Abstract
BAL30376 is a triple combination comprising a siderophore monobactam, BAL19764; a novel bridged monobactam, BAL29880, which specifically inhibits class C β-lactamases; and clavulanic acid, which inhibits many class A and some class D β-lactamases. The MIC90 was ≤4 μg/ml (expressed as the concentration of BAL19764) for most species of the Enterobacteriaceae family, including strains that produced metallo-β-lactamases and were resistant to all of the other β-lactams tested. The MIC90 for Stenotrophomonas maltophilia was 2 μg/ml, for multidrug-resistant (MDR) Pseudomonas aeruginosa it was 8 μg/ml, and for MDR Acinetobacter and Burkholderia spp. it was 16 μg/ml. The presence of the class C β-lactamase inhibitor BAL29880 contributed significantly to the activity of BAL30376 against strains of Citrobacter freundii, Enterobacter species, Serratia marcescens, and P. aeruginosa. The presence of clavulanic acid contributed significantly to the activity against many strains of Escherichia coli and Klebsiella pneumoniae that produced class A extended-spectrum β-lactamases. The activity of BAL30376 against strains with metallo-β-lactamases was largely attributable to the intrinsic stability of the monobactam BAL19764 toward these enzymes. Considering its three components, BAL30376 was unexpectedly refractory toward the development of stable resistance.
Resistance toward β-lactam antibiotics has been increasing in many Gram-negative bacteria and has reached a level that is causing concern (1, 12, 15, 19, 21). Resistance toward β-lactams in Gram-negative bacteria is frequently caused by the expression of one or more β-lactamases, which destroy the antibiotic (12, 19, 20, 22). It can be exacerbated by mutation or altered expression of outer membrane proteins responsible for the influx of the antibiotics and by increased expression of efflux pumps that remove the antibiotics from the periplasm, where they act against their lethal target, the penicillin-binding proteins (19, 22).
Combinations comprising a β-lactam antibiotic and a β-lactamase inhibitor have proved clinically useful for overcoming some types of β-lactamase-mediated resistance (3, 6, 17, 20). There are six such combinations, based around three β-lactamase inhibitors, currently available for clinical use in various parts of the world. These are amoxicillin plus clavulanic acid, ticarcillin plus clavulanic acid, ampicillin plus sulbactam, sultamicillin (a mutual prodrug between ampicillin and sulbactam), cefoperazone plus sulbactam, and piperacillin plus tazobactam. In some countries, sulbactam is also available alone for use in combination. Clavulanic acid is a potent inhibitor of class A β-lactamases and also inhibits some class D enzymes; it has little activity or no activity against class B and class C β-lactamases (6, 17). The penam sulfone sulbactam is also active against class A β-lactamases, and although it is not as potent as clavulanic acid, sulbactam is considerably more stable (6, 17). Like clavulanic acid, it has little antibacterial activity itself, except against Acinetobacter species. Tazobactam is a more recent extension of the penam sulfone class of inhibitors that has more potent activity than sulbactam, but it is still active against only a few class C β-lactamases (2). Many experimental β-lactamase inhibitors have been described, representing attempts to extend activity toward class C and D β-lactamases (3, 6, 17, 20), but most have encountered difficulty in obtaining significant improvement in activity against Pseudomonas aeruginosa because of limited stability, restricted penetration, or efflux (6, 17). An alternative strategy is represented by the bridged monobactams such as BAL29880, which are specific class C β-lactamase inhibitors that could be optimized independently for their penetration properties (9). Inhibition of class B metallo-β-lactamases can be achieved by compounds with metal ion ligating groups, such as bifunctional carboxylic acids and mercaptans (6, 17). However, the monobactams such as BAL19764, also known as PTX2416 (7), are considered to be less labile toward class B enzymes and to be inhibitors of class C enzymes (7, 9).
Siderophore monobactams were developed with the hypothesis that including an iron-chelating group would enable the compounds to mimic natural siderophores and allow them to exploit the intrinsic iron uptake systems of bacteria to circumvent resistance due to restricted penetration, especially in P. aeruginosa (7, 14). They therefore make an interesting starting point for a combination product with broad stability toward the spectrum of β-lactamases found in Gram-negative bacteria. BAL19764, like other monobactams, lacks significant activity against Gram-positive bacteria (7, 10). We show here that combining the siderophore monobactam BAL19764 with specific inhibitors of class A and class C β-lactamases produces a combination that has a broad spectrum of activity against Gram-negative pathogenic bacteria and that is relatively refractory to resistance development.
MATERIALS AND METHODS
Antimicrobial compounds.
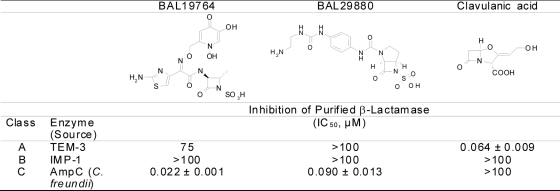
BAL19764, BAL29880 (Fig. 1), tazobactam, and piperacillin were prepared in the research laboratories of Basilea Pharmaceutica International Ltd., Basel, Switzerland. The other compounds were obtained from commercial sources: imipenem from MSD, meropenem from Astra Zeneca, cefepime from Bristol-Myers Squibb, ceftazidime from GSK, amoxicillin from Fluka, ticarcillin from SmithKline Beecham (now GSK), and clavulanic acid from Biochemie CS-Downstream. Routine quality control by high-performance liquid chromatography with UV and Fourier transform infrared spectroscopy was performed on all samples.
FIG. 1.
Structures and β-lactamase-inhibitory properties of the components of BAL30376.
Bacterial strains.
Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853, routinely used for quality control purposes, were obtained from the American Type Culture Collection. A number of strains with specific β-lactamases were obtained from D. Livermore at the Health Protection Agency (London, United Kingdom), V. Miriagou at the Hellenic Pasteur Institute (Athens, Greece), and P. Nordmann at the Hôpital de Bicêtre (Le Kremlin-Bicêtre, France). Otherwise, the isolates used in the test panels were obtained from various hospitals, mainly in Europe, Japan, and the United States: the strains were collected in more than 25 institutions in at least 7 countries over a span of 24 years. They were identified by API strip (bioMérieux, France) testing and, if necessary, also by 16S rRNA sequencing and were kept as stock cultures at −70°C or below. The strains comprising the test panels were selected on the basis of their resistotypes, and no isolates from the same source with identical antibiotic resistance patterns were included. Preference was given to strains proven (by sequencing) or suspected (from antibiotic resistance pattern) to have one or more of the following: class A extended-spectrum β-lactamases, carbapenemases, or inhibitor-resistant variants, class B metallo-β-lactamases, class C cephalosporinases, and class D carbapenemases. The MIC50s and MIC90s of the comparators for these stringent challenge panels may therefore appear to be unusually high. The strains used in the in vivo experiments were clinical isolates from the general collection and were tested for their ability to cause infection in mice before use.
MIC determination.
MICs were determined by a broth dilution method in microtiter plates, according to the general recommendation of CLSI (4), except that Iso-Sensitest broth supplemented with 16 mg/liter 2,2′-bipyridyl (BPL) (IST_BPL medium) to complex iron and thereby induce iron uptake systems (23) was used.
Bactericidal activity.
Overnight cultures of the test strains were diluted into fresh IST_BPL medium to yield an inoculum of approximately 106 CFU/ml. Drug was added to the inoculum, and incubation at 37°C was initiated. Drug concentrations of 0 (control), 0.5, 1, 2, 4, 8, and 16 times the MIC were used. Samples were taken immediately and at 3, 6, 24, and 28 h after addition of drug and were plated out onto IST_BPL agar plates to estimate the number of viable bacteria.
Population analysis.
Overnight cultures of the test strains were diluted into fresh medium to yield inocula of approximately 106 CFU/ml and approximately 109 CFU/ml. Aliquots of 0.1 ml were plated onto IST_BPL agar plates containing increasing concentrations (between 1 and 64 times the MIC) of the compound to be tested. Each experiment was performed in duplicate, and the numbers of colonies appearing on the plate after 48 h of incubation at 37°C were counted and the numbers averaged.
