Abstract
Antibiotic penetration to the infection site is critical for obtaining a good clinical outcome in patients with ventilator-associated pneumonia (VAP). Surprisingly few studies have quantified the penetration of β-lactam agents into the lung, as measured by the ratio of area under the concentration-time curve (AUC) in epithelial lining fluid (ELF) to AUC in plasma (AUCELF/AUCplasma ratio). These have typically involved noninfected patients. This study examines the penetration and pharmacodynamics of meropenem in the ELF among patients with VAP. Meropenem plasma and ELF concentration-time data were obtained from patients in a multicenter clinical trial. Concentration-time profiles in plasma and ELF were simultaneously modeled using a three-compartment model with zero-order infusion and first-order elimination and transfer (big nonparametric adaptive grid [BigNPAG]). A Monte Carlo simulation was performed to estimate the range of ELF/plasma penetration ratios one would expect to observe in patients with VAP, as measured by the AUCELF/AUCplasma ratio. The range of AUCELF/AUCplasma penetration ratios predicted by the Monte Carlo simulation was large. The 10th percentile of lung penetration was 3.7%, while the 90th percentile of penetration was 178%. The variability of ELF penetration is such that if relatively high ELF exposure targets are required to attain multilog kill or resistance suppression for bacteria like Pseudomonas aeruginosa, then even receiving the largest licensed dose of meropenem with an optimal prolonged infusion may not result in target attainment for a substantial fraction of the population.
Ventilator-associated pneumonia (VAP) remains a frequent cause of morbidity and mortality among intensive care unit patients despite advances in antimicrobial therapy, better supportive care modalities, and the use of a wide range of preventive measures (1, 26). Prompt delivery of empirical therapy for patients likely to have VAP is of paramount importance, since delays in appropriate antibiotic therapy have been associated with deleterious outcomes (1, 18, 19, 24, 25). An important consideration when selecting empirical therapy for VAP is the agent's ability to adequately penetrate the infected site and achieve sufficient concentrations for the desired endpoint. For extracellular respiratory tract pathogens, the determination of drug concentration in epithelial lining fluid (ELF) currently provides the best estimate for ascertaining the degree of antibiotic exposure for these organisms in patients with VAP (3-8, 12, 15-17, 21, 25, 27, 28).
While it is well established that the efficacy of an antibiotic regimen largely depends on its penetration in the infection site, relatively few studies have focused on the penetration of antibiotics into the ELF (3-8, 12, 15-17, 21, 23, 25, 27, 28). Of those available, most have been among patients treated with fluoroquinolones and macrolides (12, 15, 16, 23, 27, 28). A surprisingly small body of data is available for the penetration of β-lactam agents into the ELF. In the older literature, β-lactam penetration was quantified by lung biopsy (2, 8). This approach is severely flawed in that accurate penetration information is obtained very infrequently, especially for agents, such as β-lactams, that penetrate cells poorly. Cars and Ogren (7) showed definitively that traditional biopsy-with-grinding methods severely underestimated lung penetration because of dilution. The study found that dilution of 4 or 5 to 1 occurs because 80% of a biopsy specimen is cellular and, due to poor penetration, very little β-lactam is present in the intracellular fluid after grinding.
Among studies that employed the proper methodology to assess β-lactam ELF penetration among hospitalized patients, a wide variation has been noted. With ertapenem (6), an ELF penetration of about 30% was found using free drug for the plasma area under the concentration-time curve (AUC) (this drug is over 90% protein bound). In two studies where continuous infusion of β-lactams was employed, ELF and serum were sampled at steady state. Ceftazidime produced an ELF penetration of approximately 21% (5), while cefepime had an ELF penetration slightly in excess of 100% (3).
This study describes the pharmacokinetics (PK) of meropenem in the plasma and ELF among intubated patients with nosocomial pneumonia. Population PK modeling and Monte Carlo simulation were used to estimate the range of ELF concentration-time profiles (exposures) relative to those in plasma that one would observe in patients with VAP, as measured by the ratio of the AUC in the ELF to the AUC in the plasma (AUCELF/AUCplasma ratio).
