Abstract
This study identified optimal daptomycin dosing for patients receiving thrice-weekly hemodialysis (HD). Twelve adult patients on HD received daptomycin at 6 mg/kg of body weight intravenously (i.v.) one time; plasma and dialysate samples were collected over 3 days. A 2-compartment model with separate HD and non-HD clearance terms was fit to the data. A series of 9,999-subject Monte Carlo simulations (MCS) was performed to identify HD dosing schemes providing efficacy and toxicity profiles comparable to those obtained for MCS employing the daptomycin population pharmacokinetic (PK) model derived from patients in the Staphylococcus aureus bacteremia-infective endocarditis (SAB-IE) study. For efficacy, we selected the HD dosing scheme which generated an area-under-the-curve (AUC) exposure profile comparable to that for the SAB-IE population model. For toxicity, we selected HD dosing schemes that minimized trough concentrations of ≥24.3 mg/liter. Separate HD dosing schemes were developed for each FDA-approved regimen and for two weekly interdialytic periods (48 and 72 h). Administration of the same parent daptomycin dose intra-HD and post-HD resulted in AUC, maximum concentration of drug in serum (Cmax), and Cmin values most comparable to those for SAB-IE simulations for the 48-hour interdialytic period. In contrast, all candidate HD dosing schemes provided AUC48-72 values that were at least 50% lower than the SAB-IE AUC48-72 values. Increasing the parent dose by 50% provided more comparable AUC48-72 values while maintaining acceptable Cmin values. Administration of the daptomycin parent dose intra-HD or post-HD was optimal for the 48-h interdialytic period. For the 72-h interdialytic period, clinicians should consider increasing the dose by 50% to achieve more comparable AUC48-72 values.
Daptomycin is a lipopeptide approved for the treatment of Staphylococcus aureus bacteremia and complicated skin and skin structure infections, both of which are common in patients receiving hemodialysis (HD) (1, 4, 14-18). While the pharmacokinetic (PK) properties of daptomycin are well described for the general population, there are scant data for patients on HD (12, 13). An understanding of how PK changes during HD is essential to determining optimal dosing schemes for these patients. Furthermore, such dosage adjustments necessitate cognizance of interactions between antibiotic exposure, efficacy, and toxicity. Given the dearth of treatment options available against methicillin-resistant S. aureus (MRSA), it is imperative that daptomycin regimens be optimized for those on HD.
The two objectives of this study were (i) to characterize the PK profile of daptomycin in patients receiving traditional thrice-weekly hemodialysis and (ii) to identify the optimal dosing scheme for daptomycin among patients on HD. With respect to the latter, the intent was to identify HD dosing schemes with efficacy and toxicity profiles comparable to those obtained from Monte Carlo simulations (MCS) employing the daptomycin population PK model derived from patients enrolled in the Staphylococcus aureus bacteremia-infective endocarditis (SAB-IE) clinical study for doses of 4 mg/kg of body weight given intravenously (i.v.) every 24 h (q24h) and 6 mg/kg given i.v. q24h (2, 7).
Separate dosing schemes were developed for both FDA-approved daptomycin dosing regimens (4 mg/kg i.v. q24h and 6 mg/kg i.v. q24h) and for the two interdialytic periods (48 and 72 h). For efficacy, the primary exposure target was the area under the curve (AUC). Animal model data suggest that daptomycin is a concentration-dependent antibiotic, and the AUC/MIC ratio is the pharmacodynamic (PD) parameter that best describes its activity (3, 5, 9, 11). However, the AUC/MIC ratio associated with maximal effect has varied (3, 5, 9, 11). Given the lack of a definitive AUC/MIC threshold, especially for patients with bloodstream infections, the distribution of AUC values (the PD target most closely linked to effect) for any candidate HD dosing scheme should at least be comparable to what is typically observed in non-HD patients. This study uses the distribution of AUC values from MCS derived from the SAB-IE population PK model as the referent or “typical patient” AUC exposure distribution (2, 7).
For toxicity, we focused on identifying HD regimens that minimized trough concentrations in excess of 24.3 mg/liter. Animal studies have demonstrated an association between daptomycin trough concentrations and elevations in creatine phosphokinase (CPK) (6, 10). More recently, a priori examination of patients from the SAB-IE study substantiated this relationship and demonstrated that elevations in daptomycin troughs, especially above 24.3 mg/liter, are associated with an increased probability of a CPK elevation (2). The candidate HD administration scheme that best achieved the exposure targets associated with efficacy and minimal toxicity was selected as the optimal HD dosing schedule for each FDA-approved daptomycin dosing regimen.
