Abstract
Bac8c (RIWVIWRR-NH2) is an 8-amino-acid peptide derived from Bac2A (RLARIVVIRVAR-NH2), a C3A/C11A variant of the naturally occurring bovine peptide, bactenecin (also known as bovine dodecapeptide), the smallest peptide with activity against a range of pathogenic Gram-positive and Gram-negative bacteria, as well as yeast. The effects of Bac8c on Escherichia coli were examined by studying its bacteriostatic and bactericidal properties, demonstrating its effects on proton motive force generation, and visually analyzing (via transmission electron microscopy) its effects on cells at different concentrations, in order to probe the complexities of the mechanism of action of Bac8c. Results were consistent with a two-stage model for the Bac8c mode of action. At sublethal concentrations (3 μg/ml), Bac8c addition resulted in transient membrane destabilization and metabolic imbalances, which appeared to be linked to inhibition of respiratory function. Although sublethal concentrations resulted in deleterious downstream events, such as methylglyoxal formation and free radical generation, native E. coli defense systems were sufficient for full recovery within 2 h. In contrast, at the minimal bactericidal concentration (6 μg/ml), Bac8c substantially but incompletely depolarized the cytoplasmic membrane within 5 min and disrupted electron transport, which in turn resulted in partial membrane permeabilization and cell death.
Cationic antimicrobial peptides (AMPs; also termed host defense peptides for their selective immunomodulatory properties) are an integral component of the immune system and are produced by organisms in all domains of life (2, 9). Natural AMPs are generally 12 to 50 amino acids in length, contain excess positively charged amino acids (lysine and arginine residues) and around 50% hydrophobic amino acids, and fold into a diversity of amphiphilic structures upon contact with microbial membranes. AMPs have recently become a focus for drug design due to high-throughput studies that generated multiple synthetic peptides active against a broad spectrum of pathogens (14). The aim of these studies was to create small peptides (6 to 20 amino acids in length) that were highly potent, demonstrated no to low resistance, and were active against a broad range of bacterial and fungal pathogens, making such compounds particularly attractive as platforms for the development of novel antimicrobials. However, while much progress has been made in creating new peptides, the modes of action of these small synthetic peptides, as well as many naturally occurring peptides, remain poorly understood. Indeed, it has been suggested that the mechanisms of action of AMPs involve either direct interaction with the cytoplasmic membrane or translocation into the cytoplasm to access nonmembrane targets; however, it is more likely that these peptides have complex mechanisms of action involving a multiplicity of targets (33). Indeed, a broad variety of studies have implicated inhibition of DNA, RNA, and protein synthesis, inhibition or specific binding to DNA, inhibition of enzymatic activity, activation of autolysins, inhibition of septum formation, and inhibition of cell wall formation as targets of various AMPs (2, 8, 42). The lack of detailed knowledge of the complexity of the AMP mode(s) of action continues to limit our understanding of structure-activity relationships and thus our ability to take full advantage of these compounds (24).
Recent studies have applied protein evolution and engineering approaches to the development of novel AMPs (12-14). These approaches work through the integration of multiple strategies for designing peptide libraries, such as quantitative structure-activity-based machine learning, high-throughput methods, inexpensive peptide array syntheses, and rapid screens for antimicrobial activity (14). We have described the use of such approaches to not only develop many AMPs with increased activity but also to improve our understanding of AMP structure-activity relationships at the level of the whole organism (14). Bac8c (RIWVIWRR-NH2) is an 8-amino-acid peptide derived from Bac2A (RLARIVVIRVAR-NH2) with four favorable amino acid substitutions (the other amino acids found in both peptides are underlined) as determined by a complete substitution analysis of Bac2A (14). It is even smaller than bactenecin (also known as bovine dodecapeptide), the smallest known broad-spectrum natural antimicrobial peptide, but it has enhanced activity against a range of pathogenic Gram-positive and Gram-negative bacteria, as well as yeast (44). The parent molecule, Bac2A, is known to cause moderate membrane permeation and depolarization, while transmission electron microscopy (TEM) images of Gram-positive cells challenged by Bac2A showed a variety of effects, including defects at the septum, cell wall fraying, mesosome formation, and nuclear condensation at the high concentrations tested, 10× the MIC (6). These results led to the conclusion that Bac2A could kill by plural effects, in a manner not dependent on membrane disruption as the first step in cell death. The current study was directed at understanding the complexity of the mechanism of action of the improved derivative, Bac8c. In initial studies, we found that Bac8c demonstrated a rapid and dramatic onset of killing of Escherichia coli over a relatively narrow concentration range. We used this property to investigate its mechanism of action by performing a series of experiments at concentrations leading to complete killing (6 μg/ml) or 50% growth inhibition with no killing (3 μg/ml). Our results were consistent with a multistage model for Bac8c. At sublethal concentrations (3 μg/ml), Bac8c addition resulted in transient membrane destabilization and metabolic imbalances, which appeared to be linked to inhibition of respiratory function. In contrast, at the minimal bactericidal concentration (MBC; 6 μg/ml), Bac8c depolarized the cytoplasmic membrane within 5 min and disrupted electron transport, increased membrane permeability, and caused >99% cell death within 150 min.
MATERIALS AND METHODS
Bacteria, plasmids, and materials.
E. coli strain Mach1-T1R (Invitrogen, Carlsbad, CA), wild-type W strain (ATCC 9637; obtained from S. A. Waksman), and Mach1-T1R F− [φ80(lacZ)ΔM15 ΔlacX74 hsdR(rK− mK+) ΔrecA1398 endA1 tonA] containing the pSMART-LCKAN empty vector were used for all control studies. Overnight cultures were grown in Luria-Bertani (LB) medium. Growth curves were carried out in 3-(N-morpholino)propanesulfonic acid (MOPS) minimal medium (28). For all experiments that required antibiotic to maintain the vector, kanamycin (KAN) was used at 30 μg/ml. Bac8c was synthesized by using N-(9-fluorenyl)methoxy carbonyl chemistry (GenScript Corporation, Piscataway, NJ). TO-PRO-3,(3,3′-diethyloxacarbocyanine iodide) carbocyanine dye [DiSCO2(3)], 3′-(p-hydroxyphenyl) fluorescein (HPF), and carbonyl cyanide m-chlorophenylhydrazone (CCCP) were purchased from Invitrogen. ATP was measured with the BacTiter-Glo microbial cell viabilty assay kit (Promega).
E. coli strain CGSC 4908 (Δhis-67 ΔthyA43 Δpyr-37), which is auxotrophic for thymidine, uridine, and l-histidine (29), was kindly supplied by the E. coli Genetic Stock Center (Yale University, New Haven, CT). The titrated precursors [methyl-3H]thymidine (25.0 Ci/mmol), [5-3H]uridine (26.0 Ci/mmol), and l-[2,5-3H]histidine (46.0 Ci/mmol) were purchased from Fisher Scientific. NAD+ and NADH standards, yeast alcohol dehydrogenase, phenazine sulfate (PES), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Fisher Scientific.
Specific growth and killing assays.