(This research was presented in part as a poster at the 48th Interscience Conference on Antimicrobial Agents and Chemotherapy/Infectious Diseases Society of America 46th Annual Meeting, Washington, DC, 25 to 28 October 2008 [14].)
MATERIALS AND METHODS
Patient population.
Plasma and ELF concentration-time data for meropenem were obtained from a multicenter clinical trial of meropenem (13). A diagnosis of VAP was made from the quantitative culture of a baseline bronchoalveolar lavage (BAL) specimen (≥104 CFU/ml) or a quantitative culture from a protected specimen brush (≥103 CFU/ml). Subjects received 2 g or 500 mg meropenem intravenously (i.v.) as a 3-h infusion every 8 h or 1 g intravenously as a half-hour infusion every 8 h. Plasma samples for meropenem concentrations at steady-state were collected from 39 patients preinfusion and at 0.5, 1, 2, 3, 4, and 6 h into the 8-h dosing interval. Bronchoscopy samples for meropenem concentrations were collected on day 7, along with an additional matching plasma sample from 17 of these 39 subjects. Based on the above-described schedule, 290 plasma samples and 17 ELF samples were anticipated; 269 plasma samples (93% of the expected number) and 17 ELF samples were available for the analysis.
Because this was a clinical protocol, BAL fluid volumes were limited to 10 ml. Four 10-ml aliquots of 0.9% normal saline were instilled into the area of pneumonia. Each specimen was immediately aspirated and placed on ice. The first aspirate was discarded because of potential contamination with cells from the proximal airway. The rest of the aliquots were pooled, the volume recorded, and the sample centrifuged at 400 × g for 5 min. The supernatant and cells were separated, and the supernatant separated into aliquots and frozen at −70°C until the assays (meropenem and urea) were performed as indicated below. As β-lactams penetrate cells poorly, cells were not examined in this evaluation. BAL fluid samples were obtained when clinically possible.
Samples were placed in an ice-bath slurry until processed, which occurred no longer than 4 h after sample acquisition, and then were placed in a −70°C freezer. Samples were batched every 3 months. No sample went through more than one freeze-thaw, as a backup sample was available and used if a repeat determination was required.
Determination of meropenem in human plasma by LC-MS-MS.
The meropenem concentrations in plasma were determined by high-performance liquid chromatography coupled with mass spectrometry (LC-MS-MS) (PE SCIEX API 3000; MDS Sciex, Concord, Ontario, Canada). All sample handling and the thawing of frozen plasma samples were done at +4°C. Plasma samples (0.1 ml) were deproteinized by adding 0.2 ml of acetonitrile containing the internal standard. After thorough mixing, the samples were centrifuged for 5 min at 3,600 rpm at approximately +4°C, and the supernatant was diluted with ammonium acetate buffer. Thirty μl of each sample was chromatographed on a reversed-phase column (Spherisorb Phenyl, 5 μm, 40 by 4.6 mm) eluted with an isocratic solvent system consisting of 0.005 M ammonium acetate buffer and acetonitrile (75:25, vol/vol) and monitored by LC-MS-MS with a selected reaction monitoring (SRM) method as follows: precursor → product ion for meropenem m/z 384 → m/z 68 and for the internal standard m/z 518 → m/z 143; both analyses were in positive mode. Under these conditions, meropenem and the internal standard were eluted after approximately 1.5 min and 1.4 min, respectively. Mac Quan software (version 1.4-noFPU, 1991-1995; PerkinElmer, Toronto, Canada) was used to evaluate chromatograms.
Plasma samples were measured against a plasma calibration row. The calibration standards were prepared by adding defined amounts of the standard solution of meropenem or of the standard of higher concentration to drug-free human plasma. Calibration was performed by weighted (1/concentration2) linear regression. Spiked quality controls (SQC) to determine interassay variation were prepared by adding defined amounts of the stock solution of meropenem or of the spiked control of higher concentration to defined amounts of tested drug-free plasma.
