Abstract
Respiratory anthrax, in the absence of early antibiotic treatment, is a fatal disease. This study aimed to test the efficiency of antibiotic therapy in curing infected animals and those sick with anthrax. Postexposure prophylaxis (24 h postinfection [p.i.]) of guinea pigs infected intranasally with Bacillus anthracis Vollum spores with doxycycline, ofloxacin, imipenem, and gentamicin conferred protection. However, upon termination of treatment, the animals died from respiratory anthrax. Combined treatment with antibiotics and active vaccination with a protective antigen-based vaccine leads to full protection even after cessation of treatment. Delaying the initiation of antibiotic administration to over 24 h p.i. resulted in treatment of animals with anthrax exhibiting various degrees of bacteremia and toxemia. Treatment with doxycycline or ciprofloxacin cured sick guinea pigs and rabbits exhibiting bacteremia levels up to 105 CFU/ml. Addition of anti-protective antigen (PA) antibodies augmented the efficiency of protection, allowing the cure of guinea pigs and rabbits with 10- to 20-fold-higher bacteremia levels, up to 7 × 105 CFU/ml and 2 × 106 CFU/ml, respectively. Treatment with ciprofloxacin and a monoclonal anti-PA antibody rescued rabbits with bacteremia levels up to 4 × 106 CFU/ml. During antibiotic administration, all surviving animals developed a protective immune response against development of a fatal disease and subcutaneous challenge with Vollum spores. In conclusion, these results demonstrate that antibiotic treatment can prevent the development of fatal disease in respiratory-anthrax-infected animals and can cure animals after disease establishment. A therapeutic time window of 40 h to 48 h from infection to initiation of efficient antibiotic-mediated cure was observed.
Anthrax, caused by Bacillus anthracis, a Gram-positive, nonmotile, spore-forming rod (6), is primarily a disease of herbivorous animals. In humans, three types of anthrax have been recorded based on the route of infection: cutaneous, gastrointestinal, and the almost always fatal respiratory disease (2). In the 2001 bioterrorism attack in the United States, envelopes containing B. anthracis Ames spores were sent by mail, causing inhalational anthrax in 10 people and cutaneous anthrax in 12 people (11). The incubation time from infection to initial onset of respiratory disease symptoms was estimated to be 4 to 6 days. Four patients succumbed to the disease in spite of massive antibiotic administration, probably because therapy started at the fulminant stage of the disease (11). Effective antibiotic-based postexposure therapy protocols preventing the establishment of fatal anthrax disease in several experimental animal models have been described in the literature. In rhesus monkeys exposed by inhalation to lethal doses of virulent B. anthracis spores, efficient treatment was obtained following administration of penicillin (3, 4, 8), doxycycline (3), ciprofloxacin (3, 13), and levofloxacin (13). In guinea pigs, treatment was with penicillin (23), doxycycline (12), tetracycline (1), ciprofloxacin (1, 12), and erythromycin (1). All animals were protected during antibiotic treatment; however, upon termination of treatment, the animals died from anthrax due to germination of the remaining spores in the lungs (1, 8, 12). This posttreatment death could be prevented by active immunization of the animals with a protective antigen (PA)-based vaccine during the antibiotic treatment (1, 3, 24). Successful curing of 21/25 rhesus monkeys exhibiting bacteremia levels up to 14,650 bacilli per ml blood was obtained by combined therapy with penicillin, streptomycin, hydrocortisone, anti-Sterne antiserum, and immunization with a PA-based vaccine (17). Vietri et al. described the curing of bacteremic rhesus monkeys by treatment with ciprofloxacin for 10 days. Initiation of treatment on days 2, 3, 4, 5, and 6 p.i. cured 2/3, 3/3, 1/2, 1/1, and 0/1 sick animals, respectively (25). Gochenour et al. treated bacteremic and nonbacteremic rhesus monkeys for 5 days with penicillin. Treatment of bacteremic animals, which started at 48 h and 72 h. p.i., cured 4/4 and 0/2 animals, respectively (4). Mice inhalationally infected with Ames spores were cured even when ciprofloxacin or doxycycline treatment was delayed until 36 h and 48 h p.i. (7).
In humans, the respiratory disease begins with nonspecific flu-like symptoms lasting 2 to 3 days, which suddenly change to severe respiratory distress. Death occurs within 24 to 36 h as a result of respiratory failure, sepsis, and shock (9). Experimental animals do not exhibit any specific symptoms indicative of disease progression until a few hours prior to death, when the animals develop severe respiratory distress. In anthrax animal models, serum bacteremia levels and PA concentrations are considered reliable markers of the severity of the disease (15).
In this study, we addressed two main goals: to define the efficiency of postexposure prophylaxis with different antibiotics in preventing respiratory anthrax and to determine the disease severity that could still be cured. We describe the effectiveness of postexposure prophylaxis with various antibiotics, members of the tetracycline, fluoroquinolone, aminoglycoside, and carbapenem families, in preventing the development of fatal anthrax disease following intranasal (i.n.) spore infection. Since the antibiotics doxycycline and ciprofloxacin are recommended by the CDC (10) for early treatment of anthrax patients, we tested their efficiency in curing anthrax-septic animals during the systemic phase of the disease, characterized by the presence for the first time of both bacteria and toxins in the circulation. These experiments were performed in two animal models, guinea pigs and rabbits, that were infected by intranasal spore inoculation. Both animal models are well established for studying various aspects of anthrax disease: virulence and correlates of protection and cure (5).