For growth rate determinations, each clone was inoculated from a −80°C stock, cultured in 5 ml LB with KAN, and incubated overnight in a 15-ml conical tube at 37°C with shaking. Each overnight culture was diluted in MOPS minimal medium (with KAN and 0.1% glucose) to an optical density at 600 nm (OD600) of 0.4 before inoculating conical tubes with 1 to 10% (vol/vol) inoculum (starting OD600, 0.1). Fifteen-milliliter conical tubes were incubated at 37°C with shaking, and absorbance was monitored routinely. Triplicate blank vector control flasks were run in parallel for all growth experiments. The specific growth rate was calculated by determining the optimal fit of linear trend lines by analyzing the R2 value. Amino acid supplements, or NAD+ precursors, were added at a concentration of 0.04% (wt/vol). Bac8c was added at the 50% inhibitory concentration (IC50) or the MBC of the control without supplementation.
MICs.
MICs were determined aerobically in a 96-well plate format as previously described (43). Overnight cultures of strains were grown aerobically shaking at 37°C in 5 ml LB (with antibiotic when required for plasmid maintenance). A 1% (vol/vol) inoculum was introduced into a 15-ml culture of MOPS minimal medium. As samples reached mid-exponential phase, the culture was diluted to an OD600 of 0.5. The cells were diluted 1:1,000, and a 90-μl aliquot was used to inoculate each well of a 96-well plate (final concentration, ∼105 CFU/ml). The plates were arranged to measure the growth of various strains and growth conditions in increasing Bac8c concentrations, from 0 to 60 μg/ml, in 2-fold increments (14). MICs were determined as the lowest concentration at which no visible growth was observed after incubation at 37°C for 18 h.
Determination of lytic properties of Bac8c.
Turbidity was evaluated with Mach1-T1R, BW25113, and BW25113recA::KAN cultures grown to early exponential phase (OD600, 0.2) in MOPS minimal medium, 2-ml aliquots were added to 15-ml conical tubes, Bac8c was added to the desired final concentration (in μg/ml), and the samples were incubated in a shaking 37°C incubator. At each time point, the OD600 was measured every hour for 8 h and again at 24 h after addition of Bac8c. The positive control for cellular lysis was the addition of 100 μl sodium hypochlorite.
Cytoplasmic membrane depolarization measures in the DiOC2(3) assay and fluorescence-activated cell sorting.
The BacLight bacterial membrane potential kit (Invitrogen) provides a fluorescent membrane potential indicator dye, DiOC2(3), along with CCCP and premixed buffer. At low concentrations, DiOC2(3) exhibits green fluorescence in all bacterial cells; however, as it becomes more concentrated in healthy cells that are maintaining a membrane potential, it causes the dye to self-associate and the fluorescence emission to shift to red. The red and green fluorescent bacterial populations are easily distinguished by using a flow cytometer. CCCP (10 μl of a 500 μM stock) is included in the kit for use as a control because it eradicates the proton gradient, eliminating the bacterial membrane potential (29). In Gram-negative bacteria, such as E. coli, a DiOC2(3) response is observed in the presence of a membrane potential, but the response does not appear to be proportional to the proton gradient intensity. The experiment was performed as described in the manual for the BacLight bacterial membrane potential kit (Invitrogen). Briefly, 10 μl of 3 mM DiOC2(3) was added to 1-ml samples containing 1 × 107 cells/ml. Samples were incubated with dye for 15 to 30 min. All samples were then measured on the CyAn ADP analyzer (Beckman Coulter), and forward scatter (FSC), side scatter (SSC), and the FL1 (488/530 nm), FL3 (488/613 nm), and FL9 (633 nm) settings were used to detect cells and dyes.
Determination of membrane permeability.
Membrane permeability was determined with 500 nM TO-PRO-3 coincident with the use of the dye DiOC2(3) to measure membrane potential. TO-PRO-3 exhibits substantially increased fluorescence on binding to intracellular nucleic acids; it normally bears two positive charges and is excluded from cells with intact membranes, but it can stain nucleic acids in cells with damaged membranes (29). Cells killed by polymyxin B or Bac2A were used as positive controls for TO-PRO-3, and CCCP was used as a negative control.
Determination of structural interference by Bac8c.
The method for negative-stained ultrathin sections was adapted from that reported by Marcellini et al. (25). Cells were grown as for the growth kinetic experiments (OD600, 0.1) in MOPS minimal medium. Fifteen-milliliter cultures were challenged with various concentrations of Bac8c for 30 min before being centrifuged (5 min at 5,000 × g), the supernatant was removed, and the pellets were washed twice with sodium phosphate buffer. The cells were fixed in 2.5% glutaraldehyde in 100 mM sodium cacodylate buffer, pH 7.4 (CB) for 2 h at 4°C, washed three times for 10 min in CB, and postfixed in 1% osmium tetroxide in CB at 4°C for 1 h. Samples were dehydrated through an ascending ethanol series followed by propylene oxide treatment and finally embedded in Epon-Araldite epoxy resin. Thin sections (60 to 70 nm) were obtained with a Leica UC6 ultramicrotome and poststained with 2% uranyl acetate and lead citrate. The sections were analyzed in a Philips CM10 TEM (FEI Inc., Hillsboro, OR) and imaged using a Gatan Bioscan digital camera.
Effects of CCCP on Bac8C activity.
Killing kinetics assays were performed in the absence or presence of the metabolic uncouplers 2,4-dinitrophenol (DNP) and CCCP to determine if energization of the cytoplasmic membrane was required for activity. Cultures were pretreated for 10 min with 5 mM DNP or 50 mM CCCP and then incubated with 6 μg/ml Bac8c in MOPS minimal medium for 60 min. At 60 min, cells were plated and CFU/ml were quantified (34).
Microbial cell viability as determined by ATP inhibition.
The BacTiter-Glo microbial cell viability assay kit (Promega) was used to measure the effects Bac8c had on the metabolic activity of E. coli cells and to quantify the amount of residual ATP. The luminescent signal generated correlates with the number of metabolically active (viable) cells present. Replicate studies were preformed to verify the trends observed, and the protocol in the manual for ATP quantification was followed.
Microbial cell viability as determined in 2,2-dipyridyl and thiourea assays.
The method for determination of microbial cell viability was adapted from that described by Kohanski et al. (20). Standard killing kinetic assays were performed (as described above), with the OD600 and CFU/ml monitored every hour for 2 h after addition of Bac8c. For the iron chelator experiments, 2,2′-dipyridyl (Sigma) was added at a concentration of 500 μM. For hydroxyl radical quenching, thiourea (Fluka, St. Louis, MO) was added to the cultures to achieve a final concentration of 150 mM in solution. Both were added to the cultures at the same time as Bac8c. The respective concentrations of chelator and quencher used here were those determined by Kohanski et al. to minimize growth inhibition (20).
Determination of NAD+/NADH.
Dinucleotide extraction and the NAD+ cycling assay were performed as previously described by Leonardo et al. (23). Briefly, after exposure to Bac8c, cells were spun down at 5,000 × g for 2 min, supernatant was removed, and the cells were immediately frozen in a dry ice-ethanol bath. NAD+ or NADH assay buffer (for acid or base extraction, respectively) was added to each cell sample, three freeze-thaw cycles were performed, and all other procedures were performed as described previously (20, 23).
Determination of inhibition of macromolecular synthesis.