MATERIALS AND METHODS
Patients receiving thrice-weekly HD were recruited from the Hortense and Louis Rubin Dialysis Center (Clifton Park, NY), a community-based, nonprofit dialysis clinic in upstate New York. Inclusion criteria were as follows: (i) ≥18 years of age, (ii) absence of an active infection (afebrile, lack of constitutional symptoms, and white blood cell count between 5,000 and 10,000 cells/mm3), (iii) chronic HD (≥1 month), (iv) prehemodialysis weight within 10% of prescribed dry weight within 1 week of the study, and (v) hemoglobin level of ≥11 g/dl.
Patients with and without residual kidney function were included. Patients with residual kidney function who were taking medications that inhibit active tubular secretion (H2 receptor antagonists, trimethoprim, and probenecid) were included after a 2-week washout period. Patients were excluded from the study if they (i) were allergic to daptomycin, (ii) were pregnant or breastfeeding, or (iii) had received antibiotics within 2 weeks of the study.
All patients received a thrice-weekly 3.5-hour HD regimen. Blood and dialysate flow rates were fixed at 300 and 600 ml/minute, respectively. An F80 dialysis filter (polysulfone membrane; surface area, 1.8 m2; ultrafiltration coefficient [Kuf] of 59 ml/h per mm Hg) (Fresenius Medical Care North America) was used; this is considered a high-flux dialysis membrane.
Three consecutive days of study commenced with the patients' regularly scheduled midweek HD. On day 1, an intravenous catheter was placed in each arm: one was for daptomycin administration, and the other was for blood sampling. On days 2 and 3, only one catheter was placed, for blood sampling.
On study day 1, after the HD session, patients received one dose of daptomycin intravenously, infused over 30 min (6 mg/kg, rounded to the nearest 5 mg).
Sampling scheme.
Each patient provided a total of 26 blood samples during the 3-day study. Blood samples were collected in standard tubes containing sodium heparin. Two samples, collected at initiation and completion of the study, measured CPK levels. The remaining 24 samples measured the daptomycin concentration in the plasma.
On study day 1, 10 blood samples were collected at the following times: pre-HD (2 samples), post-HD, immediately following daptomycin administration (time zero), and 5, 15, 60, 120, 240, and 360 min postinfusion. On study day 2 (non-HD day), four blood samples were collected, at 22, 24, 28, and 32 h postinfusion. On study day 3 (HD day), 12 blood samples were collected at the following times: pre-HD, 15, 30, 60, and 120 min after the start of the HD session, directly following HD (time zero), and 5, 15, 30, 60, and 120 min (2 samples) post-HD. All blood samples were centrifuged at room temperature at 3,000 rpm for 10 min. The plasma was withdrawn and stored at −80°C until assay.
Dialysate was collected twice from each patient during the study. The first 30-ml sample was collected during the HD session on study day 1, before daptomycin administration, to ensure that no interfering substances would invalidate the assay. On day 3, all dialysate generated during the HD session was collected in a 55-gallon drum and mixed thoroughly, the volume was recorded, and a 30-ml sample was stored frozen at −80°C until assay.
Among patients able to produce urine, two samples were collected: the first was obtained on day 1, immediately prior to daptomycin administration, and the second was a 24-hour collection commencing with daptomycin infusion.
LC/MS/MS assay methodology for human plasma and dialysate samples.
An analytical reference standard for daptomycin and the internal standard CB-186,253 were provided by Cubist Pharmaceuticals Inc. The liquid chromatography-tandem mass spectrometry (LC/MS/MS) instrumentation included an Applied Biosystems API-4000 instrument coupled with an Agilent 1100 quaternary pump and a Leap CTC autosampler. The daptomycin analogue CB-183,253 served as the internal standard for both matrix assays. Multiple-ion-reaction monitor (MRM) transitions on the mass spectrometer of 811 to 159 and 837 to 365 were used to monitor daptomycin and the internal standard, respectively.
Human sodium heparin plasma was used for plasma assay as a matrix blank. Lactate Ringer solution (Baxter, Deerfield, IL) was used as the matrix blank for analysis of the dialysate samples.
The calibration curve was 1.0 to 100 μg/ml for plasma assay and 0.05 to 10.0 μg/ml for dialysate assay. Solid-phase extraction was employed. A Waters HLB (30 mg) 96-well plate was used, and final extracts were analyzed by LC/MS/MS. The dialysate samples were diluted with the internal standard in acetonitrile and centrifuged. The final extracts were reconstituted with 1% formic acid in 90% water to 10% acetonitrile and then analyzed by LC/MS/MS.
A Waters XBridge column (4.6 mm × 50 mm × 5 μm) was used. Mobile phase A was water, mobile phase B was acetonitrile, and mobile phase C was 10% formic acid in water. The formic acid in the final gradient was 1.0% in 35% to 50% mobile phase B over a 4.6-min run.
HPLC assay methodology for urine samples.
Concentrations of daptomycin in urine samples were determined by high-pressure liquid chromatography (HPLC) as previously described (5).
