, Merck Sharp & Dohme, Pfizer, and Schering Plough. He has been paid for talks on behalf of Gilead Sciences, Merck Sharp & Dohme, Pfizer, and Schering Plough. G.G.-E., G.D., and A.G.L. have no conflicts of interest.
Footnotes
Published ahead of print on 18 January 2011.
REFERENCES
- 1.Alexander, B. D., et al. 2007. Comparative evaluation of Etest and Sensititre YeastOne panels against the Clinical and Laboratory Standards Institute M27-A2 reference broth microdilution method for testing Candida susceptibility to seven antifungal agents. J. Clin. Microbiol. 45:698-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almirante, B., et al. 2006. Epidemiology, risk factors, and prognosis of Candida parapsilosis bloodstream infections: case-control population-based surveillance study of patients in Barcelona, Spain, from 2002 to 2003. J. Clin. Microbiol. 44:1681-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arendrup, M., T. Horn, and N. Frimodt-Moller. 2002. In vivo pathogenicity of eight medically relevant Candida species in an animal model. Infection 30:286-291. [DOI] [PubMed] [Google Scholar]
- 4.Arendrup, M. C., et al. 2009. Breakthrough Aspergillus fumigatus and Candida albicans double infection during caspofungin treatment: laboratory characteristics and implication for susceptibility testing. Antimicrob. Agents Chemother. 53:1185-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arendrup, M. C., et al. 2010. Echinocandin susceptibility testing of Candida species: comparison of EUCAST EDef 7.1, CLSI M27-A3, Etest, disk diffusion, and agar dilution methods with RPMI and Isosensitest media. Antimicrob. Agents Chemother. 54:426-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baixench, M. T., et al. 2007. Acquired resistance to echinocandins in Candida albicans: case report and review. J. Antimicrob. Chemother. 59:1076-1083. [DOI] [PubMed] [Google Scholar]
- 7.Chryssanthou, E., and M. Cuenca-Estrella. 2002. Comparison of the Antifungal Susceptibility Testing Subcommittee of the European Committee on Antibiotic Susceptibility Testing proposed standard and the E-test with the NCCLS broth microdilution method for voriconazole and caspofungin susceptibility testing of yeast species. J. Clin. Microbiol. 40:3841-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cisterna, R., et al. 2010. Nationwide sentinel surveillance of bloodstream Candida infections in 40 tertiary care hospitals in Spain. J. Clin. Microbiol. 48:4200-4206. doi: 10.1128/JCM.00920-10. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard, 3rd ed. CLSI document M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA.
- 10.Dannaoui, E., et al. 2008. Comparative in vitro activities of caspofungin and micafungin, determined using the method of the European Committee on Antimicrobial Susceptibility Testing, against yeast isolates obtained in France in 2005-2006. Antimicrob. Agents Chemother. 52:778-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desnos-Ollivier, M., et al. 2008. Mutations in the fks1 gene in Candida albicans, C. tropicalis and C. krusei correlate with elevated caspofungin MICs uncovered in AM3 medium using the EUCAST method. Antimicrob. Agents Chemother. 52:3092-3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Effron, G., S. K. Katiyar, S. Park, T. D. Edlind, and D. S. Perlin. 2008. A naturally occurring proline-to-alanine amino acid change in Fks1p in Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis accounts for reduced echinocandin susceptibility. Antimicrob. Agents Chemother. 52:2305-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Effron, G., S. Park, and D. S. Perlin. 2009. Correlating echinocandin MIC and kinetic inhibition of fks1 mutant glucan synthases for Candida albicans: implications for interpretive breakpoints. Antimicrob. Agents Chemother. 53:112-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Effron, G., S. Park, and D. S. Perlin. 2009. Improved Candida sp. echinocandin susceptibility determination by the addition of bovine serum albumin, abstr. M-352. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 15.Garey, K. W., et al. 2006. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin. Infect. Dis. 43:25-31. [DOI] [PubMed] [Google Scholar]
- 16.Hajjeh, R. A., et al. 2004. Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J. Clin. Microbiol. 42:1519-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horn, D. L., et al. 2009. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin. Infect. Dis. 48:1695-1703. [DOI] [PubMed] [Google Scholar]