The assay for determination of inhibition of macromolecular synthesis was adapted from that of Patrzykat et al. (32). Overnight cultures of E. coli CGSC 4908 were diluted in synthetic medium and allowed to grow to the exponential phase (OD600, 0.3). The cultures were spun down and resuspended in warm MOPS medium, and 500-μl aliquots were incubated with 15 μl of either [3H]thymidine, [3H]uridine, or l-[3H]histidine and an excess of the remaining two nonlabeled supplements. After 5 min of incubation at 37°C, Bac8c was added at the specified concentrations, and samples of 50 μl were removed at 0, 5, 10, 20, 40, and 60 min and then immediately added to 5 ml of ice-cold 10% trichloroacetic acid (Fisher Scientific). After 40 min on ice and 15 min at 37°C, the samples were collected via vacuum over Whatman 25-mm GF/C glass microfiber filters (Fisher Scientific, Fair Lawn, NJ) and washed twice with 10 ml of ice-cold 10% trichloroacetic acid. The filters were dried and placed in 5-ml scintillation vials with ReadySafe liquid scintillation cocktail (Beckman, Fullerton, CA), and counts were obtained in a Beckman scintillation counter for 5 min for each filter.
Methylglyoxal formation detection.
The presence of methylglyoxal (MG) in cell extracts was assayed enzymatically with glyoxalase I (Sigma) by a modified method described by Zhu et al. (46). In this assay MG was quantitatively converted to S-d-lactoyl-glutathionine (Sigma) by glyoxalase I in the presence of reduced glutathione. The increase in S-d-lactoyl-glutathione concentration was measured by the change of absorbance at 240 nm. The reaction mixture (1 ml) contained 100 mM K2HPO4-KH2PO4 buffer, 2.5 mM reduced glutathione, 1.4 kU/liter glyoxalase I, and 100 μl of samples containing MG. Extracellular MG was measured in 100 μl of the supernatant of the broth from which the cells were removed by centrifugation as described above. The cell pellet was resuspended in 200 μl phosphate buffer, and the intracellular MG concentration was measured. The cells were lysed by three cycles of freeze-thawing, with thawing of samples on ice, and the supernatant was filtered with a 10,000 molecular weight cutoff filter (Millipore) before 100-μl aliquots of samples were used for the assay.
Hydroxyl radical formation detection using flow cytometry.
To detect hydroxyl radical formation following the exposure to Bac8C, the fluorescent reporter dye HPF (Invitrogen) was used at a concentration of 5 μM. HPF is a dye that is oxidized in the presence of hydroxyl radicals. In all experiments, cells were grown as described above. This assay has been used previously to measure hydroxyl radical formation caused by other bactericidal antibiotics that inhibit macromolecular synthesis or cell wall formation (20, 21).
RESULTS
Kinetics of Bac8c-mediated inhibition and killing.
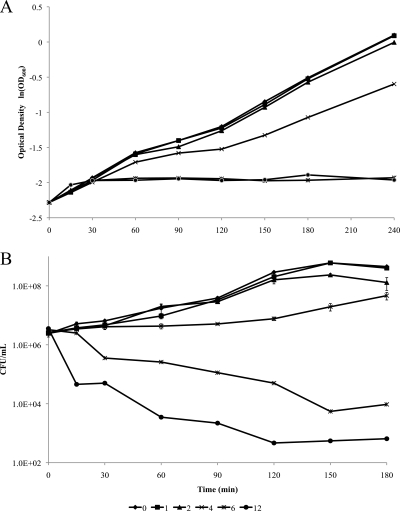
Bac8c is a synthetic AMP that was selected for this study on the basis of improved microbial killing compared to its parent peptide. Initial studies were directed at confirming its potency (the MIC, MBC, and dynamics of Bac8c killing) toward the specific E. coli strains to be used in the evaluation. The MBC was found to be 6 μg/ml and noninhibitory concentrations were ≤2 μg/ml, while growth-inhibitory (sublethal) concentrations were ∼3 μg/ml. Time-kill studies demonstrated that Bac8c killing was rapid, with >99% lethality within 15 and 150 min at 12 and 6 μg/ml, respectively (Fig. 1 A and B). Based on these data, studies were designed to identify the mechanisms of Bac8c inhibition and killing of E. coli. Our strategy was to perform mechanistic studies over a range of Bac8c concentrations that corresponded to killing, inhibitory, and noninhibitory regimens, with a specific focus on attempting to delineate the relevance of cytoplasmic and/or membrane targets in growth inhibition versus killing.
FIG. 1.
Effects of Bac8c on growth and survival of E. coli cells. (A) Effects of Bac8c on the specific growth rate of E. coli (Mach-1 T1; Invitrogen). Bac8c was added to exponentially growing cultures (time zero [x axis], OD600 of 0.1), and the growth rate was determined at intervals of 15 to 30 min for 4 h after Bac8c addition. (B) Effects of Bac8c exposure on survival of exponentially growing E. coli cells. Bac8c was added at time zero, and cells were monitored over 3 h (OD600, 0.1).
Membrane depolarization and integrity.
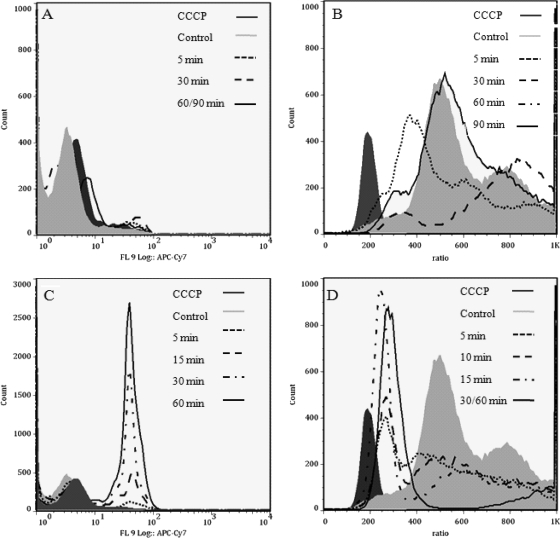
The parent peptide Bac2A is able to interact with the cytoplasmic membrane and cause moderate depolarization, in contrast to the complete depolarization reported for pore-forming peptides (6). Therefore, the membrane effects resulting from sublethal/inhibitory and bactericidal exposures to Bac8c were studied by measuring the uptake of a membrane-impermeable dye (TO-PRO-3). TO-PRO-3 is a laser-excited DNA stain that has been proposed to be impermeable to the cytoplasmic membranes of viable bacteria due to a net +2 positive charge (38). It is of note that TO-PRO-3 can reveal permeability even when there is not a change in membrane potential and can show partial effects of up to 50% for bacteriostatic drugs (e.g., tetracycline at 50%, but none with chloramphenicol) (30); thus, we consider it a test of cytoplasmic membrane permeabilization to this probe rather than an indicator of viability or lysis. Using TO-PRO-3, we found that at sublethal growth concentrations of Bac8c, E. coli remained dye impermeable (Fig. 2 A). This is consistent with the observation that no significant cell death occurred (Fig. 1B). In contrast, at bactericidal concentrations, the membrane became partially permeable to TO-PRO-3 after 30 min, which was consistent with the onset of cell death (Fig. 2C).
FIG. 2.