Pharmacokinetic data analysis.
All data were analyzed in a population PK model by use of BigNPAG (8). The PK model was parameterized as a two-compartment model with zero-order infusion and first-order intercompartmental transfer and elimination. Both non-HD clearance and HD clearance (CLHD) were modeled simultaneously. CLHD was treated as a piecewise input function, turned on during HD and off during non-HD. The model included a differential equation to account for the amount of daptomycin recovered from the dialysate generated during HD on day 3.
The inverse of the estimated assay variance was used as the first estimate of weights. Weighting assumed that total observation variance was proportional to assay variance, which was determined on a between-day basis. Adaptive gamma was utilized.
Upon attaining convergence, Bayesian estimates for each patient were obtained using the “population of one” utility in BigNPAG (8). For each model, the mean, median, and modal values were used as measurements of the central tendency of the population parameter estimates and were evaluated in the maximum a posteriori probability (MAP) Bayesian analysis. Scatter plots were examined for individual patients and the overall population. Goodness of fit was assessed by regression with an observed versus predicted plot, coefficients of determination, and log-likelihood values. Predictive performance was based on the weighted mean bias and bias-adjusted weighted mean precision.
MCS.
A series of 9,999-subject MCS using ADAPT II was performed to identify HD dosing schemes providing efficacy and toxicity profiles comparable to those obtained from the SAB-IE simulations (7). For efficacy, we selected the HD dosing scheme which generated an AUC exposure profile (cumulative and noncumulative daily AUCs) over the entire dosing interval that was comparable to that for the SAB-IE population model (7). For toxicity, we identified HD dosing schemes that minimized trough concentrations in excess of 24.3 mg/liter (2). Separate HD dosing schemes were developed for each FDA-approved daptomycin dosing regimen and for the two interdialytic periods (48 h and 72 h) observed during a week. The candidate HD administration scheme that best achieved these exposure targets was selected as the optimal HD dosing regimen for each FDA-approved daptomycin dosing regimen.
The daptomycin candidate regimens were administered 1 h prior to HD (pre-HD), 1 h prior to the end of HD (intra-HD), and following HD (post-HD).
Several different daptomycin doses were considered for each administration strategy. The FDA-approved single doses of 4 and 6 mg/kg were initially evaluated, and other doses were considered to identify HD dosing schemes most closely approximating the referent AUC distributions for 4 and 6 mg/kg i.v. q24h and having a low probability of a Cmin of ≥24.3 mg/liter (2, 7). The actual amount (milligrams) of daptomycin used in the MCS was determined by using the distribution of patient weights observed in the HD study.
For each simulated HD scheme, the AUC, concentration immediately prior to the next dose (Cmin), and concentration 1 h after completion of infusion (Cmax) were determined. Both cumulative (AUC0-48 and AUC0-72) and noncumulative, daily (AUC0-24, AUC24-48, and AUC48-72) AUC values were simulated. The Cmin values simulated were for 48 h (Cmin,48) and 72 h (Cmin,72). For each interdialytic period (48 h and 72 h), these simulated AUC, Cmin, and Cmax values were compared to those obtained from the Monte Carlo simulations derived from the SAB-IE PK model for 4 and 6 mg/kg i.v. every 24 h, given as a 30-min infusion.
For all simulations, the population simulation without process noise option was utilized. Both normal and log-normal distributions were evaluated, and the distribution selected was based on the ability to best recreate the original mean parameter vector and associated distribution.
Monte Carlo simulation was also used to evaluate the predictive performance of the population pharmacokinetic model. The mean parameter vector values were used to simulate the steady-state plasma concentration-time profile for 488 mg daptomycin infused over 30 min as a one-time dose. The dose was based on providing 6 mg/kg for the average weight (81 kg) observed in the study. The fidelity by which the concentration-time curves mirrored the raw data was assessed by visual inspection.
RESULTS
Twelve patients completed the study between October 2008 and January 2009 (Table 1). Seven (58.3%) were males, and the average age among the patients was 61.8 years. Patients received HD for a median of 51 months. Only 4 (33.3%) patients produced at least 100 ml of urine during the 24-hour urine collection period; among these patients, approximately 0.7% ± 0.6% of the total daptomycin dose was recovered in urine. The mean (standard deviation [SD]) amount of daptomycin recovered in dialysate was 40.7 (9.5) mg, which represented 8.4% of the total dose.
TABLE 1.