Resistance development in serial passage.
Development of resistance to BAL30376, meropenem, cefepime, and ciprofloxacin was studied by serial passage over increasing concentrations in 0.1 ml in microtiter plates. The initial inoculum was 106 CFU/ml, and 0.01-ml aliquots were taken from the well with the highest concentration of drug at which significant turbidity appeared (optical density at 600 nm, 0.1 to 0.5) after 2 days of incubation. The aliquots were distributed between a new set of graded concentrations of the drug. The serial transfer was continued until the bacteria were growing in at least 64 μg/ml of the antibiotic. Glycerol to 25% (vol/vol) was added to cultures growing in the presence of concentrations of BAL30376 of ≥64 μg/ml, and they were frozen at −20°C for subsequent reculturing. Duplicate experiments were performed in parallel starting from the same initial inoculum.
An aliquot from the frozen culture was inoculated into fresh medium without antibiotic and cultured overnight. The MIC of the overnight culture was determined according to the standard method, and an aliquot was used as the inoculum for a fresh culture of antibiotic-free medium. The subculturing was continued for five passages in antibiotic-free medium or until the MIC had returned to within 1 dilution step of the MIC of the parent strain before resistance selection.
Experimental septicemia in mice.
The efficacies of meropenem, aztreonam, and BAL19764 combined with BAL29880 and clavulanic acid against different Gram-negative bacterial strains were tested in a septicemia model using immunocompetent male NMRI mice (Harlan, Netherlands). The conduct of the experiments was in full accord with the Swiss governmental regulations covering such studies. After determination of the appropriate infection dose (virulence test), a control group of three to five animals (infected, not treated) and test groups of five animals were infected by intraperitoneal injection of 750 μl inoculum consisting of 1/3 bacterial suspension in NaCl (0.9%) and 2/3 porcine mucin solution. The final mucin concentration was strain dependent and ranged from 1.7 to 3.3% (wt/vol). The test substances were administered intravenously in the tail vein 1, 3, and 5 h after infection. The respective starting doses were chosen under consideration of the MIC values. The maximum dose was 50 mg/kg of body weight at 5 ml/kg. The doses were titrated down by a factor of 2 until at least three out of five animals died within 5 days.
Inhibition of β-lactamases.
The AmpC β-lactamase from Citrobacter freundii (K) and TEM-3 and IMP-1 β-lactamases were expressed and purified using ion-exchange chromatography as previously described (16, 18). Inhibition was determined with nitrocefin as the reporter substance using standard methods (16). Protein concentration was determined using Bradford protein assay reagents (Bio-Rad, Basel, Switzerland).
RESULTS
MIC determination.
The iron-chelating agent BPL was added to the growth medium for all tests to decrease the available iron concentration (18, 23). Under iron limitation, the iron uptake systems of bacteria are induced and siderophores are expressed in large amounts to compete with the BPL for iron (23). The iron-limited growth condition is believed to mimic the in vivo situation (14), where iron is bound by a variety of proteins and has been used previously for assessing the activity of siderophore antibiotics (7, 11, 18). In our investigations of siderophore monobactams (5), we found that concentrations of BPL up to 16 μg/ml (the MIC for BPL against E. coli ATCC 25922 is ≥64 μg/ml) had no effect on the growth rates of bacteria or on MICs of nonsiderophore antibiotics (data not shown). The production of pyoverdine (the most readily monitored siderophore) by P. aeruginosa is induced 8- to 15-fold, according to the strain (data on file). The activity of BAL19764 against some strains of P. aeruginosa (about 20% of all strains tested) was enhanced 2- to 16-fold by the addition, consistent with earlier observations using conalbumin to chelate iron (11). Therefore, the addition of 16 μg/ml BPL to all growth media was adopted as a standard procedure.
The MIC of BAL19764 toward strains with class A β-lactamases, except PSE-1, was decreased by the addition of clavulanic acid at 2 μg/ml, typically from >32 μg/ml to ≤4 μg/ml (Table 1). Conversely, the MIC of BAL19764 toward strains with class C β-lactamases was decreased by the addition of BAL29880 at 4 μg/ml, typically from >32 μg/ml to ≤4 μg/ml (Table 1). These activities were additive, so that the combination of BAL29880 (4 μg/ml) and clavulanic acid (2 μg/ml) brought the MICs of BAL19764 for most class A- and class C-producing organisms to ≤4 μg/ml (Table 1).
TABLE 1.
In vitro activities of components of BAL30376
| Strain | Enzyme(s) present | MIC of BAL19764 (μg/ml) in combination with: |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alone | BAL29880 at 4 μg/ml | Clavulanate at 2 μg/ml | BAL29880 at 4 μg/ml + clavulanate at 2 μg/ml |
BAL29880 + clavulanate |
||||||||
| 1:1:1 (wt/wt/wt) | 5:4:1 (wt/wt/wt) | 5:3:1 (wt/wt/wt) | 5:2:1 (wt/wt/wt) | 10:10:3 (wt/wt/wt) | 10:10:2 (wt/wt/wt) | 10:10:1 (wt/wt/wt) | ||||||
| Acinetobacter baumannii J2 | ADC | 32 | 4 | 32 | 4 | 8 | 4 | 8 | 16 | NTa | NT | NT |
| Acinetobacter junii 15218 | None | 0.5 | 0.5 | 0.5 | NT | NT | NT | 0.5 | NT | NT | NT | NT |
| Citrobacter freundii GN7391 | AmpC | >32 | 4 | >32 | 4 | 16 | 8 | 16 | 32 | NT | NT | NT |
| Enterobacter aerogenes B1 | AmpC | >32 | 2 | >32 | 2 | 2 | 4 | 4 | 8 | NT | NT | NT |
| Enterobacter cloacae P99 | AmpC | 32 | 2 | 32 | 2 | 4 | 4 | 4 | 8 | NT | NT | NT |
| E. cloacae 908R | AmpC | >32 | 1 | 32 | 1 | 2 | 1 | 1 | 4 | NT | NT | NT |
| E. cloacae R947 | TEM-1, IMP-8, AmpC | >32 | 32 | 2 | 2 | 2 | 2 | 4 | 8 | 2 | 4 | 8 |
| E. cloacae S9615 | AmpC | 32 | 2 | >32 | NT | NT | NT | 2 | NT | NT | NT | NT |
| E. cloacae S4741B | AmpC | 8 | 1 | 8 | NT | 1 | 1 | 0.5 | NT | NT | NT | NT |
| E. cloacae S9639 | AmpC | 32 | 2 | >32 | NT | NT | NT | 2 | NT | NT | NT | NT |
| Escherichia coli HB101(pAT268) | TEM-5 | >32 | >32 | 1 | 1 | 1 | 4 | 4 | 4 | 2 | 4 | 4 |
| Klebsiella oxytoca 1082E | K1 | >32 | >32 | 0.5 | 1 | 1 | 8 | 8 | 8 | 4 | 8 | 16 |
| Klebsiella pneumoniae CF104 | TEM-3 | >32 | >32 | 2 | NT | NT | NT | 2 | NT | NT | NT | NT |
| K. pneumoniae CF504 | A | >32 | >32 | 0.5 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 4 |
| K. pneumoniae 55 | SHV-1, VIM-1 | >32 | >32 | 8 | NT | NT | NT | 4 | NT | NT | NT | NT |
| K. pneumoniae 848 | SHV-1, VIM-1 | >32 | >32 | 4 | 4 | 4 | 4 | 8 | 16 | 8 | 8 | 16 |
| Pseudomonas aeruginosa BA | AmpCb | 2 | 1 | 2 | 1 | 2 | 2 | 1 | 1 | NT | NT | NT |
| P. aeruginosa 18 S/H | AmpC | 16 | 2 | >32 | 2 | 4 | 4 | 8 | 8 | NT | NT | NT |
| P. aeruginosa 527 | IMP-1, PSE-1, AmpCb | 2 | 2 | 4 | NT | 4 | NT | 2 | NT | NT | NT | NT |
| P. aeruginosa 860 | AmpC | 8 | 1 | 8 | 2 | 2 | 4 | 4 | 4 | NT | NT | NT |
| P. aeruginosa 1973E (PSE1) | PSE1, AmpC | 8 | 8 | 8 | 4 | NT | NT | 8 | NT | NT | NT | NT |
| P. aeruginosa 1937E (PSE2) | PSE2, AmpC | 8 | 8 | 4 | 4 | NT | NT | 2 | NT | NT | NT | NT |
| P. aeruginosa 6067 | AmpC | 8 | 4 | 8 | NT | 4 | NT | 4 | NT | NT | NT | NT |
| P. aeruginosa 143724R | AmpC, porin alteration | 32 | NT | NT | NT | 8 | NT | 8 | NT | NT | NT | NT |
| P. aeruginosa 143811R | AmpC | >32 | 2 | >32 | 2 | 4 | 4 | 4 | 8 | NT | NT | NT |
| P. aeruginosa (560546)9 M.P. | AmpC | 16 | 2 | 16 | NT | 2 | 2 | 2 | NT | NT | NT | NT |
| Stenotrophomonas maltophilia 1AC736 | L1, L2 | 2 | 2 | 0.125 | NT | NT | NT | 0.125 | NT | NT | NT | NT |
NT, not tested.