Membrane permeabilization (A and C) and depolarization (B and D). We examined the role of membrane permeability by using the membrane-impermeable dye TO-PRO-3. (A) Bac8c at an inhibitory concentration (3 μg/ml) did not cause an increase in membrane permeability over time. (C) After 15 min, Bac8c at the MBC caused a 1-log increase in membrane permeability in approximately 14.5% of the population, while after 60 min 65% of the population was permeabilized (see Table 1). (B and D) In parallel, membrane depolarization over time was examined in E. coli by using the dye DiSCO(3)2. (B) At the Bac8c IC50, membrane depolarization occurred within 5 min, and the population recovered from the insult within 90 min. (D) Membrane depolarization occurred within 5 min at the Bac8c MBC, and over time depolarization increased to 64% after 30 min. After 60 min of exposure, depolarization increased to 74% (data not shown). OD600, 0.1.
In parallel, we performed studies using the probe DiOC2(3) (Fig. 2B and D) to assess the effects of Bac8c on cytoplasmic membrane polarization. DiOC2(3) works by shifting from green to red fluorescence as it accumulates in cells with an intact electrical potential gradient (38), while a shift from red to green indicates membrane depolarization. We found that Bac8c at the inhibitory concentration caused partial membrane depolarization within 5 min, followed by an apparent shift back toward untreated profiles at 90 min. In contrast, at the MBC, E. coli was rapidly depolarized and was unable to reestablish membrane polarization after 30 min. These dynamics correlated in part with the cell survival dynamics (Fig. 1A), where growth restarted 90 to 120 min after inhibitory Bac8c exposure, while at the MBC, cell killing followed rapidly upon Bac8c addition. These data are summarized in Table 1.
TABLE 1.
Percentages of cells that were permeabilized or depolarizeda
| Treatment | % of gated population that showed: |
|
|---|---|---|
| Permeabilizationb | Depolarizationc | |
| None | 1.88 | 3.8 |
| CCCP (control) | 1.95 | 100 |
| Bac8c (5 min)d | 4.56 | 33.5 |
| Bac8c (10 min)d | 14.5 | 33.8 |
| Bac8c (30 min)d | 35.6 | 52.3 |
| Bac8c (60 min)d | 65 | 74 |
Results summarized here correspond to data illustrated in Fig. 2C and D.
The cutoff for permeabilization was 101 labeled cells.
The cutoff for depolarization was a ratio of 400. The ratio corresponds to the ratiometric parameter (red [depolarized cells]/green [polarized cells] used, which was calculated as follows: red value − green value + 384.
Time after Bac8c addition to cultures.
Based on these observations, we suspected that the Bac8c mode of E. coli killing involved disruption of cytoplasmic membrane function, either through direct interaction with the cytoplasmic membrane or through targets in the cytoplasm whose inhibition could result in depolarization and in turn decrease membrane stability. Thus, we next sought to characterize the effects of Bac8c on membrane stability.
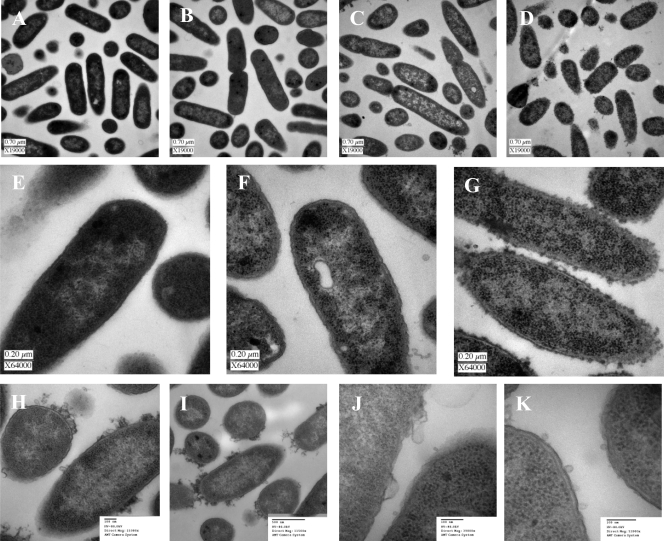
Visual interpretation of Bac8c cell envelope effects.
Electron microscopy was performed to assess any structural differences in the bacterial cells following exposure to inhibitory or bactericidal Bac8c concentrations. Images of negatively stained E. coli cells were obtained from semithin TEM sections following 30 min of bactericidal exposure. With an inhibitory concentration of Bac8c (3 μg/ml) (Fig. 3 B), no destabilization of the cell envelope was observed. In contrast, at concentrations at or above the MBC (6 μg/ml and 30 μg/ml were tested) (Fig. 3C, D, F, G, and H to K), a variety of membrane perturbations were observed. At the MBC (6 μg/ml) (Fig. 3C, F, H to K), membrane roughening and blebbing were noted, as well as some increased separation of the cytoplasmic and outer membranes, as indicated by a light ring around each cell (Fig. 3H to K). In contrast, this was not observed in control cells (Fig. 3A, no Bac8c exposure) or at inhibitory concentrations (Fig. 3B and E). Additionally, at or above the MBC there was little evidence of lysis, as revealed by little dissolution of cytoplasmic contents, evidenced by the minimal loss in density. No increases in inhibition of septum formation or lysis of cells within any samples were observed. The lack of a lytic effect was further confirmed by observations of stable optical density readings throughout 24 h of exposure to Bac8c at concentrations as high as 60 μg/ml (data not shown).
FIG. 3.
Transmission electron microscopy findings. Logarithmically growing E. coli cells were cultured in MOPS minimal medium in the presence or absence of various concentrations of Bac8c. Samples were taken after 30 min, and cells were fixed in glutaraldehyde immediately and then processed for TEM. (A) Control; (B) 3 μg/ml (IC50); (C) 6 μg/ml (MBC); (D) 30 μg/ml (5× the MBC). (E to G) At a higher magnification, the control (E), cells exposed to 6 μg/ml Bac8c (F), and cells exposed to 30 μg/ml Bac8c (G) are shown. (H to K) Alternate images of cells at the MBC (6 μg/ml), focused on the membrane effects of Bac8c.
Cytoplasmic targeting.
The studies above indicated that Bac8c killing occurs at around the same time as the perturbations of the E. coli (cytoplasmic and outer) membranes that resulted in a loss of membrane potential followed by increased permeability to a cationic dye and altered membrane morphology. Notably, these effects were observed at the MBC (6 μg/ml) but were either not observed or only transiently observed at the inhibitory concentration, which was only 2-fold lower (3 μg/ml). We were thus interested in understanding if the mechanisms of inhibition and killing were decoupled, with inhibitory mechanisms realized via inhibition of certain cytoplasmic functions.
E. coli typically maintains a proton motive force (PMF) that supports energy generation and the transport of various compounds into the E. coli cytoplasm, which includes but is not limited to certain antimicrobial peptides (42, 44) and aminoglycosides, including streptomycin and gentamicin (3, 4). To assess the potential relevance of cytoplasmic targets, we performed killing kinetic assays in the presence of CCCP, which is known to alter the PMF by shuttling protons across the membrane and abolishing the proton motive force. We found that preincubation with CCCP caused at least an 18-fold increase in survival in the presence of bactericidal levels of Bac8c after 1 h of incubation with the peptide (data not shown). These data support that Bac8c action involves either translocation into the cytoplasm or cytoplasmic membrane targeting.
Inhibition of macromolecular synthesis.