Demographic characteristics of hemodialysis patients evaluated in this study
| Characteristicc | Value (n = 12) |
|---|---|
| Mean (SD) age (yr) | 61.8 (14.1) |
| No. (%) of males | 7 (58.3) |
| No. (%) of Caucasians | 10 (83.3) |
| No. (%) of patients of African racial origin | 2 (16.7) |
| No. (%) of nonanuric patientsa | 4 (33.3) |
| Mean (SD) wt (kg) | 81.2 (11.9) |
| Median (IQR) dialysis vintage (mo) | 51 (29-68) |
| Mean (SD) daptomycin dose (mg) | 487.1 (71.9) |
| No. (%) of patients with cause of end-stage renal disease | |
| Hypertension | 4 (33.3) |
| Diabetes mellitus | 2 (16.7) |
| Otherb | 6 (50.0) |
Production of ≥100 ml of urine over 24-h collection period.
Other causes of end-stage renal disease were medication (n = 1), congestive heart failure (n = 1), immunologic kidney disease (n = 1), and unknown (n = 3).
IQR, interquartile range.
Population pharmacokinetic analysis.
The mean (SD) population parameter estimates identified by BigNPAG for the HD population PK model and the previously published SAB-IE population PK model are provided in Table 2 (2, 7). The mean nondialytic clearance observed in the HD model was one-third the estimated mean clearance in the SAB-IE model. In contrast, the mean dialytic clearance in the HD model was identical to the mean clearance for the SAB-IE model.
TABLE 2.
PK parameters from population PK analysis
| Parametera | Mean (SD) value for model |
|
|---|---|---|
| HD | SAB-IE (non-HD) | |
| Vc (liters) | 4.77 (1.08) | 6.56 (3.10) |
| CL (liters/h) | 0.25 (0.06) | 0.96 (0.47) |
| K12 (h−1) | 3.37 (4.20) | 1.67 (3.94) |
| K21 (h−1) | 3.37 (4.45) | 1.34 (3.40) |
| CLHD (liters/h) | 0.87 (0.23) | |
CL, nondialytic clearance from the central compartment; CLHD, hemodialysis clearance from the central compartment; K12, transfer rate constant from central compartment to peripheral compartment; K21, transfer rate constant from peripheral compartment to central compartment; Vc, volume in the central compartment.
The regression line equations from the observed-expected plots and measures of bias and precision for plasma and dialysate after the Bayesian step for the HD population PK model are displayed in Table 3. Using the population mean parameter values as the measure of central tendency, the overall fit of the model to the data was good, and the observed-predicted plots for plasma and dialysate after the Bayesian step were highly acceptable.
TABLE 3.
Goodness of fit and predictive performance of daptomycin hemodialysis population model for plasma and dialysate compartments
| Parameter | Value or description |
|
|---|---|---|
| Plasma | Dialysate | |
| Regression linea | Observed = 1.02 × predicted + 0.21 | Observed = 1.03 × predicted + 0.24 |
| R2b | 0.97 | 0.90 |
| Mean weighted error (mg/l) | −0.11 | −0.23 |
| Bias-adjusted mean weighted square error (mg/l)2 | 1.01 | 0.33 |
Best-fit regression line for the observed-predicted plot after the Bayesian step.
Coefficient of determination for the best-fit linear regression line for the predicted-observed plot after the Bayesian step.
Monte Carlo simulations.
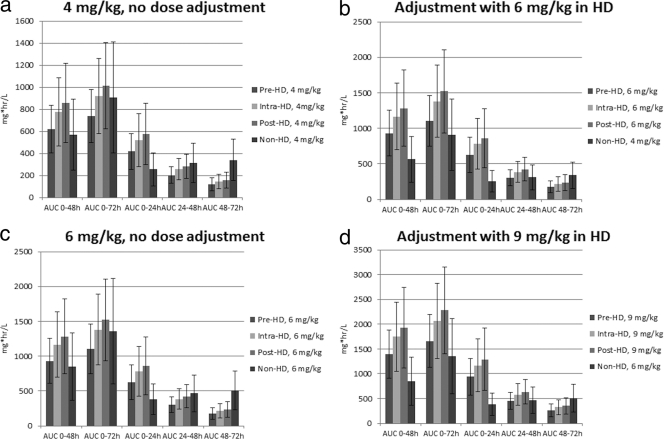
The AUC distributions for the candidate HD regimens and the MCS derived from the SAB-IE population PK model for 4 and 6 mg/kg i.v. q24h are displayed in Fig. 1 (2, 7). At both 4 mg/kg and 6 mg/kg, the mean cumulative AUC0-48 values for all HD daptomycin candidate administration strategies were similar to or slightly higher than the mean cumulative AUC0-48 simulated from the SAB-IE model (Fig. 1a and c). While pre-HD daptomycin administration resulted in lower mean AUC0-72 values than those for the SAB-IE simulations, intra- and post-HD daptomycin administration yielded mean AUC0-72 values comparable to those for the SAB-IE simulations.
FIG. 1.