There was no interference observed in plasma for meropenem or the internal standard. The limit of quantification for plasma samples was 0.0200 μg/ml. The response from calibration standards was linear from 0.0200 to 40.00 μg/ml, and the coefficient of correlation for all measured sequences was at least 0.996. The interday precision and analytical recovery of the meropenem assay during sample analysis ranged from 3.7 to 12.0% and from 95.2 to 103.3%, respectively.
Determination of meropenem in BAL fluid by LC-MS-MS.
The meropenem concentrations in BAL fluid were determined by LC-MS-MS (PE SCIEX API 3000). All sample handling and the thawing of frozen BAL samples were done at +4°C. BAL samples (0.05 ml) were mixed with 0.05 ml of acetonitrile containing the internal standard and 0.4 ml ammonium acetate buffer. After thorough mixing, 50 μl of each sample was analyzed as described above for human plasma.
BAL samples were measured against a calibration row in Ringer's solution. Calibration standards were prepared by adding defined amounts of the standard solution of meropenem or of the standard of higher concentration to Ringer's solution. Calibration was performed by weighted (1/concentration2) linear regression. SQC to determine interassay variation were prepared by adding defined amounts of the stock solution of meropenem or of the spiked control of higher concentration to defined amounts of Ringer's solution.
There was no interference observed in BAL fluid for meropenem or the internal standard. The limit of quantification for BAL samples was 0.00447 μg/ml. The response from the calibration standards was linear from 0.00447 to 0.925 μg/ml, and the coefficient of correlation for all measured sequences was at least 0.999. The interday precision and analytical recovery of the meropenem assay during sample analysis ranged from 3.3 to 8.2% and from 95.9 to 100.2%, respectively.
Determination of urea in BAL fluid by LC-MS-MS.
ELF volume determinations were performed using urea as an endogenous marker and concentrations measured in bronchoalveolar lavage fluid corrected for dilution (15). Urea concentrations in BAL fluid were determined by LC-MS-MS (PE SCIEX API 3000; MDS Sciex, Concord, Ontario, Canada). To BAL samples (1 ml), 0.1 ml internal standard solution and 1 ml methanol were added. The samples were evaporated to dryness at room temperature. An amount of 0.2 ml trifluoroacetic acid anhydride was added. After a reaction time of 30 min at 25°C, the solution was evaporated to dryness. The residual was redissolved with 0.3 ml formic acid-acetonitrile. Ten (10) microliters of each sample was chromatographed on a reversed-phase column (YMC-Cyano, 3 μm, 50 by 4 mm) eluted with an isocratic solvent system consisting of formic acid and acetonitrile (90:10, vol/vol) and monitored by LC-MS-MS with an SRM method as follows: precursor → product ion for urea m/z 157 → m/z 114 and for the internal standard m/z 161 → m/z 115; both analyses were in positive mode. Under these conditions, urea and the internal standard were eluted after approximately 1.1 min. Mac Quan software (version 1.4-noFPU, 1991-1995; PerkinElmer, Toronto, Canada) was used to evaluate chromatograms.
BAL samples were measured against a calibration row in Ringer's solution. The calibration standards were prepared by adding defined amounts of the standard solution of urea or of the standard of higher concentration to Ringer's solution. Calibration was performed by weighted (1/concentration2) linear regression. SQC determine interassay variation were prepared by the addition of defined amounts of the stock solution of urea or of the spiked control of higher concentration to defined amounts Ringer's solution.
There was no interference observed in BAL fluid for urea or the internal standard. The limit of quantification for BAL samples was 0.198 μg/ml. The response from the calibration standards was linear from 0.198 to 5.99 μg/ml, and the coefficient of correlation for all measured sequences was at least 0.998. The interday precision and analytical recovery of the urea assay during sample analysis ranged from 7.0 to 11.5% and from 98.5 to 102.7%, respectively.
Population pharmacokinetic modeling.
All data were analyzed in a population pharmacokinetic model using the big nonparametric adaptive grid (BigNPAG) with adaptive γ program of Leary et al. (20). A three-compartment model was used, with zero-order infusion into the first compartment. This design was selected based on Akaike's information criterion and rule of parsimony (29). Elimination from the central compartment and all intercompartmental distribution processes were modeled as first-order processes.