We next performed assays to examine the extent to which essential cytoplasmic processes were affected by Bac8c addition. The rate of macromolecular synthesis is dependent on a number of different factors, including ATP availability. On the other hand, in E. coli the inhibition of the membrane potential gradient with CCCP does not have an immediate effect on ATP concentrations or macromolecular synthesis per se (32). Thus, we reasoned that the dynamics of any inhibition of macromolecular synthesis by Bac8c would help delineate between possible cytoplasm and cytoplasmic membrane targets.
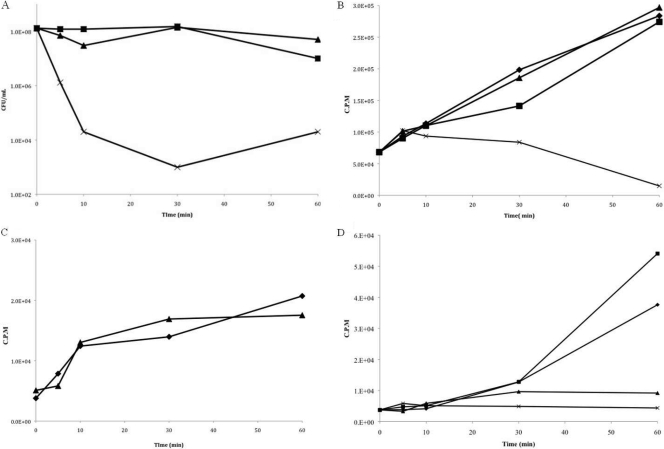
The incorporation of radiolabeled macromolecular precursors thymidine (Fig. 4 B), uridine (Fig. 4C), and histidine (Fig. 4D) was measured over 1 h, using early-log-phase E. coli cultures. At sublethal inhibitory concentrations of Bac8c (Fig. 4A, <6 μg/ml), only translation was inhibited, while at much higher concentrations (Fig. 4A, 40 μg/ml), all macromolecular processes were inhibited. Overall, we can conclude that even at inhibitory sublethal concentrations, Bac8c is able to enter the cell (as judged by the inhibition of translation) and that translation inhibition follows transient membrane depolarization (Fig. 2D).
FIG. 4.
Macromolecular synthesis. Incorporation of 3H-labeled radioactive isotopes was measured over time. (A) The CFU/ml was sampled at the same time isotope incorporation was measured. (B) Thymidine incorporation was measured to study the effects of Bac8c on DNA replication. Below the MBC, incorporation was unaffected. (C) Uridine incorporation was measured to study the effects of Bac8con transcription. Effects on transcription were seen at 40 μg/ml. (D) Histidine incorporation was measured to study the effects of Bac8c on translation. All concentrations of Bac8c had some effect on translation. In panels B to D, the y axis represents counts per minute (CPM) of radioactivity. Symbols: squares, control; diamonds, 2 μg/ml Bac8c; triangles, 4 μg/ml Bac8c; crosses, 40 μg/ml Bac8c (all in MOPS minimal medium). Cultures were started at an OD600 of 0.2.
Bac8c inhibition of ATP synthesis and electron transport chain activity.
Inhibition of translation can be achieved directly through inhibition of ribosome function (3, 45), which results in an increase in ATP pools (37), or indirectly by substrate (ATP) limitations, as would result from a loss in PMF (41). To discriminate between these two possibilities, we next measured the dynamics of ATP pools after Bac8c addition. Intracellular ATP levels were examined over 1 h following Bac8c addition at either inhibitory or bactericidal concentrations (Fig. 5 A). ATP depletion began 15 min after addition of Bac8c at inhibitory concentrations, while in comparison, at the MBC, the ATP level did not continue to rise after Bac8c addition, and depletion began within 30 min.
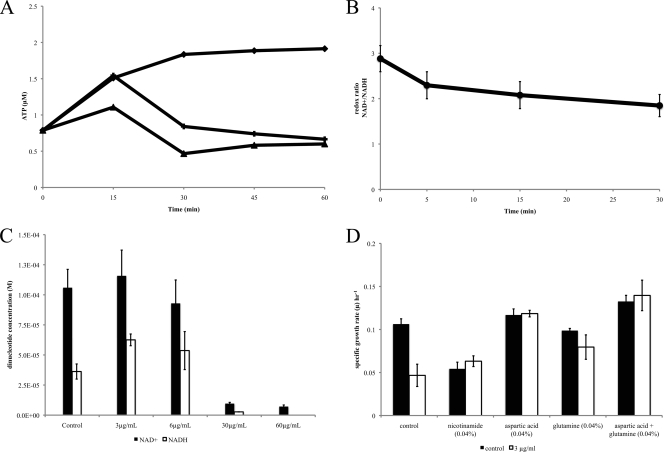
FIG. 5.
Bac8c inhibition of ATP synthesis and electron transport chain activity. (A) ATP concentration in logarithmically grown E. coli cells under normal conditions (diamonds), at the inhibitory concentration (crosses; 3 μg/ml), and the MBC (triangles; 6 μg/ml). (B) E. coli NAD+/NADH ratios after exposure to Bac8c (6 μg/ml) decreased as a function of time, reflecting decreased redox or electron transport. (C) The decrease in redox was a function of an increase in NADH and was concentration dependent. (D) Conditions that stimulated NAD+ biosynthesis also showed an increase in sublethal resistance to Bac8c. We tested the effects of supplementation with the NAD+ precursors nicotinamide (NAD+ salvage pathway), aspartate (NAD+ biosynthesis I), and glutamine (NAD+ biosynthesis I, glutamine dependent).
Based on these results, we hypothesized that at sublethal concentrations, Bac8c destabilizes the cytoplasmic membrane to a lesser extent than at the MBC. We speculated that moderate disruption of cytoplasmic membrane function could alter electron transport chain function, which if decreased would result in reduced ATP levels and possibly result in inefficient turnover in the electron transfer from NADH to NAD+. To explore this hypothesis, we next measured NAD+/NADH ratios in Bac8c-treated and untreated E. coli. We observed that at sublethal concentrations of Bac8c, the ratio of NAD+/NADH diminished rapidly after exposure to Bac8c, due to an increase in available NADH (Fig. 5B and C). Above the MBC, we observed a rapid loss of both dinucleotides (NAD+, NADH) and an abolished redox potential (at 5× and 10× the MIC). This is consistent with the loss of ATP above the MBC, which we suspect is due to membrane disruption. As with ATP, we performed MIC assays to confirm the role of NAD+/NADH in Bac8c inhibition. We found that the addition of compounds that stimulate NAD+ biosynthesis (nicotinamide, aspartic acid, and glutamine) (Fig. 5D) resulted in an increase in sublethal growth but did not increase the MIC.
The results thus far suggested that at sublethal concentrations, Bac8c disrupts normal electron flow, presumably through nonlethal destablization of the cytoplasmic membrane, resulting in a decrease in NADH turnover (Fig. 5B and C) and at least transient hyperpolarization of the cytoplasmic membrane (Fig. 2B, 30 to 90 min). Hyperpolarization of the membrane would result in the formation of superoxide radicals, which have recently been implicated in antimicrobial-based killing, as documented by Kohanski et al. (20) and observed by others (16, 20). We thus next performed tests to elucidate any role of radicals in Bac8c inhibition and/or killing.
Effect of Bac8c on free radical formation.