MATERIALS AND METHODS
B. anthracis strain.
The B. anthracis strain used in this study was ATCC 14578 (Vollum) (Tox+ Cap+) from the Israel Institute for Biological Research (IIBR) collection (16).
Animals.
Hartley guinea pigs (300 to 400 g) were obtained from Charles River, Germany. New Zealand White rabbits (2.5 to 3.5 kg) were obtained from Harlan (Israel). The animals received food and water ad libitum. All animals were cared for according to the 1997 NIH guidelines for the care and use of laboratory animals. All experimental protocols were approved by the IIBR committee on the ethics of animal experiments.
The animals were inoculated via the respiratory route by i.n. instillation. The estimated i.n. 50% lethal doses (LD50s) in rabbits and guinea pigs are 3 × 105 CFU and 4 × 104 CFU, respectively (1, 26). In the cure experiments, guinea pigs and rabbits were inoculated i.n. with 3 × 106 (75 LD50) and 2 × 106 to 6 × 106 (10 LD50) Vollum spores, respectively.
Guinea pig model.
In postexposure prophylaxis experiments, the infected animals were treated with various antibiotics starting 24 h p.i. for a period of 30 days. In cotreatment experiments with antibiotics and a PA-based vaccine, animals were vaccinated during antibiotic treatment on days 8 and 22 p.i. For the cure of septic animals, antibiotic administration started at 30 h p.i. and continued every 4 h to 6 h thereafter up to 54 h p.i. Treatment was applied twice daily for a period of 21 days. After the cessation of treatment, the animals were monitored for survivors for an additional 30 days, followed by evaluation of acquired protective immunity probed by a subcutaneous (s.c.) challenge with 5 × 103 (100 LD50) Vollum spores.
Rabbit model.
Rabbits were monitored for development of bacteremia by following the presence of PA in the circulation. In a previous study (15), we demonstrated that PA is a reliable marker for the level of bacteremia and the severity of systemic anthrax disease. The PA concentration was determined in blood samples drawn from the animals' ear veins starting at 26 h p.i. and every 2 to 3 h thereafter. After evaluation of the level of bacteremia, the animals were treated with antibiotics. The bacteremic animals were treated with antibiotics twice a day for a period of 14 days. After treatment termination, the animals were observed for survivors for an additional 30 days. The surviving animals were tested for acquired protective immunity by s.c. challenge with 2 × 104 (4,000 LD50) Vollum spores.
Antibiotics.
The following antibiotics were administered to guinea pigs twice daily at the indicated concentrations: doxycycline hydrochloride (Sigma D-9891), 10 mg/kg of body weight; ciprofloxacin (Ciproxin 100; 2 mg/ml; Bayer), 10 mg/kg; ofloxacin hydrochloride (Tarivid 200; Hoechest AG, Germany), 10 mg/kg; gentamicin-IKA (Teva, Hungary S.L.E.), 10 mg/kg; and imipenem (Tienam; Merck Sharp & Dohme, B.V. Haarlem, Netherlands), 10 mg/kg.
Rabbits were treated twice daily with doxycycline hydrochloride (Sigma D-9891), 15 mg/kg, or with ciprofloxacin (Ciproxin; Bayer; 10 g/100 ml suspension) given per os at 30 mg/kg.
PA vaccine.
Purified PA, isolated from strain ATCC 14185, was absorbed to Alhydrogel (Superfos Biosector) as previously described (21). Vaccination was carried out by s.c. injection of 0.5 ml vaccine.
Anti-PA antibodies.
Anti-PA (α-PA) antibodies were prepared in guinea pigs and rabbits injected with a PA-based vaccine (21, 26). In both species, the sera exhibited cytotoxicity neutralization antibody titers of 12,800 to 25,600. Monoclonal anti-PA number 29 was obtained from the Biotechnology Department, IIBR, and exhibited a cytotoxicity neutralization titer of 106 (22). In combined treatment with antibiotics and anti-PA antibodies, animals were injected (s.c. for guinea pigs and intravenously [i.v.] for rabbits) with the antibodies (a single injection) immediately prior to initiation of antibiotic administration.
ELISA for PA.
PA levels were determined by direct enzyme-linked immunosorbent assay (ELISA) in 96-well microtiter plates (Nunc, Roskilde, Denmark), using purified PA (21) as the reference standard. Plates were coated with rabbit α-PA antibody diluted in NaHCO3 buffer (50 mM; pH 9.6) and subsequently blocked with 5% skim milk (Becton Dickinson, Sparks, MD). The plates were washed with phosphate-buffered saline containing 0.05% Tween 20 (PBST) and incubated with the tested sera (diluted 1:2 in 0.5% skim milk) for 1 h at 37°C. For the standard curve, known concentrations of purified PA in 50% serum were used. The plates were washed with PBST and incubated with guinea pig α-PA antibody. Following a PBST rinse, the plates were developed with alkaline phosphatase-conjugated goat α-guinea pig immunoglobulin G (IgG) (Sigma, St. Louis, MO) and p-nitrophenyl phosphate (Sigma, St. Louis, MO) as the substrate. Absorbance at 405 nm was determined using a Spectromax 190 microplate reader (Molecular Devices, Sunnyvale, CA). The endpoint was defined as the highest dilution at which the absorbance was >3 standard deviations (SD) above that of the negative control. The sensitivity of the assay was determined to be 10 ng/ml PA.
Statistical analysis.
The significance of the differences in survival rates between the experimental and control groups and between experimental groups was determined using Fisher's exact test, two-tailed.