Low-level expression.
Investigation of the optimum ratio between the three components showed that a high proportion of clavulanic acid (weight ratio 1:1:1) tended to antagonize the protective effect of BAL29880 in strains with an inducible AmpC β-lactamase (for example, Citrobacter freundii GN7391 in Table 1). The protective effect of BAL29880 dropped off sharply when the proportion was less than five parts by weight of BAL19764 to three parts by weight of BAL29880. Similarly, the protective effect of clavulanic acid diminished when its proportion was less than five parts by weight of BAL19764 to one part by weight of clavulanic acid. Therefore, the mixture with a composition of five parts by weight of BAL19764, three parts by weight of BAL29880, and one part by weight of clavulanic acid, hereafter referred to as BAL30376, was taken for further characterization of in vitro antimicrobial activity. The MIC value was taken as the concentration of the antibiotic component (BAL19764) in the test solution.
The antibacterial activity of BAL30376 was compared with those of the marketed β-lactam-β-lactamase inhibitor combinations piperacillin-tazobactam (with fixed ratio 8:1, wt/wt), amoxicillin-clavulanic acid (with fixed ratio 2:1, wt/wt), and ticarcillin-clavulanic acid (with fixed ratio 15:1, wt/wt), as well as those of cefepime, ceftazidime, imipenem, and meropenem (Table 2). For Acinetobacter spp., the activities of the β-lactam-β-lactamase inhibitor combination ampicillin-sulbactam (with fixed ratio 2:1, wt/wt) and polymyxin B were also compared with the activity of BAL30376, and for P. aeruginosa, the activity of polymyxin B was also compared with that of BAL30376.
TABLE 2.
Activity of BAL30376 against strains with β-lactam-resistant phenotypes
| Organism (no. of isolates) and compound | MICa (μg/ml) |
||
|---|---|---|---|
| 50% | 90% | Range | |
| Citrobacter freundii (21) | |||
| BAL30376 | 2 | 8 | ≤0.06-16 |
| Imipenem | 1 | 2 | 0.5-4 |
| Meropenem | ≤0.06 | 0.25 | ≤0.06-4 |
| Cefepime | 1 | 4 | ≤0.06-8 |
| Ceftazidime | >32 | >32 | >32 |
| Piperacillin-tazobactam (8:1) | >32 | >32 | >32 |
| Amoxicillin-clavulanic acid (2:1) | >32 | >32 | >32 |
| Ticarcillin-clavulanic acid (15:1) | >32 | >32 | >32 |
| Enterobacter aerogenes (15) | |||
| BAL30376 | 0.5 | 2 | ≤0.06-8 |
| Imipenem | 2 | 16 | 0.5-32 |
| Meropenem | 0.25 | 4 | ≤0.06-16 |
| Cefepime | 0.25 | 16 | ≤0.06-16 |
| Ceftazidime | >32 | >32 | 0.125->32 |
| Piperacillin-tazobactam (8:1) | 32 | >32 | 4->32 |
| Amoxicillin-clavulanic acid (2:1) | >32 | >32 | >32 |
| Ticarcillin-clavulanic acid (15:1) | >32 | >32 | >32 |
| Enterobacter cloacae (25) | |||
| BAL30376 | 1 | 4 | ≤0.06-16 |
| Imipenem | 2 | 4 | 0.25-16 |
| Meropenem | ≤0.06 | 0.25 | ≤0.06-16 |
| Cefepime | 0.25 | 4 | ≤0.06->32 |
| Ceftazidime | 16 | >32 | 0.125->32 |
| Piperacillin-tazobactam (8:1) | 16 | >32 | 4->32 |
| Amoxicillin-clavulanic acid (2:1) | >32 | >32 | >32 |
| Ticarcillin-clavulanic acid (15:1) | >32 | >32 | >32 |
| Escherichia coli (18) | |||
| BAL30376 | 1 | 4 | ≤0.06->32 |
| Imipenem | 0.25 | 0.5 | ≤0.06->32 |
| Meropenem | ≤0.06 | 0.5 | ≤0.06->32 |
| Cefepime | 8 | >32 | ≤0.06->32 |
| Ceftazidime | 32 | >32 | 1->32 |
| Piperacillin-tazobactam (8:1) | 32 | >32 | 4->32 |
| Amoxicillin-clavulanic acid (2:1) | 32 | >32 | 16->32 |
| Ticarcillin-clavulanic acid (15:1) | 32 | >32 | 32->32 |
| Klebsiella oxytoca (10) | |||
| BAL30376 | 0.5 | 4 | ≤0.06->32 |
| Imipenem | 0.5 | 1 | 0.5->32 |
| Meropenem | 0.25 | 8 | ≤0.06->32 |
| Cefepime | 2 | 8 | ≤0.06->32 |
| Ceftazidime | 1 | 8 | ≤0.06->32 |
| Piperacillin-tazobactam (8:1) | 4 | >32 | 2->32 |
| Amoxicillin-clavulanic acid (2:1) | 8 | >32 | 4->32 |
| Ticarcillin-clavulanic acid (15:1) | 8 | >32 | 4->32 |
| Klebsiella pneumoniae (39) | |||
| BAL30376 | 0.5 | 4 | ≤0.06->32 |
| Imipenem | 1 | 16 | 0.5->32 |
| Meropenem | ≤0.06 | 16 | ≤0.06->32 |
| Cefepime | 4 | >32 | ≤0.06->32 |
| Ceftazidime | >32 | >32 | 0.125->32 |
| Piperacillin-tazobactam (8:1) | 32 | >32 | 1->32 |
| Amoxicillin-clavulanic acid (2:1) | >32 | >32 | 8->32 |
| Ticarcillin-clavulanic acid (15:1) | >32 | >32 | 8->32 |
| Proteus spp. (21)b | |||
| BAL30376 | 0.125 | 1 | ≤0.06-2 |
| Imipenem | 4 | 8 | 0.5-8 |
| Meropenem | 0.125 | 0.5 | ≤0.06-0.5 |
| Cefepime | 0.125 | 1 | ≤0.06-32 |
| Ceftazidime | ≤0.06 | 0.25 | ≤0.06-8 |
| Piperacillin-tazobactam (8:1) | 1 | 4 | ≤0.06-32 |
| Amoxicillin-clavulanic acid (2:1) | 32 | >32 | 8->32 |
| Ticarcillin-clavulanic acid (15:1) | 8 | >32 | 1->32 |
| Providencia spp. (20)c | |||
| BAL30376 | ≤0.06 | 0.5 | ≤0.06-4 |
| Imipenem | 4 | 16 | 1->32 |
| Meropenem | 0.25 | >32 | ≤0.06->32 |
| Cefepime | ≤0.06 | >32 | ≤0.06->32 |
| Ceftazidime | 1 | >32 | ≤0.06->32 |
| Piperacillin-tazobactam (8:1) | 4 | 32 | 0.5->32 |
| Amoxicillin-clavulanic acid (2:1) | >32 | >32 | >32 |
| Ticarcillin-clavulanic acid (15:1) | 16 | >32 | 0.5->32 |
| Serratia marcescens (12) | |||