Radical formation in E. coli can occur either through leakage of the electron chain (6a, 7, 41) or degradation of MG, which forms as a result of disrupted glycolysis and energy metabolism (18). Thus, to further confirm a role of Bac8c in inhibition of energy metabolism, we assessed the formation of both MG and hydroxyl radicals at various Bac8c concentrations.
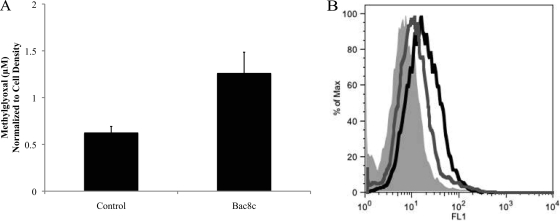
Intracellular accumulation of MG was measured by a modification of the method of Zhu et al. (46). We found that significant MG formation only occurred after 2 h of exposure at sublethal concentrations (Fig. 6 A). Similarly, by using the dye HPF, we found that hydroxyl radical formation only increased after 2 h of exposure at the MBC and increased modestly at the sublethal concentrations (Fig. 6B). These results suggested that MG and/or radical formation is a consequence of Bac8c target inhibition and/or killing rather than a cause of inhibition or killing. To test this inference, we determined if addition of compounds that reduce superoxide formation (the iron chelator 2,2-dipyridyl and the peroxide scavenger thiourea) would increase survival. Neither the killing rate nor the MIC was significantly affected by these compounds (data not shown).
FIG. 6.
Methylglyoxal accumulation and hydroxyl radical formation. (A) Intracellular accumulation of MG occurs after 2 h of incubation with sublethal levels of Bac8c (sub-MIC level of Bac8c or 6 μg/ml; OD, 0.5). (B) Flow cytometry measurement of the increase in hydroxyl radical formation by the dye HPF after 2 h with control (filled light gray), cells exposed to the IC50 (3 μg/ml; dark gray), and cells exposed to the MBC (6 μg/ml; black). Hydroxyl radical formation after 60 min of exposure to Bac8c was not significantly different from the control. OD600, 0.1.
DISCUSSION
The objective of this research was to improve our understanding of the mode of inhibition of a synthetic antimicrobial peptide, Bac8c, on E. coli. The complex sequence of observed events that are involved in the bacteriostatic and bactericidal action(s) of Bac8c are presented in Fig. 7. Interestingly, we observed that Bac8c completely and rapidly kills at an MBC of 6 μg/ml, while below this concentration (<4 μg/ml) Bac8c has a comparatively moderate inhibitory effect. Our observations were consistent with a two-stage model for Bac8c action. At or above the MBC, Bac8c appears to disrupt the proper functioning of the cytoplasmic membrane, causing rapid depolarization, which results in membrane permeation, simultaneous inhibition of all macromolecular synthesis, and rapid cell death (Fig. 7). At a sublethal concentration (3 μg/ml), cytoplasmic membrane integrity was maintained but the PMF was transiently disrupted, and cell growth continued. However, ATP synthesis, NAD+/NADH levels, and protein synthesis all decreased. This suggests that the mechanisms through which Bac8c disrupts cell function change over a small concentration range. For example, at the MBC the integrities of the outer and cytoplasmic membranes were disrupted. However, at sublethal concentrations, the function of the cytoplasmic membrane could be restored over time even though the functions of membrane-based processes (such as the electron transport chain [ETC]) appeared to be inhibited.
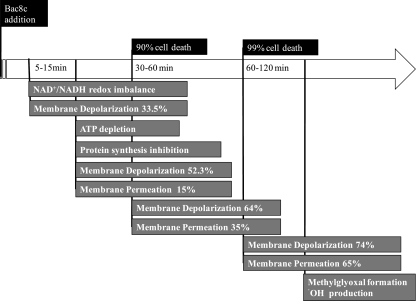
FIG. 7.
Bac8c MOA timeline. After addition of Bac8c, the first two events that occur are a decrease in the redox potential, due to an increase in NADH, and partial membrane depolarization. Within 15 min, ATP is depleted, and inhibition of macromolecular synthesis occurs. At this time, over 50% of cells have completely depolarized, yet only 15% of this population is permeabilized. Within 30 min, there is less than 10% cell survival, and there is a corresponding increase in membrane depolarization and permeabilization. Within 60 min, most of the population observed is depolarized and permeated, and 99% of the population is nonrecoverable. After 60 min, the accumulation of both MG and free radicals can be observed.
It has been proposed that many AMPs can kill bacterial cells through the disruption of membrane integrity. Here, we observed that at the MBC, we had a complete and rapid increase in membrane depolarization, which was then followed by cytoplasmic membrane permeabilization. The kinetics of these changes correlated well with those of cell killing at the time in which depolarization, not permeability alteration, signaled the lethal event (Fig. 7). TEM images of Bac8c interacting with E. coli at or above the MBC indicated that Bac8c addition caused minimal dissolution of the cytoplasmic space but significant roughing of the membrane and membrane blebbing within 30 min of exposure. Membrane blebbing, also called micellization, is indicative of the lipopolysaccharide being released from the cell surface. Membrane blebbing is caused by a variety of other AMPs, antibiotics, and conditions, such as streptomycin, ciprofloxacin, and heat-induced killing (17, 19), albeit to a lesser extent than observed for AMPs. We expect that these changes in the membrane were a major factor in killing by Bac8c but were not sufficient to result in significant loss of cytoplasmic components that would indicate lysis as demonstrated by spectrophotometry and TEM. Interestingly, at the IC50 we did not observe the same effects on membrane morphology or cytoplasmic components, suggesting that the basic mechanisms at work in killing are different from those at work in growth inhibition. We speculate that these observations could be due to Bac8c insertion into, and more profound translocation across, the cytoplasmic membrane and that a critical concentration is required for structural changes that result in killing. This type of critical concentration has been shown for several pore-forming peptides, as summarized in the review by Hancock et al. (10), but the studies reported here provide one of the best illustrations of how suddenly this occurs. Alternatively, Bac8c may largely lie parallel to the surface of the membrane at sublethal concentrations and translocate poorly across the membrane to give rise to inhibition of cytoplasmic targets, but only at or above a certain concentration, the MBC, can it more profoundly insert into the membrane and substantially disrupt critical events that require intact membranes (e.g., cell wall biosynthesis or cell division) or translocate to inhibit a broader range of cytoplasmic targets that collaborate with the membrane events to bring about killing. To obtain further information about these events, we performed a series of studies focused on macromolecular synthesis, electron trafficking, and energy metabolism.
AMPs have been shown to bind to intracellular targets, such as DNA or proteins (15), and to inhibit protein biosynthesis (22, 34, 39). We found that translation was inhibited at sublethal concentrations of Bac8c, while DNA, RNA, and protein synthesis were all inhibited at higher concentrations. While translation was inhibited, these results did not provide any insights into whether Bac8c worked in a direct or indirect manner to do so. Protein synthesis consumes more ATP than any other metabolic process (36). As ATP is produced, it is instantly consumed to make new proteins as the cell grows and divides, and therefore ATP limitation can indirectly affect translation. In contrast, if translation is inhibited, one possible outcome might be the buildup of ATP and NAD+, due to supercharging of the ETC (20, 37). With this in mind, we next measured ATP and NAD+/NADH levels at sublethal concentrations and the MBC.