RESULTS
In this study, we evaluated the efficiencies of different therapeutic approaches to experimentally cure respiratory anthrax. We tested both the efficiencies of these treatments in preventing the development of fatal disease in infected animals and their efficiencies in curing animals in which the disease developed into a systemic septic phase.
Postexposure prophylaxis experiments.
The efficacy of antibiotic-based postexposure prophylaxis was evaluated in guinea pigs intranasally infected with Vollum spores (Fig. 1). During the treatment period, all antibiotics provided good protection against the development of respiratory disease (P < 0.0001 versus control untreated animals) (Fig. 1A to C and E). However, differences in the final outcome of the infection were observed upon termination of antibiotic administration. Gentamicin did not provide long-lasting protection, and 90% of the animals died from respiratory anthrax within 8 days after cessation of antibiotic treatment (Fig. 1A). This result indicates failure of the particular antibiotic to eradicate the infecting spores and the lack of development of an efficient immune response. In the imipenem-treated group (Fig. 1B), following cessation of treatment, only 3/8 of the animals died (P = 0.009 versus control untreated animals). The remaining animals developed anti-PA antibodies with a geometric mean titer (GMT) ± SD of 64,505 ± 28,112, which conferred full protection against a subsequent s.c. challenge with Vollum spores (P = 0.005 versus control animals). In the doxycycline-treated group (Fig. 1C), 50% of the animals died after the termination of antibiotic administration, and the survivors (P = 0.0325 versus control untreated animals) did not develop anti-PA antibodies and succumbed to subsequent s.c. challenge. On the other hand, cotreatment with doxycycline and a PA-based vaccine (Fig. 1D) induced the development of anti-PA antibodies with a GMT of >256,000, which conferred full protection after cessation of treatment (P < 0.0005 versus doxycycline treatment) and upon challenge with Vollum spores (P < 0.0001 versus control animals). Thus, vaccine addition improved the efficiency of doxycycline treatment, as demonstrated by animal survival both after the cessation of treatment and upon challenge. In the ofloxacin-treated group (Fig. 1E), 11/12 animals survived after cessation of treatment (P < 0.0001 versus control untreated animals) and developed anti-PA antibodies with a GMT of 2,884 ± 15,407, which provided protection to 70% of the surviving group upon subsequent s.c. challenge (P = 0.0014 versus control animals). The combined treatment with ofloxacin and PA vaccine (Fig. 1F) elicited anti-PA antibodies with a GMT ± SD of 34,970 ± 11,661, which conferred full protection against an s.c. challenge (P < 0.0001 versus control animals; P = 0.2143 versus the ofloxacin-treated group).
FIG. 1.
Efficiency of postexposure prophylaxis of guinea pigs intranasally infected with Vollum spores. Guinea pigs were infected intranasally with Vollum spores (n = 8 to 12 per group). The animals were treated for 30 days with gentamicin (A), imipenem (B), doxycycline (C), doxycycline and PA-based vaccine (Vac.) (D), ofloxacin (E), or ofloxacin and PA-based vaccine (F). After cessation of treatment, the animals were monitored for survival for an additional 30 days, subsequently rechallenged with an s.c. injection of Vollum spores, and monitored for an additional 14 days.
Efficiency in curing septic animals.
After demonstrating that early antibiotic treatment can prevent the development of fatal anthrax disease (this study and reference 1), we tested whether septic animals could also be cured by a similar treatment. Toxemia was used as a marker for the development of anthrax (15) due to the absence of disease symptoms in any of the animal models. The efficiency of treatment with doxycycline and ciprofloxacin, either alone or supplemented with anti-PA antibodies, in curing septic animals was studied in two animal models, guinea pigs and rabbits.
Guinea pig model. (i) Development of anthrax systemic disease.
In order to assess disease progression in guinea pigs, 24 h after intranasal instillation of Vollum spores and every 4 to 6 h thereafter, groups of 6 to 8 guinea pigs were bled and the serum bacterial loads and PA concentrations were determined. Septic animals were detected from 30 h postinfection, and at each time point thereafter, the percentage of bacteremic animals increased until all animals became bacteremic (between 48 h and 60 h). Concomitant with the development of bacteremia, higher levels of serum PA were observed. At each time point postinfection, variations in the levels of bacteremia and toxemia between different animals were observed (in agreement with our previous results [15]). Death of untreated animals occurred from 48 h p.i., and the animals died with a mean time to death (MTTD) of 52 h to 54 h.
(ii) Efficiency of treatment with doxycycline.
Delaying the initiation of doxycycline administration to over 24 h p.i. resulted in the need to treat septic animals (70 guinea pigs). Treatment with doxycycline cured 40/41 guinea pigs exhibiting bacteremia levels of up to 3.5 × 104 CFU/ml. Partial cure (6/12) was observed in animals exhibiting bacteremia levels of 4.4 × 104 CFU/ml to 4.0 × 105 CFU/ml, and no cure was obtained in animals exhibiting higher levels of bacteremia (Fig. 2A). Based on the toxemic status of the animals, treatment with doxycycline cured 33/33 animals with toxemia levels of up to 52 ng PA/ml, partial cure (9/23) was obtained in animals exhibiting PA concentrations between 60 ng/ml and 1,160 ng PA/ml, and no cure was obtained in animals with higher levels of toxemia (Fig. 2D). In summary, treatment of guinea pigs with respiratory anthrax with doxycycline efficiently protected animals with bacteremia levels up to ∼105 CFU/ml and toxemia levels up to 52 ng PA/ml.
FIG. 2.