(a) Mean AUC values without dose adjustment for 72-h interdialytic period for a 4-mg/kg dose of daptomycin. (b) Mean AUC values after dose adjustment with an additional 50% daptomycin dose to accommodate the 72-h interdialytic period. (c) Mean AUC values without dose adjustment for 72-h interdialytic period for a 6-mg/kg dose of daptomycin. (d) Mean AUC values after dose adjustment with an additional 50% daptomycin dose to accommodate the 72-h interdialytic period.
The AUC values partitioned into 24-h increments (AUC0-24, AUC24-48, and AUC48-72) were not as comparable (Fig. 1a and c). Of the three partitioned AUC values, the first two were higher than or comparable to those for the SAB-IE simulations. In contrast, all candidate AUC48-72 values were lower than the SAB-IE AUC48-72 values, by at least 50%. To compensate for the low AUC48-72 values that would be expected during a 72-hour interdialytic period, we examined whether increasing the dose by 50% would achieve more comparable AUC48-72 values (Fig. 1b and d). For the supplemental dose-adjusted regimens, the HD AUC0-24 values were still higher than the non-HD AUC0-24 values and the AUC24-48 values were still comparable. The supplemental dose resulted in HD AUC48-72 values that were ∼70% of the non-HD AUC48-72 values when the dose of daptomycin was administered intra-HD or post-HD.
The results of the Cmax and Cmin MCS analyses are displayed in Table 4. All HD administration strategies resulted in Cmax values that were comparable to the simulated SAB-IE Cmax values at both doses evaluated. For the Cmin simulation analyses, pre-HD or intra-HD administration of daptomycin resulted in simulated Cmin,48 values that were most isometric to simulated Cmin,48 values observed in the non-HD population PK model. Post-HD administration of daptomycin resulted in Cmin,48 values that were higher than those for the SAB-IE model at both doses evaluated. All values simulated for HD were slightly lower than the SAB-IE Cmin,72 values. The probability of Cmin values exceeding 24.3 mg/liter was low for all HD candidate dosing regimens (Table 5). For the 9-mg/kg postdialysis supplemental dose regimen, the probabilities of a Cmin of ≥24.3 mg/liter at hour 72 were low, ranging from 0.3% to 2%.
TABLE 4.
Cmax and Cmin values from Monte Carlo simulation analyses
| Regimen and parametera | Mean (SD) value (μg/ml) |
|
|---|---|---|
| 4 mg/kg | 6 mg/kg | |
| Pre-HD | ||
| Cmax | 31.4 (17.7) | 47.2 (26.5) |
| Cmin,48 | 6.3 (2.7) | 9.4 (4.1) |
| Cmin,72 | 3.8 (2.2) | 5.7 (3.3) |
| Intra-HD | ||
| Cmax | 30.4 (16.8) | 45.6 (25.2) |
| Cmin,48 | 7.8 (3.2) | 11.7 (4.8) |
| Cmin,72 | 4.6 (2.5) | 6.9 (3.7) |
| Post-HD | ||
| Cmax | 34.0 (19.8) | 51.0 (29.6) |
| Cmin,48 | 8.5 (3.5) | 12.7 (5.3) |
| Cmin,72 | 4.9 (2.6) | 7.5 (3.9) |
| SAB-IE (non-HD) | ||
| Cmax | 27.5 (18.8) | 41.2 (28.2) |
| Cmin,48 | 5.4 (4.5) | 8.0 (6.8) |
| Cmin,72 | 6.2 (5.3) | 9.3 (7.9) |
Simulations for HD candidate regimens were based on one dose. Simulations for SAB-IE (non-HD) regimens were based on intravenous dosing q24h for 3 days.
TABLE 5.
Probabilities of Cmin,48 and Cmin,72 values exceeding 24.3 mg/liter
| Regimen and parametera |
P value |
|
|---|---|---|
| 4 mg/kg | 6 mg/kg | |
| Pre-HD | ||
| Cmin,48 | 0.0000 | 0.0011 |
| Cmin,72 | 0.0000 | 0.0000 |
| Intra-HD | ||
| Cmin,48 | 0.0004 | 0.0098 |
| Cmin,72 | 0.0000 | 0.0002 |
| Post-HD | ||
| Cmin,48 | 0.0006 | 0.0230 |
| Cmin,72 | 0.0000 | 0.0007 |
| SAB-IE (non-HD) | ||
| Cmin,48 | 0.0039 | 0.0293 |
| Cmin,72 | 0.0089 | 0.0516 |
Simulations for HD candidate regimens were based on one dose. Simulations for non-HD regimens were based on intravenous dosing q24h for 3 days.
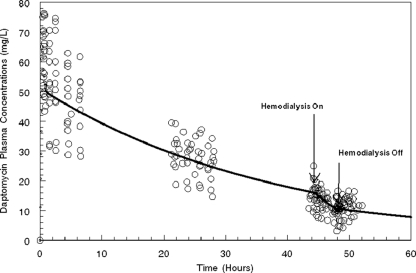
The simulated plasma concentration-time profile for 488 mg daptomycin infused over 30 min as a one-time dose is displayed in Fig. 2. The simulated plasma concentration-time curve mirrored the central tendency of the raw data reasonably well, as the vast majority of the data points were evenly distributed around the plasma concentration-time curve.