| BAL30376 | 1 | 2 | 0.5-4 |
| Imipenem | 2 | 8 | 0.5->32 |
| Meropenem | 0.125 | 4 | ≤0.06->32 |
| Cefepime | 1 | 2 | 0.125-16 |
| Ceftazidime | 1 | 2 | 0.125->32 |
| Piperacillin-tazobactam (8:1) | >32 | >32 | 4->32 |
| Amoxicillin-clavulanic acid (2:1) | >32 | >32 | >32 |
| Ticarcillin-clavulanic acid (15:1) | >32 | >32 | 8->32 |
| Acinetobacter spp. (68) | |||
| BAL30376 | 4 | 16 | ≤0.06->32 |
| Imipenem | 0.25 | 32 | ≤0.06->32 |
| Meropenem | 1 | 16 | ≤0.06->32 |
| Cefepime | 16 | >32 | ≤0.06->32 |
| Ceftazidime | 16 | >32 | ≤0.06->32 |
| Piperacillin-tazobactam (8:1) | >32 | >32 | 8->32 |
| Amoxicillin-clavulanic acid (2:1) | >32 | >32 | 8->32 |
| Ampicillin/sulbactam (2:1) | 16 | >32 | 2->32 |
| Polymyxin B | 4 | 32 | ≤0.06->32 |
| Burkholderia spp. (20) | |||
| BAL30376 | 0.5 | 16 | 0.25->32 |
| Imipenem | 32 | >32 | 16->32 |
| Meropenem | 2 | 4 | 0.25-8 |
| Cefepime | 8 | 16 | 4-32 |
| Ceftazidime | 2 | 8 | 0.25-8 |
| Piperacillin-tazobactam (8:1) | 8 | 32 | 2->32 |
| Amoxicillin-clavulanic acid (2:1) | >32 | >32 | >32 |
| Ticarcillin-clavulanic acid (15:1) | >32 | >32 | >32 |
| Pseudomonas aeruginosa (158) | |||
| BAL30376 | 2 | 8 | ≤0.06->32 |
| Imipenem | 4 | 16 | 0.5->32 |
| Meropenem | 1 | 8 | ≤0.06->32 |
| Cefepime | 8 | 32 | 0.5->32 |
| Ceftazidime | 4 | >32 | 0.25->32 |
| Piperacillin-tazobactam (8:1) | 16 | >32 | 0.5->32 |
| Amoxicillin-clavulanic acid (2:1) | >32 | >32 | >32 |
| Ticarcillin-clavulanic acid (15:1 | >32 | >32 | >32 |
| Polymyxin B | 4 | 4 | 1->32 |
| Stenotrophomonas maltophilia (105) | |||
| BAL30376 | 0.25 | 2 | ≤0.06-16 |
| Imipenem | >32 | >32 | 32->32 |
| Meropenem | 8 | >32 | 0.25->32 |
| Cefepime | 16 | >32 | 2->32 |
| Ceftazidime | 2 | 16 | 0.5->32 |
| Piperacillin-tazobactam (8:1) | 32 | >32 | 4->32 |
| Amoxicillin-clavulanic acid (2:1) | >32 | >32 | >32 |
| Ticarcillin-clavulanic acid (15:1) | 2 | 16 | 0.25->32 |
With MIC90 values in the range of from 0.5 μg/ml (for Providencia spp.) to 8 μg/ml (for C. freundii), BAL30376 showed consistently better activity than the other β-lactam-β-lactamase inhibitor combinations, all of which, except for piperacillin-tazobactam against Proteus spp., had MIC90 values of ≥32 μg/ml. The activity of BAL30376 against most Enterobacteriaceae family members was more potent than that of ceftazidime and cefepime (except for the latter against C. freundii, Proteus spp., and Serratia marcescens). BAL30376 also had more potent activity than either cefepime or ceftazidime against Acinetobacter spp., P. aeruginosa, and Stenotrophomonas maltophilia. Although it was also potent against susceptible Burkholderia spp. (MIC50, 0.25 μg/ml), there were a number of strains with MICs greater than 32 μg/ml. Among the Enterobacteriaceae, the MIC90 values of BAL30376 were lower than those of imipenem for the panels of Enterobacter aerogenes (2 μg/ml and 16 μg/ml, respectively), Klebsiella pneumoniae (4 μg/ml and 16 μg/ml, respectively), Proteus spp. (1 μg/ml and 8 μg/ml, respectively), Providencia spp. (0.5 μg/ml and 16 μg/ml, respectively), and S. marcescens (2 μg/ml and 8 μg/ml, respectively). The MIC90s of BAL30376 and imipenem were comparable for the panel of Enterobacter cloacae strains, and imipenem had better activity against the panels of C. freundii (8 μg/ml and 2 μg/ml, respectively), E. coli (4 μg/ml and 0.5 μg/ml, respectively), and Klebsiella oxytoca (4 μg/ml and 1 μg/ml, respectively) strains. BAL30376 had lower MIC90s against all the nonfermenters. BAL30376 had lower MIC90s than meropenem against E. aerogenes (2 μg/ml and 4 μg/ml, respectively), K. oxytoca (4 μg/ml and 8 μg/ml, respectively), K. pneumoniae (4 μg/ml and 16 μg/ml, respectively), Providencia spp. (0.5 μg/ml and >32 μg/ml, respectively), S. marcescens (2 μg/ml and 4 μg/ml, respectively), and S. maltophilia (2 μg/ml and >32 μg/ml, respectively). The two drugs had comparable activity against Acinetobacter spp. and P. aeruginosa, although the individual strains had different susceptibilities according to their resistotypes (see below). Meropenem had lower MIC90s against C. freundii (8 μg/ml and 0.25 μg/ml, respectively), E. cloacae (4 μg/ml and 0.25 μg/ml, respectively), E. coli (4 μg/ml and 0.5 μg/ml, respectively), Proteus spp. (1 μg/ml and 0.5 μg/ml, respectively), and Burkholderia cepacia (16 μg/ml and 4 μg/ml, respectively).
BAL30376 had very good activity against most strains where a metallo-β-lactamase contributed largely to the β-lactam resistance (Table 3). In particular, panresistant isolates, such as K. pneumoniae 848 from Greece (13), were only inhibited by BAL30376. The combination was also active against Acinetobacter strains with OXA-27 and OXA-58 class D carbapenemases but not against strains with OXA-25 and OXA-26 enzymes. BAL30376 was also active against some strains with class A carbapenemases but not against the one strain tested with a KPC-2 carbapenemase.
TABLE 3.