Efficacy of antibiotic-mediated cure of septic guinea pigs. Animals were intranasally infected with Vollum spores, and following the development of septicemia, the animals were treated for 21 days with doxycycline (A and D), ciprofloxacin (B and E), or ciprofloxacin and anti-PA antibodies (C and F). The results are expressed as individual bacteremia levels (A to C) and serum PA levels (D to F) of each animal prior to initiation of treatment. GPαPA, guinea pig anti-PA.
(iii) Efficiency of treatment with ciprofloxacin.
Treatment with ciprofloxacin was applied to 59 bacteremic animals. Antibiotic administration cured 26/29 animals exhibiting bacteremia levels up to 9 × 104 CFU/ml. Partial cure (6/16) was obtained in animals exhibiting bacteremia levels of 1.3 × 105 CFU/ml to 4.5 × 106 CFU/ml. No protection was obtained in animals with higher levels of bacteremia (Fig. 2B). Treatment with ciprofloxacin cured 17/22 animals exhibiting toxemia levels up to 50 ng PA/ml and cured 10/23 animals with PA concentrations between 50 ng/ml and 780 ng/ml. No protection was observed in animals with higher levels of toxemia (Fig. 2E). In summary, treatment with ciprofloxacin cured animals with respiratory anthrax with bacteremia levels up to 9 × 104 CFU/ml and toxemia levels up to 50 ng PA/ml.
The observed MTTD of the sick guinea pigs that were not cured by treatment with either doxycycline or ciprofloxacin indicates that animals with bacteremia levels of 105 to 106 CFU/ml died 65.6 ± 28.5 h and 75.6 ± 72.7 h after initiation of treatment, respectively. Animals with bacteremia levels of 106 to 107 CFU/ml died 38.0 ± 12.0 h and 45.6 ± 34.8 h after initiation of treatment, respectively. Animals with bacteremia levels of >107 CFU/ml died a few hours after initiation of treatment. To improve the efficiency of cure of animals exhibiting bacteremia levels higher than 105 CFU/ml, a combined treatment with ciprofloxacin and anti-PA antibodies to neutralize the activities of the lethal and edema toxins was tested.
(iv) Efficiency of treatment with ciprofloxacin and anti-PA antibodies.
Passive immunization with anti-PA antibodies protected guinea pigs from development of fatal anthrax disease when administered 24 h p.i. (14). The efficiency of curing septic guinea pigs by a combined treatment of ciprofloxacin and anti-PA antibodies (neutralization titer, 2,000) was evaluated for 31 septic animals. The combined treatment cured all animals (11/11) with bacteremia levels up to 1.0 × 105 CFU/ml, provided partial protection to animals (8/13) with bacteremia of 105 CFU/ml to 1.4 × 106 CFU/ml, and failed to cure animals with higher levels of bacteremia (Fig. 2C). The combined treatment cured 12/13 animals with PA concentrations up to 150 ng/ml, partially cured 7/14 animals with toxemia levels of 200 ng PA/ml to 3,000 ng PA/ml, and failed to cure animals with higher levels of toxemia (Fig. 2F). In summary, the advantage of cure by combined treatment with ciprofloxacin and anti-PA antibodies over treatment with ciprofloxacin alone was evaluated on bacteremic and toxemic animals. While ciprofloxacin administration cured 38.4% of animals exhibiting bacteremia levels of 105 CFU/ml to 106 CFU/ml, the combined treatment cured 64.2% of the septic animals with the same levels of bacteremia (P = 0.272 versus ciprofloxacin-treated animals). Based on the toxemia levels of the treated animals, while ciprofloxacin treatment cured animals harboring soluble-PA concentrations of 50 ng/ml, the combined treatment cured animals exhibiting at least 3-fold-higher PA concentrations (P = 0.0624 versus ciprofloxacin-treated animals), indicating a trend toward increased efficiency with the addition of antibodies.
(v) Protective immunity in surviving guinea pigs.
Surviving animals were observed for 30 days after cessation of antibiotic administration. The animals were tested for acquired protective immunity by monitoring their anti-PA antibody levels and their resistance to s.c. challenge with lethal doses of Vollum spores. All the cured septic animals survived after cessation of antibiotic administration. All ciprofloxacin-treated animals developed high anti-PA antibody titers, with GMTs of 51,200 to 344,560. All the surviving animals acquired protective immunity and resisted s.c. challenge with Vollum spores (Fig. 3).
FIG. 3.
Development of protective acquired immunity in septic guinea pigs. Animals were grouped based on their pretreatment bacteremia levels as follows: up to 104 (blue), 104 to 105 (green), 105 to 106 (orange), 106 to 107 (red), and above 107 (black) CFU/ml. The treatment regime was doxycycline (A), ciprofloxacin (B), or ciprofloxacin and anti-PA antibodies (C) for 21 days. After cessation of antibiotic administration, the animals were monitored for 30 days, rechallenged s.c. with Vollum spores, and observed for an additional 14 days.
To summarize, appropriate antibiotic administration can prevent the development of fatal respiratory disease and cure anthrax-septic guinea pigs. Efficient postexposure prophylaxis requires combined treatment with antibiotics and active immunization with PA-based vaccines. Delaying initiation of antibiotic treatment till 2 days postinfection, when all the animals are septic and toxemic, can still cure sick animals with a systemic bacterial load of up to ∼105 CFU/ml and toxemia levels of ∼50 ng PA/ml. Addition of anti-PA antibodies to the antibiotic treatment allows efficient curing of animals with higher levels of bacteremia and toxemia.
Rabbit model.
The rabbit model enables continuous monitoring of the respiratory disease progression into the systemic phase by monitoring of the PA concentration in the circulation (15). Treatment was initiated after evaluation of the animal's bacteremia level.