We determined that ATP decreases rapidly after Bac8c addition at either sublethal concentrations or the MBC. It is known that within minutes after a dramatic loss of membrane integrity, cells lose the ability to synthesize ATP, and endogenous ATPases would destroy any remaining ATP. If cytoplasm-based ETC (i.e., respiration) and ATP synthesis were inhibited, then one would expect to observe a decrease in electron turnover via NADH oxidation, and thus a decrease in the ratio of NAD+ to NADH. Indeed, we observed a rapid decrease in this ratio after addition of Bac8c at both the sublethal concentrations and the MBC. As a further check on the effects of respiration inhibition, we determined that under anaerobic conditions the Bac8c MIC doubled. Finally, we found that activating NAD+ biosynthesis pathways increased resistance to Bac8c at sub-MIC levels. It is tempting to speculate that this resistance could come from metabolic rerouting and removing some pressure from the ETC to make ATP, or from an increased availability of dinucleotides that may leach out of the cell even at low concentrations of Bac8c. Collectively, these results demonstrate that the Bac8c mode of action involves multiple targets (i.e., direct interaction membrane, ATP depletion, redox imbalance), with evidence pointing toward direct or indirect disruption of respiratory functions located within the cytoplasmic membrane.
As the cellular respiration and electron transport chain is the main source of oxygen radicals, its functions must be tightly regulated (35). Kohanski et al. reported that hydroxyl radical formation is an important player in cell death caused by bactericidal antibiotics (5, 20, 21, 26), and they proposed that exposure to antibiotics stimulates cell respiration and consequentially accelerates endogenous formation of reactive oxygen species (ROS), resulting in cell death (11). A number of factors are known to influence the rate of hydroxyl radical generation, including the TCA cycle, NADH levels, and iron sulfur cluster assembly, which can also affect the rate of cell death (16). In addition to the factors listed above, another well-studied mechanism of hydroxyl radical formation is the production of MG, a highly toxic electrophile that is an intermediate involved in a glycolysis bypass pathway that is activated by buildup of phosphorylated sugars that are created in upstream glycolytic reactions (1).
We found that both MG accumulation and hydrogen radical formation occurred at high concentrations of Bac8c, but not significantly until 2 h after incubation with the peptide. This suggests that both MG accumulation and hydroxyl radical formation are not contributors to cell death but are by-products that build up after the cell is committed to death. We further confirmed this inference by testing Bac8c potency in conjunction with two established means of blocking hydroxyl radical formation: the application of iron chelators (16) and a hydroxyl radical scavenger (31). We showed that reduction of superoxide formation by an iron chelator or a peroxide scavenger did not increase resistance to Bac8c, or limit the rate of cell death by Bac8c, thereby further supporting the conclusion that Bac8c does not kill in a hydroxyl radical-dependent manner. These results are consistent with our results with NAD+/NADH and the accumulation of NADH in cells below the MBC. Instead of supercharging the ETC, as suggested for various antibiotics by Kohanski et al. (20), Bac8c appears to stall the ETC and thus increase relative NADH levels. If these effects were not mitigated, both superoxide formation (through electron leakage from carriers in the electron transport chains) and MG formation (through active upper glycolytic metabolism yet stalled respiration) would likely occur as downstream events (18).
We have been unable to obtain more than very moderate levels of resistance to Bac8c through extensive knockout and overexpression library screenings (unpublished data), indicating that a single gene/protein target may not exist. These results are consistent with our model of targeting cytoplasmic membrane-dependent functions, which involve the action of a large variety of proteins and other macromolecules. This makes Bac8c an attractive peptide for use in drug combinations. As an example, most efflux pumps require the PMF to function when conferring resistance to antibiotics, such as tetracycline, erythromycin, or chloramphenicol, among others (27). Addition of Bac8c, which we have shown can disrupt the PMF both transiently at low concentrations and more profoundly at higher concentrations, along with a traditional antibiotic affected by efflux mechanisms could increase efficacy and decrease the emergence of resistance. Continued studies of the mechanism of action of antimicrobial peptides are required to further develop similar strategies for taking advantage of the attractive qualities that AMPs offer as antimicrobial platforms.
Acknowledgments
Funding from the Canadian Institutes for Health Research and Advanced Foods and Materials Network to R.E.W.H. is gratefully acknowledged. R.E.W.H. holds a Canada Research Chair.
Footnotes
Published ahead of print on 31 January 2011.
REFERENCES
- 1.Booth, I. R., et al. 2003. Bacterial production of methylglyoxal: a survival strategy or death by misadventure? Biochem. Soc. Transact. 31:1406-1408. [DOI] [PubMed] [Google Scholar]
- 2.Brogden, K. A. 2005. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 3:238-250. [DOI] [PubMed] [Google Scholar]
- 3.Bryan, L. E., and S. Kwan. 1983. Roles of ribosomal binding, membrane potential, and electron transport in bacterial uptake of streptomycin and gentamicin. Antimicrob. Agents Chemother. 23:835-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Damper, P. D., and W. Epstein. 1981. Role of the membrane potential in bacterial resistance to aminoglycoside antibiotics. Antimicrob. Agents Chemother. 20:803-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farr, S. B., and T. Kogoma. 1991. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol. Rev. 55:561-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedrich, C. L., D. Moyles, T. J. Beveridge, and R. E. W. Hancock. 2000. Antibacterial action of structurally diverse cationic peptides on gram-positive bacteria. Antimicrob. Agents Chemother. 44:2086-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Gennis, R. B., and V. Stewart. 1996. Respiration, p. 217-261. In F. C. Neidhardt et al. (ed.), Escherichia Coli and Salmonella: cellular and molecular biology, vol. 1. Asm Press, Washington, DC. [Google Scholar]
- 7.Gonzalez-Flecha, B., and B. Demple. 1995. Metabolic sources of hydrogen peroxide in aerobically growing Escherichia coli. J. Biol. Chem. 270:13681-13687. [DOI] [PubMed] [Google Scholar]
- 8.Hale, J. D., and R. E. Hancock. 2007. Alternative mechanisms of action of cationic antimicrobial peptides on bacteria. Expert Rev. Anti Infect. Ther. 5:951-959. [DOI] [PubMed] [Google Scholar]
- 9.Hancock, R. E. W., and D. S. Chapple. 1999. Peptide antibiotics. Antimicrob. Agents Chemother. 43:1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hancock, R. E. W., and A. Rozek. 2002. Role of membranes in the activities of antimicrobial cationic peptides. FEMS Microbiol. Lett. 206:143-149. [DOI] [PubMed] [Google Scholar]