Activity of BAL30376 against strains with identified β-lactamases as the sole determinant of β-lactam resistance
| Organism | Enzyme(s) present | MICa (μg/ml) |
||
|---|---|---|---|---|
| BAL30376 | MEM | IMP | ||
| E. coli HB101(pAT268) | TEM-5 | 2 | ≤0.06 | 0.25 |
| E. coli HB101(pAT266) | TEM-4 | 8 | ≤0.06 | 0.5 |
| E. coli K802N(pAT251) | TEM-3 | 0.5 | ≤0.06 | 0.25 |
| E. coli AC133 | CTX-M-15 | 1 | ≤0.06 | 1 |
| K. pneumoniae 1357E | TEM-10 | 0.25 | ≤0.06 | 0.5 |
| K. pneumoniae HPA721 | TEM-29, SHV-14 | 0.125 | ≤0.06 | 1 |
| K. pneumoniae 26768WU | SHV-5 | 4 | ≤0.06 | 1 |
| K. oxytoca 1028E | K1 | 4 | 0.125 | 0.5 |
| P. aeruginosa 1973E | PSE-1, AmpC | 8 | 4 | 2 |
| P. aeruginosa 1559E | PSE-4, AmpC | 2 | 1 | 2 |
| S. marcescens S6 | SME-1, AmpC | 0.25 | 32 | >32 |
| E. coli NMCA | NMCA | 2 | >32 | >32 |
| K. pneumoniae YC | KPC-2, SHV-2, TEM-1 | >32 | >32 | >32 |
| A. baumannii HPA74510 | IMP-4 | 0.5 | 32 | 16 |
| E. cloacae R947 | IMP-8, TEM-1, AmpC | 2 | 1 | 8 |
| P. aeruginosa HPA101-1477 | IMP-1, AmpC | 1 | >32 | >32 |
| P. aeruginosa HPA10586 | VIM-1, AmpC | 2 | >32 | >32 |
| P. aeruginosa HPA996 | VIM-2, AmpC | 0.5 | 32 | >32 |
| K. pneumoniae 848 | VIM-1, SHV-1 | 2 | 32 | >32 |
| P. rettgeri J96 | IMP-1 | 1 | >32 | >32 |
| S. maltophilia B715 | L1, L2 | 0.25 | >32 | >32 |
| E. cloacae 908R | AmpC | 1 | 0.125 | 1 |
| E. cloacae ATCC 13047 | AmpC | 1 | 1 | 1 |
| E. cloacae ATCC 13047/CRO-R | AmpC, porin- | 16 | 2 | 8 |
| E. coli Libyien | CMY-2 | 1 | 0.125 | 1 |
| P. aeruginosa 143811R | AmpC | 2 | 0.125 | 0.5 |
| P. aeruginosa 18S/H | AmpC | 2 | 2 | 4 |
| P. aeruginosa 143724R | AmpC | 8 | 4 | 8 |
| S. marcescens 1T9 | AmpC | 1 | 0.125 | 1 |
| P. aeruginosa 1937E | PSE-2, AmpC | 2 | 4 | 4 |
| A. baumannii HPA327009 | OXA-25 | 32 | >32 | >32 |
| A. baumannii HPA4737 | OXA-26 | >32 | >32 | >32 |
| A. baumannii HPAI-16 | OXA-27 | 2 | >32 | >32 |
| A. baumannii MAD (PN) | OXA-58 | 1 | 8 | >32 |
Bactericidal activity.
The bactericidal effect of BAL30376 against the following strains selected for the β-lactamases that they express was investigated (Tables 1 and 3): A. baumannii HPA74510, E. cloacae S9615, E. cloacae R947, P. aeruginosa BA, P. aeruginosa HPA10586, P. aeruginosa 527, P. aeruginosa 860, P. aeruginosa (560546)9 M.P., and P. aeruginosa 143724R. Inspection of the time courses of decreases in viable bacteria indicated that most strains exhibited a biphasic reaction with the antibiotics (Fig. 2). There was an initial phase of reaction, during which a decrease of 90 to 99.9% in the culture density was achieved within 6 h. The second phase of the reaction, which in most cases ran to complete sterilization of the culture within 28 h, was markedly slower. The initial phase of the reaction appears to represent killing of the growing, planktonic cells present in the initial culture. The second phase of the reaction may represent the reactivation and subsequent killing of a nongrowing population present at the beginning of the experiment. There was no indication that the second phase had a different concentration dependence of killing than the initial phase, and therefore, it does not appear to represent an intrinsically resistant population.
FIG. 2.
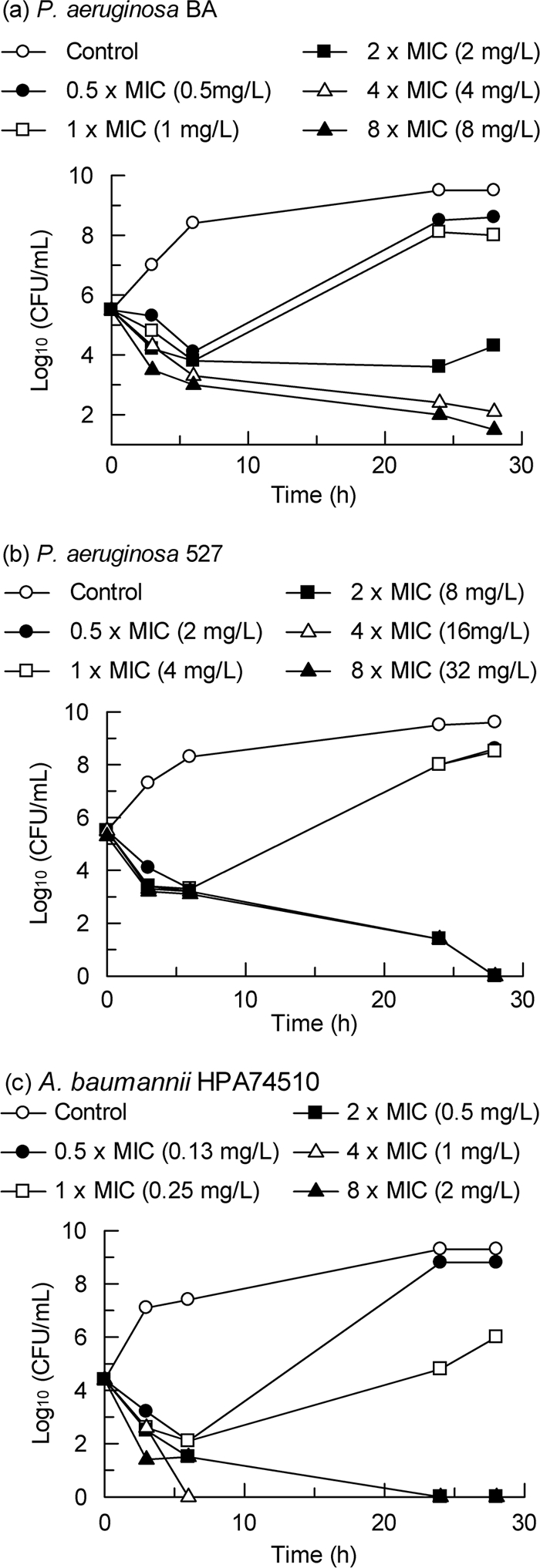
Bactericidal effect of BAL30376. (a) P. aeruginosa BA; (b) P. aeruginosa 527; (c) A. baumannii HPA74510.
The minimal bactericidal concentrations (MBCs) of BAL30376 for the susceptible strains of Enterobacter spp. were generally higher than those for meropenem against the same strains (Table 4). There was less overall difference between BAL30376 and meropenem in the MBCs for the P. aeruginosa strains (4 to >32 and 2 to > 32 μg/ml for BAL30376 and meropenem, respectively). The average MBCs for meropenem (arithmetic mean, 7.5 μg/ml; geometric mean, 3 μg/ml) and BAL30376 (arithmetic mean, 7.3 μg/ml; geometric mean, 5 μg/ml) were similar. Related to the MICs, they were 3× and 5× MIC, respectively. Generally, the MBCs were increased at higher inoculum densities. Some strains (e.g., the metallo-β-lactamase producer E. cloacae R947) showed a stronger inoculum effect with meropenem than with BAL30376, but the converse was true for other strains (e.g., the class C-hyperexpressing strain P. aeruginosa 860). The bactericidal effect of both antibiotics was significantly diminished at an inoculum density above 5 × 105 CFU/ml.
TABLE 4.
Minimum bactericidal concentrations determined by time-kill analysis
| Strain | Inoculum (CFU/ml) | Minimum bactericidal concn |
|||
|---|---|---|---|---|---|
|
BAL30376a |
Meropenem |
||||
| μg/ml | Multiple of MIC | μg/ml | Multiple of MIC | ||
| A. baumannii HPA74510 | 2 × 104 | 0.5 | 2 | >128b | |
| 3 × 106 | 2 | 8 | >128b | ||
| E. cloacae S9615 | 4 × 105 | 4 | 2 | 2 | 1 |
| E. cloacae R947 | 1 × 105 | 8 | 4 | 2 | 4 |
| 6 × 106 | 8 | 4 | 8 | 16 | |
| P. aeruginosa BA | 1 × 105 | 2 | 2 | 2 | 4 |
| 6 × 106 | >16 | >16 | 4 | 8 | |
| P. aeruginosa HPA10586 | 2 × 104 | 8 | 4 | >128b | |
| 7 × 106 | >32 | >16 | >128b | ||
| P. aeruginosa 527 | 3 × 105 | 4 | 2 | 32 | 8 |
| P. aeruginosa 860 | 4 × 104 | 8 | 2 | 4 | 2 |
| 5 × 106 | 32 | 16 | >32 | >16 | |
| P. aeruginosa (560546)9 M.P. | 2 × 105 | 2 | 1 | 2 | 1 |
| 2 × 107 | 32 | 16 | 32 | 16 | |
| P. aeruginosa 143724R | 8 × 106 | 32 | 4 | 8 | 2 |
Expressed as the concentration of BAL19764.
Data represent MIC in mg/ml.
Population analysis.
The frequency of spontaneous mutation to resistance toward BAL30376 in E. cloacae R947 was approximately 10−7 (Table 5). The frequency of mutation to resistance toward meropenem was 10-fold higher, suggesting that induction of an existing resistance mechanism, probably the IMP-8 metallo-β-lactamase, may play a role. No spontaneous mutation to resistance toward BAL30376 or meropenem was observed in K. pneumoniae CF104 (with the TEM-3 class A β-lactamase). Breakthrough resistance to BAL30376 occurred at a frequency of 10−9 to 10−8 (Table 5) in the Acinetobacter species tested. The frequency of resistance development toward meropenem in Acinetobacter junii 15218 was significantly higher at 1.6 × 10−7. No spontaneous mutation to resistance toward BAL30376 was observed in S. maltophilia 1AC736 (with class A and class B β-lactamases). The rate of mutation was 10−6.5 with ceftazidime. The P. aeruginosa strains showed higher rates of resistance development toward all the antibiotics tested. BAL30376 had the lowest frequency of resistance development against the two strains with an inducible class C β-lactamase (P. aeruginosa BA with low-level expression after induction and P. aeruginosa 860 with high-level expression after induction). The frequencies of spontaneous resistance were higher in the two strains with constitutive hyperexpression of the class C β-lactamase [P. aeruginosa 6067 and P. aeruginosa (560546)9 M.P.].
TABLE 5.
Frequency of spontaneous resistance to BAL30376
| Organism | Inoculum (CFU) | Compound | Highest concn at which growth occurred (mg/liter) | Frequency |
|---|---|---|---|---|
| A. baumannii HPA74510 | 109 | BAL30376 | No resistant colonies | |
| A. junii 15218 | 109 | BAL30376 | No resistant colonies | |
| Meropenem | 1 | 10−6.8 | ||
| E. cloacae R947 | 109 | BAL30376 | 8 | 10−6.2 |
| Meropenem | 16 | 10−5.2 | ||
| K. pneumoniae CF104 | 109 | BAL30376 | No resistant colonies | |
| Meropenem | No resistant colonies | |||
| Ceftazidime | No resistant colonies | |||
| Cefepime | No resistant colonies | |||
| P. aeruginosa BA | 109 | BAL30376 | 16 | 10−7.7 |
| Meropenem | 16 | 10−6.8 | ||
| Ceftazidime | 16 | 10−6.5 | ||
| Cefepime | 16 | 10−5.9 | ||
| P. aeruginosa 860 | 107 | BAL30376 | 16 | 10−6.3 |
| Meropenem | No resistant colonies | |||
| Ceftazidime | 16 | 10−3.4 | ||
| Cefepime | 16 | 10−4.0 | ||
| P. aeruginosa 6067 | 107 | BAL30376 | 16 | 10−3.9 |
| Meropenem | 8 | 10−5.3 | ||
| P. aeruginosa (560546)9 M.P. | 107 | BAL30376 | 16 | 10−5.4 |
| Meropenem | 8 | 10−6.1 | ||
| S. maltophilia 1AC736 | 109 | BAL30376 | No resistant colonies | |
| Ceftazidime | 16 | 10−6.4 |
Resistance development in serial passage.
Repeated passaging of the enterobacterial test strains in cultures containing BAL30376 yielded strains with >4-fold elevated MICs after 10 to 20 passages (Table 6). Strains with >4-fold elevated MICs toward cefepime or meropenem appeared within 4 passages under the same conditions. Many of the resistant isolates obtained in these studies had increased MICs for ceftazidime and cefepime, suggesting that they had higher levels of expression of β-lactamase (Table 7). Mutants derived from A. junii 15218, which does not express a β-lactamase, also had increased ciprofloxacin and meropenem MICs and may have upregulated efflux systems. Resistant cultures were obtained more rapidly with the two P. aeruginosa test strains: after 6 to 7 passages with P. aeruginosa 527 (with IMP-1, PSE-1, and a low-level, inducible class C β-lactamase) and after 4 to 10 passages with P. aeruginosa (560546)9 M.P. (with a high-level, constitutive class C β-lactamase) (Fig. 3). High-level resistance to meropenem was achieved within three passages with both strains. Thus, BAL30376 appears to be relatively refractory to resistance development even when the strains already have mechanisms that confer high-level resistance to BAL19764 alone.
TABLE 6.
Resistance development in serial passage
| Organism |
BAL30376a |
Meropenem selection |
Cefepime selection |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Selection |
Reversion |
|||||||||||
| MIC (μg/ml) |
No. of passages | MIC (μg/ml) |
No. of passages | MIC (μg/ml) |
No. of passages | MIC (μg/ml) |
No. of passages | |||||
| Initial | Final | Initial | Final | Initial | Final | Initial | Final | |||||
| A. baumannii HPA74510 | 0.5 | 64 | 20 | Not tested | Not tested | Not tested | ||||||
| 64 | 9 | 4 | 0.5 | 3 | ||||||||
| A. junii 15218 | 0.5 | 2 | 20 | Not tested | 0.125 | 0.5 | 20 | 0.5 | 8 | 20 | ||
| E. cloacae R947 | 2 | 8 | 20 | Not tested | 0.5 | 64 | 4 | Not tested | ||||
| 64 | 4 | 8 | 8 | 5 | 64 | 5 | ||||||
| E. cloacae S4741B | 1 | 8 | 20 | Not tested | 0.03 | 8 | 20 | 0.03 | 256 | 9 | ||
| 64 | 16 | |||||||||||
| E. cloacae S9639 | 2 | 8 | 20 | Not tested | 0.03 | 16 | 20 | 0.03 | 8 | 20 | ||
| K. pneumoniae 55 | 1 | 2 | 20 | Not tested | Not tested | Not tested | ||||||
| 64 | 7 | 16 | 4 | 5 | ||||||||
| K. pneumoniae CF104 | 4 | 8 | 20 | Not tested | 0.015 | 1 | 20 | Not tested | ||||
| P. aeruginosa 527 | 4 | 128 | 6 | 32 | 32 | 5 | 2 | 128 | 2 | 4 | 64 | 2 |
| 64 | 7 | 32 | 32 | 5 | ||||||||
| P. aeruginosa | 2 | 64 | 4 | 2 | 2 | 64 | 3 | Not tested | ||||
| (560546)9 M.P. | 2 | 16 | 10 | 16 | 16 | 16 | ||||||
| S. maltophilia 1AC736 | 0.125 | 16 | 20 | Not tested | 1 | 256 | 4 | |||||
| 64 | 7 | 8 | 8 | 5 | ||||||||
MIC expressed as the concentration of BAL19764.
TABLE 7.