(i) Efficiency of treatment with doxycycline.
Following intranasal infection with 10 LD50 of Vollum spores (34 to 40 h p.i.), 26 animals exhibiting bacteremia levels of 2 CFU/ml to 4.7 × 108 CFU/ml were treated with doxycycline. Control untreated animals died with an MTTD of 41.3 ± 9.8 h. The treatment cured 6/6 animals with bacteremia levels up to 1.0 × 104 CFU/ml, protected 5/9 animals with bacteremia levels of 5 × 104 CFU/ml to 2 × 106 CFU/ml, and failed to protect animals with higher levels of bacteremia (Fig. 4A). When the PA concentration was used as a parameter for toxemia, the administration of doxycycline cured 10/12 animals with toxemia levels up to 570 ng PA/ml and did not protect animals with higher levels of toxemia (Fig. 5A). After cessation of antibiotic administration, all the cured animals developed anti-PA antibody titers (GMT, 25,599 ± 20,293) that provided full protection against subsequent intradermal (i.d.) inoculation of 400 LD50 of Vollum spores (with challenge 30 days after termination of treatment) (Fig. 6A).
FIG. 4.
Efficacy of curing bacteremic rabbits. Animals were intranasally infected with Vollum spores, and following the development of septicemia, the animals were treated for 14 days with doxycycline (A), ciprofloxacin (B), doxycycline and rabbit (Rb) anti-PA antibodies (C), ciprofloxacin and rabbit anti-PA antibodies (D), or ciprofloxacin and monoclonal antibody (MAb) number 29 (E). The results are expressed as individual bacteremia levels prior to initiation of antibiotic administration.
FIG. 5.
Efficacy of curing toxemic rabbits. Animals were intranasally infected with Vollum spores, and following the development of septicemia, the animals were treated for 14 days with doxycycline (A), ciprofloxacin (B), doxycycline and rabbit anti-PA antibodies (C), ciprofloxacin and rabbit anti-PA antibodies (D), or ciprofloxacin and monoclonal antibody number 29 (E). The results are expressed as individual serum PA levels prior to initiation of antibiotic administration.
FIG. 6.
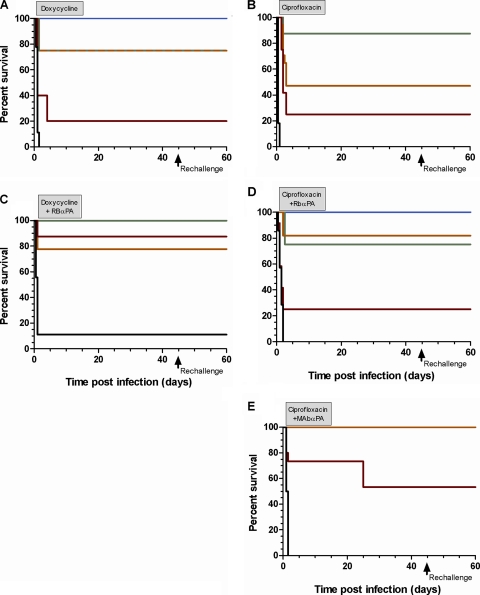
Development of protective acquired immunity in septic rabbits. Animals were grouped based on their pretreatment bacteremia levels as follows: up to 104 (blue), 104 to 105 (green), 105 to 106 (orange), 106 to 107 (red), and above 107 (black) CFU/ml. The treatment regimes (21 days) were doxycycline (A), ciprofloxacin (B), doxycycline and rabbit anti-PA antibodies (C), ciprofloxacin and rabbit anti-PA antibodies (D), and ciprofloxacin and number 29 anti-PA monoclonal antibody (E). After cessation of antibiotic administration, the animals were monitored for an additional 30 days, rechallenged s.c. with Vollum spores, and monitored for an additional 14 days.
Animals that succumbed and that exhibited bacteremia levels lower than 107 CFU/ml at the initiation of treatment died with an MTTD of 30.6 ± 33.5 h after initiation of treatment, while animals with bacteremia higher than 107 CFU/ml died with an MTTD of 14.8 ± 9.8 h after initiation of treatment.
(ii) Efficiency of treatment with ciprofloxacin.
The efficiency of ciprofloxacin in curing sick rabbits was tested on 48 bacteremic animals that were treated between 30 and 40 h p.i. after exposure to 10 LD50 of Vollum spores. The treatment cured 8/9 animals with bacteremia levels up to 1.0 × 105 CFU/ml, cured 10/27 animals with bacteremia levels of 1.1 × 105 CFU/ml to 5.0 × 106 CFU/ml, and failed to protect animals with higher levels of bacteremia (Fig. 4B). Based on toxemia levels, treatment with ciprofloxacin cured 11/11 animals with toxemia levels up to 150 ng PA/ml, protected 7/13 animals with toxemia levels of 150 ng PA/ml to 450 ng PA/ml, and did not protect animals with higher levels of toxemia (Fig. 5B). All the animals that were protected during ciprofloxacin administration survived after termination of treatment (Fig. 6B).
Monitoring the MTTD of the animals that succumbed indicates that animals that prior to initiation of treatment exhibited bacteremia levels lower than 107 CFU/ml died 55.6 ± 23.6 h after initiation of treatment, whereas animals with bacteremia loads of >107 CFU/ml died 12.2 ± 17.6 h after initiation of treatment.
(iii) Efficiency of treatment with antibiotics and anti-PA antibodies.