FIG. 2.
Simulated plasma concentration-time profile for 488 mg daptomycin (6 mg/kg for an 81-kg patient) over 30 min as a one-time dose, determined from the mean parameter vector values. The solid black line represents the simulated concentration-time profile for the mean parameter vector. The open circles represent the raw data points observed among the 12 study subjects.
Safety analysis.
All patients were contacted via telephone 30 days after receiving daptomycin, and no adverse effects were reported during this period. No elevations in CPK were noted.
DISCUSSION
This study distinguishes itself by using a model-based approach to determine the optimal dosing of an antibiotic for patients receiving HD, rather than the largely empirical processes used previously. Often, a predefined supplemental post-HD dose is recommended for HD patients, without a clear understanding of its dialytic clearance. Furthermore, previous evaluations failed to incorporate the relationship between antibiotic exposure and effect in the HD dose selection process. Advances in mathematical modeling, namely, population pharmacokinetic modeling and Monte Carlo simulation, now make it possible to design antibiotic dosing regimens that ensure a high probability of success with a minimal likelihood of toxicity among patients receiving HD. These mathematical modeling techniques have become the standard methodologies for assessing the clinical viability and determining the optimal dosing of both experimental and approved antimicrobials.
Another unique feature that distinguishes this study from previous HD pharmacokinetic studies is our integrative approach to modeling both daptomycin plasma and dialysate concentrations simultaneously. In the past, drug recovery in dialysate has been modeled separately and not integrated with plasma data. By integrating the two in the same population pharmacokinetic model, we were able to estimate both HD and non-HD clearances with greater precision than in previous attempts.
In determining optimal dosing of antibiotics for patients on thrice-weekly dialysis, it is important to recognize that the HD sessions are spaced ∼48 h apart twice a week and that once a week the interdialytic period is ∼72 h. These two distinct interdialytic periods require that separate dosing schemes be considered for each interval. Administration of the same parent daptomycin dose (4 mg/kg or 6 mg/kg, depending on the indication for therapy) intra-HD and post-HD resulted in AUC, Cmax, and Cmin values that were most comparable to those for SAB-IE simulations for the ∼48-hour interdialytic period. The similar cumulative AUC0-72 values for HD and non-HD might suggest that there is no need for a supplemental dose prior to the 72-h interdialytic period. However, our analysis of partitioned AUC values (AUC0-24, AUC24-48, and AUC48-72) revealed that the AUC48-72 values for all candidate HD administration strategies were at least 50% lower than non-HD (SAB-IE) AUC48-72 values.
In clinical practice, there are two primary ways to compensate for the low HD AUC48-72 values. First, the patient can be dosed 48 h into the 72-hour interdialytic period on a non-HD day and resume dosing with HD sessions on the following day. Second, the patient can receive a higher dose prior to the 72-h interdialytic period. While it seems intuitive, dosing 48 h into the 72-h interdialytic period is not optimal. Redosing the patient 48 h into the interdialytic period adds a fourth weekly dose of daptomycin and results in grossly excessive daptomycin exposures, including a >25% probability of Cmin values that exceed 24.3 mg/liter for prolonged periods. Outpatients also require additional medical care to receive the fourth daptomycin dose at either home or the clinic.
A supplemental 50% dose of daptomycin intra-HD or post-HD prior to the 72-h interdialytic period provided AUC48-72 values that were nearly 70% of the non-HD AUC48-72 values (Fig. 1b and d). While not completely isometric, achieving a 70% result is much more reasonable than barely achieving a 50% result. Additionally, this approach is much more convenient than redosing the patient 48 h into the interdialytic period on the non-HD day and confers a lower likelihood of having Cmin values in excess of 24.3 mg/liter. We considered higher supplemental doses (doubling [12 mg/kg] or tripling [18 mg/kg] of parent dose), but these regimens were associated with unacceptable probabilities (>10%) of Cmin values in excess of 24.3 mg/liter at hour 72. Unfortunately, the literature is inconclusive regarding the relative importance of the cumulative versus partitioned AUC values with respect to antimicrobial effect. Future inquiries should delve into this question.