The general differential equations for the model were as follows: dX(1)/dt = R(t) − [(CL/V) + K12+ K13] × X(1) + K2 × X(2) + K31× X(3); dX(2)/dt = K12 × X(1) − K21 × X(2); and dX(3)/dt = K13 × X(1) − K31 × X(3). The variables are defined as follows: X(1) is the amount of drug in the central compartment (in milligrams), X(2) is the amount of drug in the peripheral compartment (in milligrams), X(3) is the amount of drug in the ELF compartment (in milligrams), R(t) is the time-delimited zero-order drug input rate (piece-wise input function) into the central compartment (in milligrams per hour), CL is clearance from the central compartment (liters per hour), Vc and VELF are scalars and represent the apparent volumes of the central compartment and ELF compartment (in liters), respectively, and K12, K21, K13, and K31 are first-order intercompartmental transfer rate constants (in h−1).
The inverse of the estimated assay variance was used as the first estimate for weighting in the pharmacokinetic modeling. Weighting was accomplished by making the assumption that total observation variance was proportional to assay variance.
Assay variance was determined on a between-day basis. Upon attaining convergence, Bayesian estimates for each patient were obtained using the BigNPAG “population of one” utility. The mean and median values were employed as measures of central tendency for the population parameter estimates, and both were evaluated in the Bayesian analysis.
Scatter plots were examined for individual patients and for the population as a whole. Goodness of fit was assessed by regression with an observed-predicted plot, coefficients of determination, and log-likelihood values. Predictive performance evaluation was based on weighted mean error and the bias-adjusted weighted mean-squared error.
Monte Carlo simulation.
The mean parameter vector and covariance matrix from the population pharmacokinetic model were embedded in subroutine PRIOR of the ADAPT II package of D'Argenio and Schumitzky (9). The population simulation without process noise option was employed. A Monte Carlo simulation with 9,999 subjects was performed for 2 g meropenem i.v. as a single dose with a 3-h infusion. Both normal and log-normal distributions were evaluated, and these were discriminated by their ability to recreate the mean parameter vector and corresponding standard deviations from the population model. Monte Carlo simulation was used to calculate the ELF/plasma penetration ratios for 2 g meropenem i.v. as a single dose by estimating the AUCELF and AUCplasma from 0 to 8 h during the first dosing interval. This evaluation interval was chosen because a number of recent publications have quantified the importance of optimal early therapy (1, 18, 19, 24) and this interval would be a conservative evaluation. Specifically, the AUC in both ELF and plasma were calculated by integrating the concentration-time profile in each compartment from time 0 (start of administration) to 8 h after the first administration. The AUCELF/AUCplasma penetration ratio derived from the mean parameter vector from the population model was also calculated.
Systat for Windows (version 11.0) was employed for all data transformation.
RESULTS
Demographics.
As would be expected, the demographics (Table 1) of our patients were similar to those in previous studies of patients with nosocomial pneumonia (11).
TABLE 1.
Patient demographics and baseline characteristics
| Parameter | Mean (SD) | Range |
|---|---|---|
| Male (% of population) | 59 | |
| Age (yr) | 49.3 (19.4) | 20-85 |
| Weight (kg) | 83.1 (22.6) | 46-140 |
| Height (cm) | 168.2 (10.9) | 144-185 |
| APACHE II score | 19.6 (6.9) | |
| CPISa > 6 | 84% |
CPIS, clinical pulmonary infection score.
Model fit.
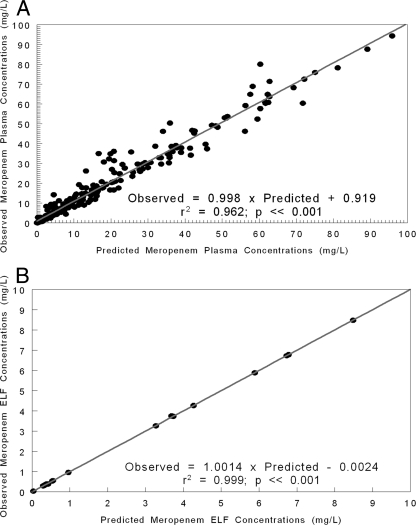
The fit of the model to the data, shown in Fig. 1, was quite acceptable, as were measures of bias and precision. For plasma, the mean weighted bias was −0.579 and the bias-adjusted mean weighted precision was 8.856. These values for ELF were 0.0901 and 0.0506, respectively. The regression between predicted and observed meropenem plasma concentrations was as follows: observed = 0.998 × predicted + 0.919; r2 = 0.962; P < 0.001; n = 269. The regression between predicted and observed meropenem ELF concentrations was as follows: observed = 1.001 × predicted − 0.0024; r2 = 0.999; P < 0.001; n = 17. The parameter values from the analysis are displayed in Table 2.
FIG. 1.
(A) Fit of the model to the data after the Bayesian step for the plasma concentration-time data for meropenem in intubated patients with nosocomial pneumonia. (B) Fit of the model to the data after the Bayesian step for the ELF concentration-time data for meropenem in intubated patients with nosocomial pneumonia. L, liter.
TABLE 2.
Population pharmacokinetic parameters for intubated patients with hospital-acquired pneumoniaa
| Parameter (n = 39) | Vc (liters) | CL (liters/h) | K12 (h−1) | K21 (h−1 h−1) | K13 (h−1) | K31 (liters) | VELF (liters) |
|---|---|---|---|---|---|---|---|
| Mean | 12.6 | 15.2 | 8.3 | 14.1 | 10.1 | 14.2 | 30.4 |
| Median | 6.7 | 13.5 | 3.2 | 11.2 | 8.0 | 15.4 | 24.2 |
| SD | 13.3 | 9.7 | 9.8 | 11.7 | 8.6 | 11.4 | 25.2 |
Vc, volume of distribution in the central compartment; CL, meropenem clearance from plasma; K12, K21, K13, and K31, first-order intercompartmental transfer rate constants; VELF, volume of distribution in the ELF compartment.
ELF penetration.
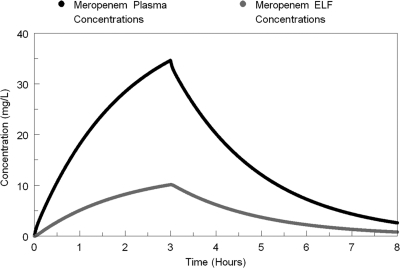
Simulations of the first-dose concentration-time profiles of meropenem in plasma and ELF from the mean parameter values from the population PK model are displayed in Fig. 2. A more robust mean exposure profile was observed in plasma than in ELF. The AUCELF/AUCplasma penetration ratio derived from the mean parameter vector from the population model was 30%.
FIG. 2.
Concentration-time profiles of meropenem in plasma (black) and ELF (gray) as calculated from the mean parameter vector. L, liter.
The findings from the 9,999-subject Monte Carlo simulation are displayed in Table 3. The mean (standard deviation) AUCELF and AUCplasma values were 82.3 (140.1) mg·h/liter and 150.8 (87.4) mg·hour/liter, respectively. The mean (standard deviation) penetration ratio was 81.6% (223.0%). The median (25th and 75th percentile values) AUCELF and AUCplasma values were 130.9 (90.1 to 189.3) mg·hour/liter and 35.0 (12.5 to 92.1) mg·hour/liter, respectively. The median AUCELF/AUCplasma penetration ratio was 25.4%, and the 25th and 75th percentile value ratios were 9.0% and 70.1%, respectively. The average value for the Monte Carlo simulation is skewed because of outliers, as is evident when one examines the median value of the penetration ratio of 25.4%, the mean ratio of 81.6%, and the large standard deviation of 223.8%. The AUCELF/AUCplasma penetration ratio derived from the mean parameter vector from the population model lines up best with the median value from the Monte Carlo simulation and further reflects the influence of outliers on the mean penetration ratio from the Monte Carlo simulation. As a reality check, we also examined the Bayesian parameter estimates for only those patients who had an ELF sample determination (n = 17). These patients had a median value for penetration of 26.4%, which is nicely concordant with the median value for penetration ratio from the Monte Carlo simulation (25.6%).
TABLE 3.
Estimation of penetration of meropenem into ELF using a Monte Carlo simulationa
| Parameter | AUCplasma (mg·h/liter) | AUCELF (m·h/liter) | Penetration ratio (%) |
|---|---|---|---|
| Mean | 150.8 | 82.30 | 81.6 |
| Median | 130.9 | 35.00 | 25.42 |
| SD | 87.40 | 140.1 | 223.0 |
| 95% CI of the mean | 149.1-152.5 | 79.55-85.04 | 77.28-86.02 |
| Percentile | |||
| 10th | 63.90 | 4.76 | 3.67 |
| 25th | 90.14 | 12.52 | 9.00 |
| 50th | 130.90 | 35.00 | 25.42 |
| 75th | 189.30 | 92.10 | 70.14 |
| 90th | 262.10 | 204.70 | 177.90 |
AUC, area under the concentration-time curve; Penetration ratio, AUCELF/AUCplasma; CI, confidence interval.
DISCUSSION
Penetration of an antibiotic to an infection site is critical for obtaining a good clinical outcome. Surprisingly little has been published on the penetration of β-lactam agents into the ELF in patients with VAP (3-6). In this evaluation, we examined 39 patients with VAP, of whom 17 had a bronchoalveolar lavage to determine the ELF concentration of the β-lactam antibiotic meropenem (13).
Interestingly, when we calculated ELF penetration using the mean parameter values identified after population modeling (Table 2), the estimate of the penetration ratio was 30%. This is in line with the results of earlier work with the carbapenem ertapenem (6) and the cephalosporin ceftazidime (5) but quite discordant with the results for the cephalosporin cefepime (3). The discordance may be due to the unique physicochemical properties of these agents. Alternatively, the differences may simply be a function of the small patient populations examined in each of these studies. Collectively, these findings highlight the importance of quantifying exposure profiles in the ELF when evaluating the utility of an agent for VAP rather than using estimates from similar agents within the antibiotic class.
Of interest, the mean plasma clearance of meropenem in this study is not clinically different from that found in previous analyses of meropenem pharmacokinetics among volunteers (10, 22). Although the mean plasma clearance was not markedly different from that in healthy subjects, the variability around parameter estimates was much greater. For example, the coefficients of variation for plasma clearance and volume of distribution in the central compartment were 73% (9.71/13.3) and 121% (15.2/12.6), respectively. Similar coefficients of variance have been noted in previous nosocomial-pneumonia PK studies (11), which speaks directly to the differing physiological states across this patient population. More importantly, the range of lung penetration ratios predicted by the Monte Carlo simulation was impressive. The 10th percentile of lung penetration was 3.7%, while the 90th percentile of penetration was 178% (Table 3). What is completely unclear is the physiological basis for such a broad range of penetration. In order to optimize therapy for this infection, this question must be addressed. Otherwise, some patients will get inadequate drug exposure at the primary infection site, likely resulting in a less-than-optimal outcome. Performing bronchoalveolar lavage on patients routinely during therapy is unlikely to occur because of the elevated morbidity of these patients. Consequently, it is a key issue to identify physiological markers that will identify patients likely to get inadequate exposure.
In summary, we have documented the behavior of meropenem in the plasma and ELF of intubated patients with nosocomial pneumonia. The mean pharmacokinetic parameter values are similar to those found in volunteers, but the variability is much greater, likely reflecting a subpopulation of an older patient population with somewhat impaired renal function being counterbalanced by a subset of hyperdynamic young patients. The penetration into the ELF was also found to be variable, as was shown previously for levofloxacin by Monte Carlo simulation (11). The pharmacokinetic parameter values identified here (Table 2) allow calculation of the time above the MIC in the ELF for meropenem, which will allow the adequacy of a specific dose to be evaluated, once the target is known (e.g., from a preclinical animal model). Clinically, the variability of the penetration is such that if relatively high-exposure targets in ELF are required to attain multilog kill or suppress resistance emergence for bacteria like Pseudomonas aeruginosa, then even receiving the largest licensed dose of meropenem with an optimal prolonged infusion may not result in target attainment for a substantial fraction of the population. This highlights the importance of identifying the proper ELF exposure targets for multilog cell kill and, most importantly, for suppression of the amplification of resistant subpopulations in order to judge the adequacy of current dose and schedule for these patients. If the probability of target attainment is found to be lower than acceptable, combination chemotherapy may be necessary.