- 18.Katiyar, S., M. Pfaller, and T. Edlind. 2006. Candida albicans and Candida glabrata clinical isolates exhibiting reduced echinocandin susceptibility. Antimicrob. Agents Chemother. 50:2892-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuse, E. R., et al. 2007. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet 369:1519-1527. [DOI] [PubMed] [Google Scholar]
- 20.Laupland, K. B., D. B. Gregson, D. L. Church, T. Ross, and S. Elsayed. 2005. Invasive Candida species infections: a 5 year population-based assessment. J. Antimicrob. Chemother. 56:532-537. [DOI] [PubMed] [Google Scholar]
- 21.Laverdiere, M., et al. 2006. Progressive loss of echinocandin activity following prolonged use for treatment of Candida albicans oesophagitis. J. Antimicrob. Chemother. 57:705-708. [DOI] [PubMed] [Google Scholar]
- 22.Miller, C. D., B. W. Lomaestro, S. Park, and D. S. Perlin. 2006. Progressive esophagitis caused by Candida albicans with reduced susceptibility to caspofungin. Pharmacotherapy 26:877-880. [DOI] [PubMed] [Google Scholar]
- 23.Mora-Duarte, J., et al. 2002. Comparison of caspofungin and amphotericin B for invasive candidiasis. N. Engl. J. Med. 347:2020-2029. [DOI] [PubMed] [Google Scholar]
- 24.Odabasi, Z., V. Paetznick, J. H. Rex, and L. Ostrosky-Zeichner. 2007. Effects of serum on in vitro susceptibility testing of echinocandins. Antimicrob. Agents Chemother. 51:4214-4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ostrosky-Zeichner, L., et al. 2003. Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob. Agents Chemother. 47:3149-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paderu, P., et al. 2007. Serum differentially alters the antifungal properties of echinocandin drugs. Antimicrob. Agents Chemother. 51:2253-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pappas, P. G., et al. 2007. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin. Infect. Dis. 45:883-893. [DOI] [PubMed] [Google Scholar]
- 28.Park, S., et al. 2005. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob. Agents Chemother. 49:3264-3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pasquale, T., J. R. Tomada, M. Ghannoun, J. Dipersio, and H. Bonilla. 2008. Emergence of Candida tropicalis resistant to caspofungin. J. Antimicrob. Chemother. 61:219. [DOI] [PubMed] [Google Scholar]
- 30.Perlin, D. S. 2007. Resistance to echinocandin-class antifungal drugs. Drug Resist. Updat. 10:121-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfaller, M. A., et al. 2008. In vitro susceptibility of invasive isolates of Candida spp. to anidulafungin, caspofungin, and micafungin: six years of global surveillance. J. Clin. Microbiol. 46:150-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pfaller, M. A., and D. J. Diekema. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20:133-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfaller, M. A., et al. 2008. Correlation of MIC with outcome for Candida species tested against caspofungin, anidulafungin, and micafungin: analysis and proposal for interpretive MIC breakpoints. J. Clin. Microbiol. 46:2620-2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33a.Pfaller, M. A., et al. Drug Resist. Updat., in press.
- 34.Pfeiffer, C. D., et al. 2010. Breakthrough invasive candidiasis in patients on micafungin. J. Clin. Microbiol. 48:2373-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez-Tudela, J. L., et al. 2008. EUCAST definitive document EDef 7.1: method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts. Clin. Microbiol. Infect. 14:398-405. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez-Tudela, J. L., et al. 2010. Comparison of caspofungin MICs by means of EUCAST method EDef 7.1 using two different concentrations of glucose. Antimicrob. Agents Chemother. 54:3056-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tortorano, A. M., et al. 2004. Epidemiology of candidaemia in Europe: results of 28-month European Confederation of Medical Mycology (ECMM) hospital-based surveillance study. Eur. J. Clin. Microbiol. Infect. Dis. 23:317-322. [DOI] [PubMed] [Google Scholar]
- 38.Wiederhold, N. P., et al. 2007. In vivo efficacy of anidulafungin and caspofungin against Candida glabrata and association with in vitro potency in the presence of sera. Antimicrob. Agents Chemother. 51:1616-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]