Several additional things should be noted in interpreting these results. First, in the absence of a well-defined AUC/MIC threshold, especially for MRSA bloodstream infections, we felt that it was prudent to design HD dosing schemes that provided AUC distributions similar to those obtained from the population PK model derived from patients in the SAB-IE clinical study (2). We believe that these are the best available data on the dispersion of AUC values that one would expect to see for the population of patients likely to receive daptomycin. Second, while we advocate that increasing the dose by 50% will help to reach the optimal AUC48-72 for the 72-h interdialytic period, the AUC0-24 will be higher than that at 24 h of exposure (AUC0-24,ss) observed for volunteers treated with a dose of 12 mg/kg. While this may not be prohibitive, since the AUC has not been linked to toxicity, caution is advisable. Third, the toxicity target was derived from an a priori analysis of the relationship between daptomycin exposure and the probability of CPK elevation among patients from the SAB-IE study. Among the 108 evaluable patients, 3 (50%) of 6 patients with Cmin values of ≥24.3 mg/liter had elevated CPK values, compared with 3 (2.9%) of 102 patients with Cmin values of <24.3 mg/liter (P = 0.002 by the Fisher exact test) (2). It should be noted that everyone in this study had a creatinine clearance (CLCR) of >30 ml/min and received daptomycin intravenously every 24 h; patients with CLCR of <30 ml/min were excluded from the trial. For patients with CLCR of <30 ml/min, the daptomycin package insert indicates that patients should receive 4 or 6 mg/kg every 48 h. The exposure target associated with CPK elevation among patients with diminished renal function receiving daptomycin intravenously every 48 h has not been elucidated. Although limited, these data are the best available to date, and further studies are sorely needed to better delineate this exposure-response relationship among patients on HD. In the absence of further data, we believe that it was prudent to assess Cmin concentrations, since this was the target identified in both animal and human studies (2, 6, 10).
Two studies performed by Salama et al. also examined intra-HD and post-HD administration of daptomycin (12, 13). On a superficial level, these studies look like they provide different results. However, several notable characteristics of these studies account for the dissimilar findings. The average weight (standard deviation) in one study of Salama et al. was 64.1 (14.1) kg (12). In contrast, the average weight in our study was 81.2 (11.9) kg. In our study, HD sessions were fixed at 3.5 h (with blood and dialysate flow rates of 300 and 600 ml/min, respectively), while the flow rates and duration of the dialysis were not fixed in the studies by Salama et al. Our study simulated intradialytic dosing during the last hour of dialysis, while Salama et al. administered daptomycin during the last half hour. There were also notable differences in the exposure end points studied (12, 13). One of the major advantages of Monte Carlo simulation is that it permits different covariate inputs, such as HD duration and weight. After standardizing our Monte Carlo simulation inputs to match the weight distribution, duration of HD, and daptomycin administration time used in the studies by Salama and colleagues, our results are largely concordant (Table 6).
TABLE 6.
Comparison of results from studies examining daptomycin PK among patients on hemodialysis
| Regimen and parameter (reference) | Mean (SD) value (crude, unstandardized) |
Mean (SD) value (standardized)a |
||
|---|---|---|---|---|
| Work of Salama et al. | Our data | Work of Salama et al. | Our data | |
| Intradialytic administration of daptomycin (12) | ||||
| Intra-HD AUC | 711.5 (125.9) | 1,164.0 (467.9) | 711.5 (125.9) | 977.7 (386.0) |
| Intra-HD Cmax | 39.7 (7.6) | 45.6 (25.2) | 39.7 (7.6) | 43.6 (23.1) |
| Postdialytic administration of daptomycin (13) | ||||
| AUC0-68 at 6 mg/kg | 1,351 (154) | 1,520 (584.6) | 1,351 (154) | 1,217 (429.5) |
| AUC0-68 at 8 mg/kg | 1,801 (205) | 2,027 (779.4) | 1,801 (205) | 1,622 (572.6) |
For intra-HD administration of daptomycin, Monte Carlo simulations were standardized to (i) administration of daptomycin during the last 30 min of HD versus the last 60 min of HD, (ii) 3-h HD session versus 3.5-h HD session, and (iii) 64-kg patient versus actual distribution of weights observed among HD patients under study. For post-HD administration of daptomycin, Monte Carlo simulations were standardized to (i) 4-h HD session versus 3.5-h HD session and (ii) 66-kg patient versus actual distribution of weights observed among HD patients under study.
With respect to interpretation, Salama and colleagues advocate the use of larger doses of daptomycin to accommodate for the interdialytic loss (12, 13). However, they did not consider the exposure profiles observed among HD patients relative to the exposures of “typical” non-HD patients and did not balance the probability of achieving the exposure targets associated with success and toxicity. Since the estimated exposure profiles between studies are largely concordant (Table 5), it is reasonable to surmise that they would have reached similar dosing recommendations if the same exposure targets for efficacy and toxicity were used in the dose selection process.
In conclusion, mathematical models show that administration of daptomycin intra-HD or post-HD seems to be optimal. These administration strategies provided the most isometric cumulative AUC, Cmax, and Cmin values relative to those for the simulations derived from the SAB-IE PK model for the same doses. To achieve similar noncumulative AUC values, particularly when the interdialytic period is ∼72 h, clinicians might consider administering an additional 50% daptomycin dose with the HD session preceding the extended interdialytic period.