Acknowledgments
This work was supported by grant R01AI079578 from NIAID to the Emerging Infections and Pharmacodynamics Laboratory.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
David Melnick is an AstraZeneca employee, and Barry Mason was an employee of AstraZeneca Pharmaceuticals.
Footnotes
Published ahead of print on 7 February 2011.
REFERENCES
- 1.American Thoracic Society. 2005. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 171:388-416. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin, D. R., R. Wise, J. M. Andrews, and D. Honeybourne. 1992. Concentrations of cefpodoxime in serum and bronchial mucosal biopsies. J. Antimicrob. Chemother. 30:67-71. [DOI] [PubMed] [Google Scholar]
- 3.Boselli, E., et al. 2003. Steady-state plasma and intrapulmonary concentrations of cefepime administered in continuous infusion in critically ill patients with severe nosocomial pneumonia. Crit. Care Med. 31:2102-2106. [DOI] [PubMed] [Google Scholar]
- 4.Boselli, E., et al. 2008. Alveolar concentrations of piperacillin/tazobactam administered in continuous infusion to patients with ventilator-associated pneumonia. Crit. Care Med. 36:1500-1506. [DOI] [PubMed] [Google Scholar]
- 5.Boselli, E., et al. 2004. Plasma and lung concentrations of ceftazidime administered in continuous infusion to critically ill patients with severe nosocomial pneumonia. Intensive Care Med. 30:989-991. [DOI] [PubMed] [Google Scholar]
- 6.Boselli, E., D. Breilh, M. C. Saux, J. B. Gordien, and B. Allaouchiche. 2006. Pharmacokinetics and lung concentrations of ertapenem in patients with ventilator-associated pneumonia. Intensive Care Med. 32:2059-2062. [DOI] [PubMed] [Google Scholar]
- 7.Cars, O., and S. Ogren. 1985. Antibiotic tissue concentrations: methodological aspects and interpretation of results. Scand. J. Infect. Dis. Suppl. 44:7-15. [PubMed] [Google Scholar]
- 8.Cazzola, M., et al. 1995. Pulmonary penetration of ceftazidime. J. Chemother. 7:50-54. [DOI] [PubMed] [Google Scholar]
- 9.D'Argenio, D. Z., and A. Schumitzky. 1997. ADAPT II. A program for simulation, identification, and optimal experimental design. User's manual. Biomedical Simulations Resource, University of Southern California, Los Angeles, CA. http://bmsr.usc.edu/.
- 10.Drusano, G. L., and M. Hutchison. 1995. The pharmacokinetics of meropenem. Scand. J. Infect. Dis. Suppl. 96:11-16. [PubMed] [Google Scholar]
- 11.Drusano, G. L., et al. 2004. Relationship between fluoroquinolone area under the curve:minimum inhibitory concentration ratio and the probability of eradication of the infecting pathogen, in patients with nosocomial pneumonia. J. Infect. Dis. 189:1590-1597. [DOI] [PubMed] [Google Scholar]
- 12.Drusano, G. L., S. L. Preston, M. H. Gotfried, L. H. Danziger, and K. A. Rodvold. 2002. Levofloxacin penetration into epithelial lining fluid as determined by population pharmacokinetic modeling and Monte Carlo simulation. Antimicrob. Agents Chemother. 46:586-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drusano, G. L., F. Sorgel, J. Quinn, B. Mason, and D. Melnick. 2005. Impact of pharmacodynamics dosing of meropenem on emergence of resistance during treatment of ventilator-associated pneumonia: a prospective clinical trial, abstr. K-127. 45th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC). American Society for Microbiology, Washington, DC.