In an attempt to improve the efficiency of cure of rabbits exhibiting bacteremia levels higher than 105 CFU/ml, we tested the effect of neutralization of the bacterial toxins concomitantly with initiation of the antibiotic treatment. In a previous study, we found that anti-PA neutralization antibody titers of 1,000 conferred protection against intranasal challenge with B. anthracis spores (26). For combined treatment with antibiotics and antibodies, we used either rabbit anti-PA antibodies conferring neutralization titers of 2,000 or the monoclonal anti-PA number 29 (22) antibody, which conferred neutralization titers of 10,000. The antibodies were injected once at treatment initiation.
(iv) Efficiency of treatment with doxycycline and anti-PA antibodies.
Rabbits were infected intranasally with 10 to 40 LD50 of Vollum spores, and septic animals were treated from 30 to 40 h postinfection with rabbit anti-PA antibodies injected i.v. and with doxycycline twice a day for a period of 14 days. The combined treatment cured 5/5 animals with bacteremia levels up to 1.4 × 105 CFU/ml, efficiently cured 6/7 animals with bacteremia levels between 4.4 × 105 CFU/ml and 2.5 × 106 CFU/ml, and failed to cure animals with higher levels of bacteremia (Fig. 4C). Based on the toxemia levels of the sick animals, the combined treatment with doxycycline and anti-PA antibodies cured 12/13 animals with toxemia levels up to 1,400 ng PA/ml and failed to protect animals with higher levels of toxemia (Fig. 5C). No significant (P = 0.294) improvement could be attributed to the addition of polyclonal antibodies to doxycycline treatment when estimated by correlation with the bacteremic levels, but improvement was significant (P = 0.0033) when evaluated based on the toxemia levels.
All protected animals survived after cessation of antibiotic administration and acquired protection against i.d. inoculation with 500 LD50 of Vollum spores (Fig. 6C).
The animals that succumbed and that exhibited bacteremia levels of ≥107 CFU/ml died with an MTTD of 17.9 ± 7.5 h after initiation of treatment, similar to the MTTD observed following treatment with doxycycline alone.
(v) Efficiency of treatment with ciprofloxacin and anti-PA antibodies.
Combined treatments with ciprofloxacin and anti-PA antibodies were performed both with polyclonal rabbit anti-PA antibodies (providing a neutralization titer of 2,000) and number 29 anti-PA monoclonal antibody (providing a neutralization titer of 10,000).
Combined treatment with ciprofloxacin and rabbit anti-PA antibodies cured 5/5 animals exhibiting bacteremia levels up to 7 × 104 CFU/ml, protected 9/12 animals with bacteremia levels of 8 × 104 CFU/ml to 9 × 105 CFU/ml, and failed to protect animals with bacteremia levels higher than 106 CFU/ml (Fig. 4D), Based on the toxemia levels of the treated animals, the combined treatment with ciprofloxacin and rabbit anti-PA antibodies cured 12/13 animals with PA concentrations up to 265 ng/ml, protected 6/14 animals with toxemia levels of 300 ng PA/ml to 1,700 ng PA/ml, and failed to cure animals with higher levels of toxemia (Fig. 5D). The increase in efficiency of cure by addition of rabbit anti-PA antibodies was not significant when evaluated based on either bacteremia levels (P = 0.585 versus ciprofloxacin-treated animals) or toxemia levels (P = 0.122 versus ciprofloxacin-treated animals). All animals that were protected during the treatment survived after cessation of antibiotic administration and acquired protection against subsequent i.d. inoculation with 500 LD50 of Vollum spores (Fig. 6D).
Treatment with ciprofloxacin and monoclonal anti-PA number 29 antibodies cured 9/9 animals with bacteremia levels up to 1.6 × 106 CFU/ml, cured 8/12 animals with bacteremia levels of 2 × 106 CFU/ml to 8.5 × 106 CFU/ml, and failed to protect animals with higher levels of bacteremia (Fig. 4E). Based on the toxemia levels of the animals, treatment with ciprofloxacin and monoclonal anti-PA number 29 antibodies protected 17/21 animals with toxemia levels up to 3,200 ng PA/ml (Fig. 5E). The treatment failed to cure animals with higher levels of toxemia. On day 10 after termination of treatment, 3 animals that at treatment onset exhibited bacteremia levels of 2.3 × 106 CFU/ml, 3.3 × 106 CFU/ml, and 8.5 × 106 CFU/ml died from anthrax. All other animals survived and developed protective immunity (Fig. 6E). Statistical analysis of the efficiency of cure by combined administration of ciprofloxacin and monoclonal anti-PA antibodies compared to treatment with ciprofloxacin alone indicated P values of 0.0056 (based on bacteremia) and of 0.0023 (based on toxemia), suggestive of a significant contribution of the monoclonal antibody to the efficiency of cure with ciprofloxacin.
To summarize, antibiotic administration can cure rabbits with respiratory anthrax at the systemic stage of the disease and exhibiting bacteremia levels of ∼105 CFU/ml and toxemia levels of 150 ng PA/ml to 500 ng PA/ml. Combined administration of anti-PA antibodies and antibiotics cured animals with a more advanced disease exhibiting bacteremia levels of 2 × 106 CFU/ml to 4 × 106 CFU/ml and toxemia levels of >1,000 ng PA/ml.
DISCUSSION
Here, we broadened the range of antibiotics that can be used for prophylaxis and that would be important additions to the main recommended antibiotics in case of mass exposure and the need to treat special populations. Postexposure prophylaxis of animals experimentally infected with B. anthracis spores has been described in the literature in different animal anthrax models and with various antibiotics (1, 3, 4, 7, 8, 12, 13, 17, 18, 23-25). Doxycycline and ciprofloxacin are considered the antibiotics of choice to treat humans (10). In this study, we tested whether administration of both of these antibiotics and additional antibiotics can prevent the development of respiratory anthrax disease. Our data indicate that postexposure prophylaxis in guinea pigs with doxycycline, tetracycline (1), ciprofloxacin (1), ofloxacin, imipenem, gentamicin, and erythromycin (1), initiated at 24 h p.i., can prevent the development of fatal anthrax respiratory disease. However, only a combined treatment with antibiotics and active immunizations with a PA-based vaccine conferred long-term protective immunity against reestablishment of the disease. Similar results were reported previously in guinea pigs (12) and rhesus monkeys (3, 8, 24).
The current study addressed the issue of whether delayed initiation of antibiotic administration (until the animals are both bacteremic and toxemic) can still rescue sick animals. Previous publications on curing sick rhesus monkeys and mice (4, 7, 17, 25) did not characterize the disease that can be cured. Our novel findings defined by bacteremia and toxemia the severity of respiratory anthrax that can be cured by either antibiotic administration or combined treatment with antibiotics and anti-PA antibodies. In the guinea pig model, respiratory disease progression could be divided into 3 stages: an incubation period from 30 h to the commencement of the systemic stage, an increase in the percentage of bacteremic animals until 48 h p.i., and the death phase from 48 h to 60 h p.i. The observed time window from bacteremia onset to death (∼18 h) is similar to that previously observed in the rabbit model (15). We tested the efficiency of curing septic guinea pigs and rabbits by treatment with doxycycline or ciprofloxacin alone and in combination with anti-PA antibodies to facilitate killing of the bacteria and neutralization of the soluble toxins in the circulation. Treatment with both antibiotics (doxycycline and ciprofloxacin) cured septic animals exhibiting bacterial concentrations of ∼105 CFU/ml in both animal models. Since untreated animals died exhibiting a circulatory bacterial load of about 109 CFU/ml (representing ∼13 additional replication cycles within approximately 7 h from 105 CFU/ml), it could be inferred that the antibiotic treatment cured severely sick animals at a stage very close to death. Treatment with doxycycline and ciprofloxacin cured guinea pigs and rabbits displaying toxemia levels of approximately 50 ng PA/ml and 150 to 400 ng PA/ml, respectively.
In this study, we demonstrated that coadministration of anti-PA antibodies and antibiotics was more beneficial than antibiotics alone in two animal models. Cotreatment of bacteremic guinea pigs with ciprofloxacin and anti-PA antibodies protected animals with bacteremia up to 7 × 105 CFU/ml and toxemia up to 3,000 ng PA/ml, representing about a 10-fold improvement in cure efficacy compared to antibiotic alone.
In rabbits, combined treatment with doxycycline or ciprofloxacin and anti-PA antibodies cured animals exhibiting bacteremia levels of 2 × 106 to 3 × 106 CFU/ml, a 10-fold increase over the efficiency of treatment with antibiotics alone. Using higher titers of neutralization antibodies enhanced the improvement of treatment efficiency, protecting animals exhibiting bacteremia levels up to 4 × 106 CFU/ml. However, after cessation of treatment with ciprofloxacin and monoclonal antibody number 29, 3 animals died on day 10 after termination of treatment (day 24 after initiation of treatment). This might be a consequence of either a decrease in the antibody circulatory concentration (half-life [t1/2] = 4 to 10 days [19, 20]) or severe damage to the animals as a result of the high bacteremia and toxemia concentrations prior to initiation of treatment.
All the rescued guinea pigs and rabbits acquired protective immunity during antibiotic administration and survived after the termination of treatment. The surviving animals developed anti-PA antibodies and resisted s.c. challenge with lethal doses of Vollum spores. Similar results were reported for ciprofloxacin-treated rhesus monkeys with inhalation anthrax (25). Seven out of the 10 protected animals also survived after cessation of treatment. The potential for antibiotic administration to cure severely septic animals is also evident from the analysis of the times of death of animals that succumbed. Animals exhibiting bacterial loads of 105 CFU/ml to 107 CFU/ml died 2 to 3 days after initiation of antibiotic administration, whereas animals exhibiting bacteremia concentrations higher than 107 CFU/ml died within a few hours after the initiation of treatment. These results indicate that a more elaborate treatment with various antibiotics, antibodies, and toxin neutralization agents may be necessary to improve the extent of cure of animals with bacteremia of up to 107 CFU/ml.
Another relevant postinfection issue is the therapeutic time window from infection until initiation of antibiotic administration that would still provide efficient cure of sick animals. Administration of doxycycline up to 48 h p.i. cured 92% of the sick animals. Treatment initiated at 48 h p.i. cured 75% of the animals. A similar therapeutic time window for initiation of treatment was observed with ciprofloxacin. Antibiotic administration up to 46 h postinfection cured 69% of the sick animals. Initiation of treatment at 46 h p.i. rescued only 68% of the sick animals. These results indicate the existence of a time span of ∼2 days from infection to initiation of antibiotic administration that still ensures efficient cure of guinea pigs with respiratory anthrax.
In conclusion, our results suggest that doxycycline and ciprofloxacin are efficient antibiotics to treat anthrax, not only as postexposure prophylaxis, but also during the systemic phase of the disease. Treatment with both antibiotics can cure guinea pigs and rabbits in an advanced stage of systemic anthrax exhibiting bacteremia levels of ∼105 CFU/ml. Cotreatment with ciprofloxacin and anti-PA antibodies improved the efficiency of treatment and cured guinea pigs with bacteremia levels up to 7 × 105 CFU/ml and rabbits with 4 × 106 CFU/ml.
Acknowledgments
We thank Avigdor Shafferman for his guidance and fruitful discussion during this study.
Footnotes
Published ahead of print on 24 January 2011.
REFERENCES
- 1.Altboum, Z., et al. 2002. Postexposure prophylaxis against anthrax: evaluation of various treatment regimens in intranasally infected guinea pigs. Infect. Immun. 70:6231-6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dixon, T. C., M. Meselson, J. Guillemin, and P. C. Hanna. 1999. Anthrax. N. Engl. J. Med. 341:815-826. [DOI] [PubMed] [Google Scholar]
- 3.Friedlander, A. M., et al. 1993. Postexposure prophylaxis against experimental inhalation anthrax. J. Infect. Dis. 167:1239-1243. [DOI] [PubMed] [Google Scholar]
- 4.Gochenour, W. S., Jr., C. A. Gleiser, and W. D. Tigertt. 1962. Observations on penicillin prophylaxis of experimental inhalation anthrax in the monkey. J. Hyg. 60:29-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goossens, P. L. 2009. Animal models of human anthrax: the quest for the holy grail. Mol. Aspects Med. 30:467-480. [DOI] [PubMed] [Google Scholar]
- 6.Hanna, P. 1998. Anthrax pathogenesis and host response. Curr. Top. Microbiol. Immunol. 225:13-35. [DOI] [PubMed] [Google Scholar]
- 7.Heine, H. S., et al. 2007. Determination of antibiotic efficacy against Bacillus anthracis in a mouse aerosol challenge model. Antimicrob. Agents Chemother. 51:1373-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henderson, D. W., S. Peacock, and F. C. Belton. 1956. Observations on the prophylaxis of experimental pulmonary anthrax in the monkey. J. Hyg. 54:28-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holty, J. E., et al. 2006. Systematic review: a century of inhalational anthrax cases from 1900 to 2005. Ann. Intern. Med. 144:270-280. [DOI] [PubMed] [Google Scholar]
- 10.Inglesby, T. V., et al. 2002. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA 287:2236-2252. [DOI] [PubMed] [Google Scholar]
- 11.Jernigan, J. A., et al. 2001. Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg. Infect. Dis. 7:933-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones, M. N., R. J. Beederman, P. C. B. Turnbull, and R. J. Manchee. 1996. Antibiotic prophylaxis for inhalational anthrax. Salisbury Med. Bull. Special Suppl. 87:127-128. [Google Scholar]
- 13.Kao, L. M., et al. 2006. Pharmacokinetic considerations and efficacy of levofloxacin in an inhalational anthrax (postexposure) rhesus monkey model. Antimicrob. Agents Chemother. 50:3535-3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobiler, D., et al. 2002. Efficiency of protection of guinea pigs against infection with Bacillus anthracis spores by passive immunization. Infect. Immun. 70:544-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobiler, D., et al. 2006. Protective antigen as a correlative marker for anthrax in animal models. Infect. Immun. 74:5871-5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy, H., M. Fisher, N. Ariel, Z. Altboum, and D. Kobiler. 2005. Identification of strain specific markers in Bacillus anthracis by random amplification of polymorphic DNA. FEMS Microbiol. Lett. 244:199-205. [DOI] [PubMed] [Google Scholar]
- 17.Lincoln, R. E., et al. 1964. Successful treatment of rhesus monkeys for septicemia anthrax. Antimicrob. Agents Chemother. 10:759-763. [PubMed] [Google Scholar]
- 18.Maples, K. R., et al. 2007. Novel semisynthetic derivative of antibiotic eremomycin active against drug-resistant gram-positive pathogens including Bacillus anthracis. J. Med. Chem. 50:3681-3685. [DOI] [PubMed] [Google Scholar]
- 19.Mohamed, N., et al. 2005. A high-affinity monoclonal antibody to anthrax protective antigen passively protects rabbits before and after aerosolized Bacillus anthracis spore challenge. Infect. Immun. 73:795-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peterson, J. W., et al. 2006. Human monoclonal anti-protective antigen antibody completely protects rabbits and is synergistic with ciprofloxacin in protecting mice and guinea pigs against inhalation anthrax. Infect. Immun. 74:1016-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reuveny, S., et al. 2001. Search for correlates of protective immunity conferred by anthrax vaccine. Infect. Immun. 69:2888-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenfeld, R., et al. 2009. Isolation and chimerization of a highly neutralizing antibody conferring passive protection against lethal Bacillus anthracis infection. PloS One 4:e6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vancurick, J. 1966. Causes of the failure of antibiotic prophylaxis of inhalation anthrax and clearance of the spores from the lungs. Folia Microbiol. (Praha) 11:459-464. [DOI] [PubMed] [Google Scholar]
- 24.Vietri, N. J., et al. 2006. Short-course postexposure antibiotic prophylaxis combined with vaccination protects against experimental inhalational anthrax. Proc. Natl. Acad. Sci. U. S. A. 103:7813-7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vietri, N. J., et al. 2009. A short course of antibiotic treatment is effective in preventing death from experimental inhalational anthrax after discontinuing antibiotics. J. Infect. Dis. 199:336-341. [DOI] [PubMed] [Google Scholar]
- 26.Weiss, S., et al. 2006. Immunological correlates for protection against intranasal challenge of Bacillus anthracis spores conferred by a protective antigen-based vaccine in rabbits. Infect. Immun. 74:394-398. [DOI] [PMC free article] [PubMed] [Google Scholar]