Cross-resistance of mutants selected during serial passage experiments
| Organism | MIC (μg/ml)a |
||||||
|---|---|---|---|---|---|---|---|
| BAL30376b | BAL19764 | MEP | IMP | FEP | CAZ | CIP | |
| A. baumannii HPA74510 | 0.5 | 1 | 32 | 16 | >32 | >32 | >32 |
| A. baumannii HPA74510 #9c | 16 | >32 | 32 | 4 | >32 | >32 | >32 |
| A. junii 15218 | 0.5 | 0.5 | 0.25 | 0.25 | 1 | 4 | 0.125 |
| A. junii 15218 #14 | 4 | 8 | 4 | 0.5 | 4 | 16 | 0.5 |
| E. cloacae R947 | 2 | >32 | 0.25 | 1 | 2 | >32 | 4 |
| E. cloacae R947 #20 | 16 | >32 | 0.125 | 1 | 32 | >32 | 4 |
| E. cloacae S4741B | 1 | 2 | ≤0.06 | 1 | ≤0.06 | 0.25 | ≤0.06 |
| E. cloacae S4741B #20 | 8 | >32 | 0.125 | 0.25 | 0.5 | >32 | ≤0.06 |
| E. cloacae S9639 | 2 | 32 | 0.125 | 1 | 0.125 | 0.5 | ≤0.06 |
| E. cloacae S9639 #20 | 8 | >32 | 0.125 | 1 | 8 | >32 | ≤0.06 |
| K. pneumoniae 55 | 1 | >32 | 8 | 8 | 32 | >32 | >32 |
| K. pneumoniae 55 #20 | 32 | >32 | 16 | 16 | >32 | >32 | >32 |
| K. pneumoniae 104 | 4 | >32 | ≤0.06 | 0.25 | 4 | >32 | 4 |
| K. pneumoniae 104 #18 | 8 | >32 | 0.125 | 1 | 8 | >32 | 4 |
| P. aeruginosa (560546)9 M.P. | 2 | 1 | 4 | 8 | 8 | 8 | 1 |
| P. aeruginosa (560546)9 M.P. #7 | 16 | >32 | 4 | 8 | 8 | 16 | 1 |
| P. aeruginosa (560546)9 M.P. #9 | 16 | >32 | 32 | 8 | 8 | 8 | 0.5 |
| S. maltophilia 1AC736 | 0.125 | 0.5 | 8 | >32 | 2 | 2 | 8 |
| S. maltophilia 1AC736 #8 | 32 | >32 | >32 | >32 | >32 | >32 | 16 |
MEP, meropenem; IMP, imipenem; FEP, cefepime; CAZ, ceftazidime; CIP, ciprofloxacin.
Expressed as the concentration of BAL19764.
Suffix #n indicates the passage step at which the mutant was isolated.
FIG. 3.
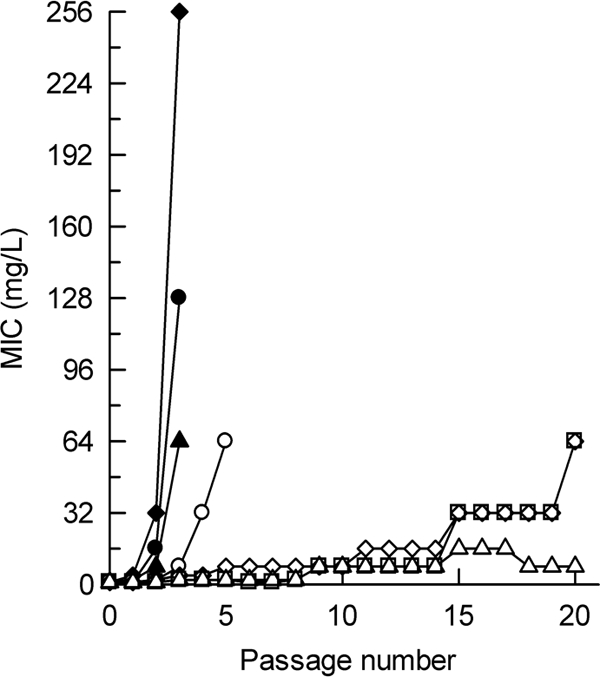
Resistance development during serial passage of bacteria producing metallo-β-lactamases. Open symbols, results obtained with BAL30376 (MIC expressed as the concentration of BAL19764); solid symbols, results obtained with meropenem; circles, P. aeruginosa 527; squares, A. baumannii HPA74510; diamonds, S. maltophilia 1AC736; triangles, E. cloacae R947.
Inhibition of β-lactamases.
As expected for a monobactam, BAL19764 inhibited the AmpC β-lactamase of C. freundii (Fig. 1), although it was less potent than aztreonam (50% inhibitory concentration, 0.005 μM). No reaction with IMP-1 was detected, while weak competition, consistent with rapid hydrolysis, was apparent with the TEM-3 β-lactamase. BAL29880 inhibited only the C. freundii AmpC β-lactamase out of the enzymes tested (Fig. 1), and clavulanic acid inhibited only TEM-3 β-lactamase with a high affinity (Fig. 1).
Experimental septicemia in mice.
The in vivo activity of the combination was tested with a mixture comprising equal proportions of the three components because only preliminary pharmacokinetic data were available and because the known instability of clavulanic acid was expected to lead to a markedly more rapid decay of this component (8, 24). The in vitro activity translated into in vivo activity against a number of strains with AmpC or a metallo-β-lactamase (Table 8). The lowest, fully protective doses of BAL19764-BAL29880-clavulanic acid (1:1:1) were 3.1 mg/kg and 0.8 mg/kg for A. baumannii J2 (with a class C β-lactamase) and E. cloacae R947 (with an IMP-8 metallo-β-lactamase), respectively.
TABLE 8.
Activities of BAL19764-BAL29880-clavulanic acid (1:1:1) and reference compounds against experimental septicemia in mice
| Strain (no. of CFU/mouse) and compound | MIC (μg/ml) | MBC (μg/ml) | Lowest dose affording protection (mg/kg t.i.d.a) |
|---|---|---|---|
| A. baumannii HPA74510 (4 × 105) | |||
| BAL19764-BAL29880-clavulanate | 0.5 | 2 | <3.1 |
| Meropenem | 32 | >32 | 3.1 |
| A. baumannii J2 (1 × 103) | |||
| BAL19764-BAL29880-clavulanate | 8 | 32 | 3.1 |
| Meropenem | 1 | 16 | <1.6 |
| Aztreonam | >32 | >32 | >3.1 |
| E. cloacae R947 (1 × 103) | |||
| BAL19764-BAL29880-clavulanate | 2 | 8 | <0.8 |
| Meropenem | 0.5 | 8 | <0.4 |
| Aztreonam | >32 | >32 | >0.4 |
| E. cloacae S9615 (1 × 107), BAL19764-BAL29880-clavulanate | 1 | 4 | 0.8 |
| P. aeruginosa 527 (1 × 107), BAL19764-BAL29880-clavulanate | 4 | 4 | 1.6 |
| P. aeruginosa HPA101-1477 (1 × 107) | |||
| BAL19764-BAL29880-clavulanate | 1 | >32 | 6.25 |
| Meropenem | >32 | >32 | >12.5 |
| Imipenem | >32 | >32 | >12.5 |
t.i.d., three times a day.
DISCUSSION
β-Lactamases are one of the major determinants of resistance of Gram-negative bacilli toward the β-lactam antibiotics. Each of the four Ambler classes of β-lactamase has a fundamentally different catalytic mechanism, and finding an efficient mechanism-based inhibitor that can encompass this mechanistic diversity has proved challenging. However, the intensive search for new inhibitors and β-lactamase-stable antibiotics has shown that it is possible to achieve potent, selective inhibitors of individual classes of enzymes and to achieve stability toward some types of β-lactamase. BAL19764 is a monobactam antibiotic and, like other monobactams such as aztreonam, acts as an inhibitor of class C β-lactamases while being refractory to hydrolysis by class B enzymes. It is somewhat more labile than aztreonam toward class C enzymes and therefore needs additional protection, especially in derepressed strains that hyperproduce these enzymes. BAL29880 is a selective inhibitor of class C β-lactamases belonging to the bridged monobactam class that was specifically designed to block the hydrolytic mechanism of these enzymes (9). We show here that BAL29880 is able to protect BAL19764 against class C β-lactamases, even in derepressed strains of Enterobacteriaceae and P. aeruginosa. Unlike some other experimental β-lactamase inhibitors that have potent activity against class C enzymes (6), BAL29880 is able to penetrate effectively into P. aeruginosa and appears to avoid the action of efflux pumps. Clavulanic acid is a well-known inhibitor of class A β-lactamases, including the majority of the extended-spectrum variants of TEM, SHV, and CTX-M enzymes. It is able to protect BAL19764 against the action of these enzymes. Interestingly, BAL19764 appears to be stable toward hydrolysis by some of the serine carbapenemases, as BAL30376 retained activity against strains with the class A enzymes NMCA and SME-1 as well as strains with the class D enzymes OXA-27 and OXA-58. Clavulanic acid does not inhibit these carbapenemases very potently and adds little to the activity of BAL19764 against these strains.