- 14.Drusano, G. L., T. P. Lodise, F. Sorgel, B. Mason, and D. Melnick. 2008. Penetration of meropenem into epithelial lining fluid of patients with ventilator-associated pneumonia, abstr. A-1899. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC. [DOI] [PMC free article] [PubMed]
- 15.Gotfried, M. H., L. H. Danziger, and K. A. Rodvold. 2003. Steady-state plasma and bronchopulmonary characteristics of clarithromycin extended-release tablets in normal healthy adult subjects. J. Antimicrob. Chemother. 52:450-456. [DOI] [PubMed] [Google Scholar]
- 16.Gotfried, M. H., L. H. Danziger, and K. A. Rodvold. 2001. Steady-state plasma and intrapulmonary concentrations of levofloxacin and ciprofloxacin in healthy adult subjects. Chest 119:1114-1122. [DOI] [PubMed] [Google Scholar]
- 17.Gotfried, M. H., et al. 2008. Intrapulmonary distribution of intravenous telavancin in healthy subjects and effect of pulmonary surfactant on in vitro activities of telavancin and other antibiotics. Antimicrob. Agents Chemother. 52:92-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iregui, M., S. Ward, G. Sherman, V. J. Fraser, and M. H. Kollef. 2002. Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator-associated pneumonia. Chest 122:262-268. [DOI] [PubMed] [Google Scholar]
- 19.Kollef, M. H., G. Sherman, S. Ward, and V. J. Fraser. 1999. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest 115:462-474. [DOI] [PubMed] [Google Scholar]
- 20.Leary, R., R. Jelliffe, A. Schumitzky, and M. van Guilder. 2001. An adaptive grid, non-parametric approach to pharmacokinetic and dynamic (PK/PD) models, pp. 389-394. Proc. 14th IEEE Symp. Comput. Based Med. Syst. IEEE Computer Society, Bethesda, MD.
- 21.Lodise, T. P. Jr., M. Gotfried, S. Barriere, and G. L. Drusano. 2008. Telavancin penetration into human epithelial lining fluid determined by population pharmacokinetic modeling and Monte Carlo simulation. Antimicrob. Agents Chemother. 52:2300-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lodise, T. P., et al. 2007. Pharmacodynamics of ceftazidime and meropenem in cerebrospinal fluid: results of population pharmacokinetic modelling and Monte Carlo simulation. J. Antimicrob. Chemother. 60:1038-1044. [DOI] [PubMed] [Google Scholar]
- 23.Lodise, T. P., et al. 2005. Pharmacodynamics of an 800-mg dose of telithromycin in patients with community-acquired pneumonia caused by extracellular pathogens. Diagn. Microbiol. Infect. Dis. 52:45-52. [DOI] [PubMed] [Google Scholar]
- 24.Luna, C. M., et al. 2006. Appropriateness and delay to initiate therapy in ventilator-associated pneumonia. Eur. Respir. J. 27:158-164. [DOI] [PubMed] [Google Scholar]
- 25.Luna, C. M., et al. 1997. Impact of BAL data on the therapy and outcome of ventilator-associated pneumonia. Chest 111:676-685. [DOI] [PubMed] [Google Scholar]
- 26.Muscedere, J. G., C. M. Martin, and D. K. Heyland. 2008. The impact of ventilator-associated pneumonia on the Canadian health care system. J. Crit. Care. 23:5-10. [DOI] [PubMed] [Google Scholar]
- 27.Rodvold, K. A., L. H. Danziger, and M. H. Gotfried. 2003. Steady-state plasma and bronchopulmonary concentrations of intravenous levofloxacin and azithromycin in healthy adults. Antimicrob. Agents Chemother. 47:2450-2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodvold, K. A., M. H. Gotfried, L. H. Danziger, and R. J. Servi. 1997. Intrapulmonary steady-state concentrations of clarithromycin and azithromycin in healthy adult volunteers. Antimicrob. Agents Chemother. 41:1399-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamaoka, K., T. Nakagawa, and T. Uno. 1978. Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J. Pharmacokinet. Biopharm. 6:165-175. [DOI] [PubMed] [Google Scholar]