- 11.Hassett, D. J., and J. A. Imlay. 2007. Bactericidal antibiotics and oxidative stress: a radical proposal. ACS Chem. Biol. 2:708-710. [DOI] [PubMed] [Google Scholar]
- 12.Hilpert, K., et al. 2006. Sequence requirements and an optimization strategy for short antimicrobial peptides. Chem. Biol. 13:1101-1107. [DOI] [PubMed] [Google Scholar]
- 13.Hilpert, K., C. D. Fjell, and A. Cherkasov. 2008. Short linear cationic antimicrobial peptides: screening, optimizing, and prediction, p. 127-159. In L. Otvos (ed.), Peptide-based drug design. Humana Press, Totowa, NJ. [DOI] [PubMed]
- 14.Hilpert, K., R. Volkmer-Engert, T. Walter, and R. E. W. Hancock. 2005. High-throughput generation of small antibacterial peptides with improved activity. Nat. Biotechnol. 23:1008-1012. [DOI] [PubMed] [Google Scholar]
- 15.Hsu, C.-H., et al. 2005. Structural and DNA-binding studies on the bovine antimicrobial peptide, indolicidin: evidence for multiple conformations involved in binding to membranes and DNA. Nucleic Acids Res. 33:4053-4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imlay, J. A., and S. Linn. 1988. DNA damage and oxygen radical toxicity. Science 240:1302-1309. [DOI] [PubMed] [Google Scholar]
- 17.Kadurugamuwa, J. L., A. J. Clarke, and T. J. Beveridge. 1993. Surface action of gentamicin on Pseudomonas aeruginosa. J. Bacteriol. 175:5798-5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalapos, M. P. 2008. The tandem of free radicals and methylglyoxal. Chem. Biol. Interact. 171:251-271. [DOI] [PubMed] [Google Scholar]
- 19.Katsui, N., et al. 1982. Heat-induced blebbing and vesiculation of the outer membrane of Escherichia coli. J. Bacteriol. 151:1523-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohanski, M. A., D. J. Dwyer, B. Hayete, C. A. Lawrence, and J. J. Collins. 2007. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130:797-810. [DOI] [PubMed] [Google Scholar]
- 21.Kohanski, M. A., D. J. Dwyer, J. Wierzbowski, G. Cottarel, and J. J. Collins. 2008. Mistranslation of membrane proteins and two-component system activation trigger antibiotic-mediated cell death. Cell 135:679-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kragol, G., et al. 2001. The antibacterial peptide pyrrhocoricin inhibits the ATPase actions of DnaK and prevents chaperone-assisted protein folding. Biochemistry 40:3016-3026. [DOI] [PubMed] [Google Scholar]
- 23.Leonardo, M. R., Y. Dailly, and D. P. Clark. 1996. Role of NAD in regulating the adhE gene of Escherichia coli. J. Bacteriol. 178:6013-6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mangoni, M. L., et al. 2004. Effects of the antimicrobial peptide temporin L on cell morphology, membrane permeability and viability of Escherichia coli. Biochem. J. 380:859-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcellini, L., M. Giammatteo, P. Aimola, and M. L. Mangoni. 2010. Fluorescence and electron microscopy methods for exploring antimicrobial peptides mode(s) of action. Methods Mol. Biol. 618:249-266. [DOI] [PubMed] [Google Scholar]
- 26.Miller, C., et al. 2004. SOS response induction by β-lactams and bacterial defense against antibiotic lethality. Science 305:1629-1631. [DOI] [PubMed] [Google Scholar]
- 27.Minahk, C. J., F. Dupuy, and R. D. Morero. 2004. Enhancement of antibiotic activity by sub-lethal concentrations of enterocin CRL35. J. Antimicrob. Chemother. 53:240-246. [DOI] [PubMed] [Google Scholar]
- 28.Neidhardt, F. 1974. Culture medium for enterobacteria. J. Bacteriol. 119:736-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novo, D., N. G. Perlmutter, R. H. Hunt, and H. M. Shapiro. 1999. Accurate flow cytometric membrane potential measurement in bacteria using diethyloxacarbocyanine and a ratiometric technique. Cytometry 35:55-63. [DOI] [PubMed] [Google Scholar]
- 30.Novo, D. J., N. G. Perlmutter, R. H. Hunt, and H. M. Shapiro. 2000. Multiparameter flow cytometric analysis of antibiotic effects on membrane potential, membrane permeability, and bacterial counts of Staphylococcus aureus and Micrococcus luteus. Antimicrob. Agents Chemother. 44:827-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novogrodsky, A., A. Ravid, A. L. Rubin, and K. H. Stenzel. 1982. Hydroxyl radical scavengers inhibit lymphocyte mitogenesis. Proc. Natl. Acad. Sci. U. S. A. 79:1171-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patrzykat, A., C. L. Friedrich, L. Zhang, V. Mendoza, and R. E. W. Hancock. 2002. Sublethal concentrations of pleurocidin-derived antimicrobial peptides inhibit macromolecular synthesis in Escherichia coli. Antimicrob. Agents Chemother. 46:605-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peschel, A., and H.-G. Sahl. 2006. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat. Rev. Microbiol. 4:529-536. [DOI] [PubMed] [Google Scholar]
- 34.Podda, E., et al. 2006. Dual mode of action of Bac7, a proline-rich antibacterial peptide. Biochim. Biophys. Acta 1760:1732-1740. [DOI] [PubMed] [Google Scholar]
- 35.Poole, R. K. 1994. Oxygen reactions with bacterial oxidases and globins: binding, reduction and regulation. Antonie Van Leeuwenhoek 65:289-310. [DOI] [PubMed] [Google Scholar]
- 36.Russell, J. B., and G. M. Cook. 1995. Energetics of bacterial growth: balance of anabolic and catabolic reactions. Microbiol. Rev. 59:48-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider, D. A., T. Gaal, and R. L. Gourse. 2002. NTP-sensing by rRNA promoters in Escherichia coli is direct. Proc. Natl. Acad. Sci. U. S. A. 99:8602-8607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shapiro, H. M. 2008. Flow cytometry of bacterial membrane potential and permeability, p. 175-186. In W. Scott Champney (ed.), New antibiotic targets. Humana Press, Totowa, NJ. [DOI] [PubMed]
- 39.Shaw, J. E., J.-R. Alattia, J. E. Verity, G. G. Prive, and C. M. Yip. 2006. Mechanisms of antimicrobial peptide action: studies of indolicidin assembly at model membrane interfaces by in situ atomic force microscopy. J. Struct. Biol. 154:42-58. [DOI] [PubMed] [Google Scholar]
- 40.Reference deleted.
- 41.Vlamis-Gardikas, A. 2008. The multiple functions of the thiol-based electron flow pathways of Escherichia coli: eternal concepts revisited. Biochim. Biophys. Acta 1780:1170-1200. [DOI] [PubMed] [Google Scholar]
- 42.Westerhoff, H. V., D. Juretic, R. W. Hendler, and M. Zasloff. 1989. Magainins and the disruption of membrane-linked free-energy transduction. Proc. Natl. Acad. Sci. U. S. A. 86:6597-6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wiegand, I., K. Hilpert, and R. E. W. Hancock. 2008. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 3:163-175. [DOI] [PubMed] [Google Scholar]
- 44.Wu, M., and R. E. W. Hancock. 1999. Improved derivatives of bactenecin, a cyclic dodecameric antimicrobial cationic peptide. Antimicrob. Agents Chemother. 43:1274-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yonath, A. 2005. Antibiotics targeting ribosomes: resistance, selectivity, synergism, and cellular regulation. Annu. Rev. Biochem. 74:649-679. [DOI] [PubMed] [Google Scholar]
- 46.Zhu, M. M., F. A. Skraly, and D. C. Cameron. 2001. Accumulation of methylglyoxal in anaerobically grown Escherichia coli and its detoxification by expression of the Pseudomonas putida glyoxalase I gene. Metab. Eng. 3:218-225. [DOI] [PubMed] [Google Scholar]