The intrinsic β-lactamase stability of the antibiotic, as well as the protection conferred by the two inhibitors, allows BAL30376 to exert a bactericidal effect against a broad range of β-lactamase-producing organisms, including strains that were refractory to other β-lactams, including aztreonam. The bactericidal effect was time and concentration dependent, similar to the effects that other β-lactams exert on susceptible strains, and there was a pronounced inoculum effect, as might be expected for β-lactamase-producing organisms.
It is vital for an antimicrobial agent that its antibacterial properties not easily be overcome by the rapid emergence of resistant strains. This could be of particular concern for a combination antibiotic with three components, each having a critical, independent role in its overall function. In our experiments, BAL30376 appeared to be relatively refractory toward selection of resistant mutants. The frequencies of resistance in population analysis (single-step or breakthrough resistance) were lower than those observed with comparators for many of the organisms studied, and in serial passage experiments, BAL30376 was always the last compound for which stable resistance appeared.
In keeping with its in vitro properties, therapeutic efficacy against a variety of β-lactamase-producing organisms could be demonstrated for BAL30376 in mouse models of septicemia. There was a reasonable correlation between the MIC observed in vitro and the lowest effective dose in vivo, although for some organisms, such as the IMP-1 producer P. aeruginosa HPA101-1477, MBC might be the more significant parameter.
In conclusion, BAL30376 is a new combination antibiotic comprising a siderophore monobactam, BAL19764, and two potent, highly selective β-lactamase inhibitors, clavulanic acid and BAL29880, a bridged monobactam inhibitor of Ambler class C β-lactamases. It has a broad spectrum of activity against aerobic Gram-negative bacteria, including many troublesome multidrug-resistant strains. In vivo experiments have confirmed the activity against a selection of β-lactamase-producing organisms, including those with multiple enzymes and metallo-β-lactamases.
Acknowledgments
We thank Alain Brendle, Beatrice Hofer, Dominique Klauer, and Caroline Müller for their expert assistance. We are grateful to D. Livermore, V. Miriagou, and P. Nordmann for providing bacterial strains.
Footnotes
Published ahead of print on 18 January 2011.
REFERENCES
- 1.Boucher, H. W., et al. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48:1-12. [DOI] [PubMed] [Google Scholar]
- 2.Bush, K., C. Macalintal, B. A. Rasmussen, V. J. Lee, and Y. Yang. 1993. Kinetic interactions of tazobactam with beta-lactamases from all major structural classes. Antimicrob. Agents Chemother. 37:851-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buynak, J. D. 2006. Understanding the longevity of the β-lactam antibiotics and of antibiotic/β-lactamase inhibitor combinations. Biochem. Pharmacol. 71:930-940. [DOI] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 7th ed. M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA.
- 5.Desarbre, E., A. de Fontanges, M. G. P. Page, F. Richalet, and P. Roussel. 2007. Antimicrobial activity of novel monobactams, poster F1-351. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 6.Drawz, S., and R. A. Bonomo. 2009. Three decades of β-lactamase inhibitors. Clin. Microbiol. Rev. 23:160-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gill, A. E., et al. 2003. Comparative in vitro activity of PTX2416, a dihydroxypyridone monobactam, against Gram-negative clinical isolates, poster F-552. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 8.Haginaka, J., T. Nakagawa, and T. Uno. 1981. Stability of clavulanic acid in aqueous solutions. Chem. Pharm. Bull. 29:3334-3341. [DOI] [PubMed] [Google Scholar]
- 9.Heinze-Krauss, I., et al. 1998. Structure-based design of β-lactamase inhibitors. 1. Synthesis and evaluation of bridged monobactams. J. Med. Chem. 41:3961-3971. [DOI] [PubMed] [Google Scholar]
- 10.Jacobus, N. V., M. C. Ferreira, and M. Barza. 1982. In vitro activity of aztreonam, a monobactam antibiotic. Antimicrob. Agents Chemother. 22:821-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Livermore, D. M., and S. Mushtaq. 2003. PTX 2416, a dihydroxypyridone monobactam, vs. Pseudomonas aeruginosa strains with characterised resistances, poster F-554. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 12.Livermore, D. M., and N. Woodford. 2006. The beta-lactamase threat in Enterobacteriaceae, Pseudomonas and Acinetobacter. Trends Microbiol. 14:413-420. [DOI] [PubMed] [Google Scholar]
- 13.Miriagou, V., E. Tzelepi, G. L. Daikos, P. T. Tassios, and L. S. Tzouvelekis. 2005. Panresistance in VIM-1-producing Klebsiella pneumoniae. J. Antimicrob. Chemother. 55:810-811. [DOI] [PubMed] [Google Scholar]
- 14.Möllmann, U., L. Heinisch, A. Bauernfeind, T. Köhler, and D. Ankel-Fuchs. 2009. Siderophores as drug delivery agents: application of the “Trojan Horse” strategy. Biometals 22:615-624. [DOI] [PubMed] [Google Scholar]
- 15.Nicasio, A. M., J. L. Kuti, and D. P. Nicolau. 2008. The current state of multidrug-resistant Gram-negative bacilli in North America. Pharmacotherapy 28:235-249. [DOI] [PubMed] [Google Scholar]
- 16.Page, M. G. P. 1993. The kinetics of non-stoichiometric bursts of β-lactam hydrolysis catalyzed by class C beta-lactamases. Biochem. J. 295:295-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Page, M. G. P. 2000. β-Lactamase inhibitors. Drug Resist. Updat. 3:109-125. [DOI] [PubMed] [Google Scholar]
- 18.Page, M. G. P., C. Dantier, and E. Desarbre. 2010. In vitro properties of BAL30072, a novel siderophore sulfactam with activity against multiresistant Gram-negative bacilli. Antimicrob. Agents Chemother. 54:2291-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paterson, D. L., and Y. Doi. 2007. A step closer to extreme drug resistance (XDR) in gram-negative bacilli. Clin. Infect. Dis. 45:1179-1181. [DOI] [PubMed] [Google Scholar]
- 20.Sandanayaka, V. P., and A. S. Prashad. 2002. Resistance to β-lactam antibiotics: structure and mechanism based design of β-lactamase inhibitors. Curr. Med. Chem. 9:1145-1165. [DOI] [PubMed] [Google Scholar]
- 21.Talbot, G. H., et al. 2006. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin. Infect. Dis. 42:657-668. [DOI] [PubMed] [Google Scholar]
- 22.Thomson, J. M., and R. A. Bonomo. 2005. The threat of antibiotic resistance in Gram-negative pathogenic bacteria: β-lactams in peril! Curr. Opin. Microbiol. 8:518-524. [DOI] [PubMed] [Google Scholar]
- 23.Wagegg, W., and V. Braun. 1981. Ferric citrate transport in Escherichia coli requires outer membrane receptor protein FecA. J. Bacteriol. 145:156-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wildfeuer, A., and K. Räder. 1996. Stability of β-lactamase inhibitors and β-lactam antibiotics in parenteral dosage forms and in body fluids and tissue homogenates: a comparative study of sulbactam, clavulanic acid, ampicillin and amoxycillin. Int. J. Antimicrob. Agents 6:S31-S34. [PubMed] [Google Scholar]