Acknowledgments
This work was supported by Cubist Pharmaceuticals, Inc. T.P.L. was the principal investigator for this grant.
Please note that Cubist only provided support to complete the project and was not involved in the following: design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation and review of the manuscript.
The manuscript greatly benefited from the thoughtful editing of Allison Krug. Analysis of daptomycin concentrations in blood and dialysates was performed by Lihong Gao.
G.L.D. has served as an advisor to Cubist Pharmaceuticals, Inc. T.P.L. serves on the speakers' bureau of and has received grant support from Cubist Pharmaceuticals, Inc.
Footnotes
Published ahead of print on 31 January 2011.
REFERENCES
- 1.Allon, M. 2004. Dialysis catheter-related bacteremia: treatment and prophylaxis. Am. J. Kidney Dis. 44:779-791. [PubMed] [Google Scholar]
- 2.Bhavnani, S. M., C. M. Rubino, P. G. Ambrose, and G. L. Drusano. 2010. Daptomycin exposure and the probability of elevations in the creatine phosphokinase level: data from a randomized trial of patients with bacteremia and endocarditis. Clin. Infect. Dis. 50:1568-1574. [DOI] [PubMed] [Google Scholar]
- 3.Bowker, K. E., A. R. Noel, and A. P. MacGowan. 2009. Comparative antibacterial effects of daptomycin, vancomycin and teicoplanin studied in an in vitro pharmacokinetic model of infection. J. Antimicrob. Chemother. 64:1044-1051. [DOI] [PubMed] [Google Scholar]
- 4.Cubist Pharmaceuticals, Inc. 2006. Cubicin (daptomycin) package insert. Cubist Pharmaceuticals, Inc., Brooks, KY.
- 5.Dandekar, P. K., P. R. Tessier, P. Williams, C. H. Nightingale, and D. P. Nicolau. 2003. Pharmacodynamic profile of daptomycin against Enterococcus species and methicillin-resistant Staphylococcus aureus in a murine thigh infection model. J. Antimicrob. Chemother. 52:405-411. [DOI] [PubMed] [Google Scholar]
- 6.Eisenstein, B. I., F. B. Oleson, Jr., and R. H. Baltz. 2010. Daptomycin: from the mountain to the clinic, with essential help from Francis Tally, MD. Clin. Infect. Dis. 50(Suppl. 1):S10-S15. [DOI] [PubMed] [Google Scholar]
- 7.Fowler, V. G., Jr., et al. 2006. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N. Engl. J. Med. 355:653-665. [DOI] [PubMed] [Google Scholar]
- 8.Leary, R., R. Jelliffe, A. Schumitzky, and M. van Guilder. 2001. An adaptive grid, non-parametric approach to pharmacokinetic and dynamic (PK/PD) models, p. 389-394. In Proceedings of the Fourteenth IEEE Symposium on Computer-Based Medical Systems. IEEE Computer Society, Bethesda, MD.
- 9.Louie, A., et al. 2001. Pharmacodynamics of daptomycin in a murine thigh model of Staphylococcus aureus infection. Antimicrob. Agents Chemother. 45:845-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oleson, F. B., Jr., et al. 2000. Once-daily dosing in dogs optimizes daptomycin safety. Antimicrob. Agents Chemother. 44:2948-2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Safdar, N., D. Andes, and W. A. Craig. 2004. In vivo pharmacodynamic activity of daptomycin. Antimicrob. Agents Chemother. 48:63-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salama, N. N., et al. 2009. Intradialytic administration of daptomycin in end stage renal disease patients on hemodialysis. Clin. J. Am. Soc. Nephrol. 4:1190-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salama, N. N., et al. 2010. Single-dose daptomycin pharmacokinetics in chronic haemodialysis patients. Nephrol. Dial. Transplant. 25:1279-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarnak, M. J., and B. L. Jaber. 2000. Mortality caused by sepsis in patients with end-stage renal disease compared with the general population. Kidney Int. 58:1758-1764. [DOI] [PubMed] [Google Scholar]
- 15.Tokars, J. I., E. R. Miller, M. J. Alter, and M. J. Arduino. 1998. National surveillance of dialysis associated diseases in the United States, 1995. ASAIO J. 44:98-107. [DOI] [PubMed] [Google Scholar]
- 16.Tokars, J. I., E. R. Miller, and G. Stein. 2002. New national surveillance system for hemodialysis-associated infections: initial results. Am. J. Infect. Control 30:288-295. [DOI] [PubMed] [Google Scholar]
- 17.United States Renal Data System. 2008. USRDS 2008 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD.
- 18.United States Renal Data System. 2005. USRDS annual data report: atlas of end-stage renal disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD.