Abstract
The in vitro and in vivo activities of modithromycin, a novel bicyclolide, against Legionella pneumophila were compared with those of telithromycin, clarithromycin, azithromycin, and levofloxacin. All the test agents decreased the intracellular growth of viable L. pneumophila bacteria over 96 h of incubation in both types of cells used, A/J mouse-derived macrophages and A549 human alveolar epithelial cells, at extracellular concentrations of 4× and 16× MIC, respectively. However, when the agents were removed from the medium after exposure for 2 h, regrowth of intracellular bacteria occurred in both cell systems when they were exposed to telithromycin, clarithromycin, and levofloxacin but not when they were exposed to modithromycin and azithromycin. Once-daily administration of modithromycin at a dose of 10 mg/kg of body weight for 5 days led to a significant decrease of intrapulmonary viable L. pneumophila bacteria in immunosuppressed A/J mice. The efficacy of modithromycin was superior to the efficacies of telithromycin and clarithromycin and comparable to the efficacies of azithromycin and levofloxacin. In addition, modithromycin and azithromycin inhibited the intrapulmonary regrowth of bacteria even at 72 h after the last treatment, but telithromycin and levofloxacin did not. These results suggested that modithromycin has longer-lasting cellular pharmacokinetic features like azithromycin. In conclusion, modithromycin, as well as azithromycin, has excellent in vitro and in vivo bactericidal activities and persistent efficacy against intracellular L. pneumophila. Modithromycin should be a useful agent for treatment of pulmonary infections caused by this pathogen.
Legionella pneumophila is a facultative intracellular pathogen that causes life-threatening pneumonia, such as Legionnaires' disease and Pontiac fever (7). This organism multiplies and survives in several types of host cells, such as phagocytes and epithelial cells (13). Therefore, antimicrobial agents for the treatment of Legionella infections should have not only in vitro antibacterial activity but also efficient penetration into the host cells. Recently, macrolides and fluoroquinolone agents have been selected for treatment of L. pneumophila infections because of their potent in vitro activity and high level of distribution into the lungs (1, 7, 18, 19, 20). In particular, in the North American consensus guidelines, azithromycin is recommended as the most preferable macrolide agent for the management of community-acquired pneumonia in adults (3, 15, 16).
Modithromycin (EDP-420, EP-013420, S-013420), a bicyclolide agent, is a novel member of the macrolide class with a broad spectrum of activity against Gram-positive and -negative respiratory pathogens, including intracellular bacteria, such as Mycoplasma pneumoniae and Chlamydia pneumoniae (12). In recent studies, we have demonstrated that modithromycin has potent in vitro activity against clinical isolates of Legionella spp., with a MIC90 of 0.031 μg/ml, which is comparable to that of levofloxacin and which makes it more active than telithromycin, clarithromycin, and azithromycin (MIC90s, 0.125, 0.063, and 1 μg/ml, respectively) (21). However, for evaluation of its clinical efficacy against this pathogen, intracellular antibacterial activity and in vivo efficacy should also be determined. These would reflect not only in vitro antibacterial activity but also intracellular penetration and tissue distribution.
In this study, the intracellular activity of modithromycin against L. pneumophila was compared with the activities of telithromycin, clarithromycin, azithromycin, and levofloxacin using two different types of cells, A/J mouse-derived bone marrow macrophages and human alveolar epithelial cell line A549. We also evaluated the therapeutic efficacy of these antimicrobial agents against pulmonary growth of this pathogen in immunosuppressed A/J mice.
(This work was presented in part at the 46th Interscience Conference on Antimicrobial Agents and Chemotherapy, abstr. E-1856, San Francisco, CA, 2006, and the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy, abstr. E-1629, Chicago, IL, 2007.)
MATERIALS AND METHODS
Antimicrobial agents.
Modithromycin and telithromycin were kindly supplied by Shionogi & Co., Ltd. (Osaka, Japan). Clarithromycin, azithromycin, and ampicillin were purchased from the U.S. Pharmacopeia (Rockville, MD). Levofloxacin was kindly supplied by Daiichi Sankyo Co., Ltd. (Tokyo, Japan). As solvents for the in vitro studies, 1 M acetate buffer (pH 4.5) was used for modithromycin, telithromycin, clarithromycin, and azithromycin, 0.1 N NaOH was used for levofloxacin, and 0.1 M phosphate buffer (pH 6.0) was used for ampicillin. These prepared solutions were diluted to the desired concentration with distilled water. To prepare suspensions for oral administration in the in vivo studies, 0.5% methylcellulose (Wako Pure Chemical Industries, Ltd., Japan) was used as the vehicle.
Bacterial strain and susceptibilities to antimicrobial agents.
The L. pneumophila Suzuki strain (serogroup 1), isolated from a Japanese medical facility, was used in this study. The MICs of modithromycin, telithromycin, clarithromycin, azithromycin, levofloxacin, and ampicillin for the Suzuki strain were 0.031, 0.125, 0.063, 0.25, 0.031, and 0.5 μg/ml, respectively, which were determined by broth microdilution method using BYEα broth [N-(2-acetamido)-2-aminoethanesulfonic acid-buffered yeast extract broth supplemented with 0.1% α-ketoglutarate, 0.04% l-cysteine, and 0.025% iron(III) diphosphate (final pH, 6.9)] (21).
Preparation of bacterial suspension.
The test strain was grown on BCYEα agar [N-(2-acetamido)-2-aminoethanesulfonic acid-buffered charcoal yeast extract agar supplemented with 0.1% α-ketoglutarate, 0.04% l-cysteine, and 0.025% iron(III) diphosphate (final pH, 6.9)] for 3 days at 35°C, and a single colony was transferred to BYEα broth, followed by incubation for 24 h at 35°C with constant shaking. The bacterial suspension was again transferred to fresh BYEα broth and incubated for 24 h under the same conditions. The suspension including post-exponential-phase bacteria was used after dilution in the test medium (RPMI 1640 [Invitrogen Corporation] supplemented with 10% heat-inactivated fetal bovine serum [Invitrogen Corporation]) or saline for the in vitro and in vivo studies, respectively (6).
Determination of in vitro intracellular activity.
The primary culture of bone marrow macrophages was prepared from bone marrow exudates of an A/J mouse (male, 6 weeks old) as described previously (26). The human alveolar epithelial cell line A549 (CCL-185) was purchased from the American Type Culture Collection. These cells (macrophages, 1.3 × 105 cells/0.1 ml/well; A549 cells, 1.6 × 104 cells/0.1 ml/well) were incubated in the test medium for 24 h at 37°C in humidified air containing 5% CO2 to make cell monolayers.
In this study, two types of experiments (retention experiment and removal experiment) were performed, as follows. A suspension of the Suzuki strain (0.1 ml) was added to each well containing the cell monolayer (inoculum, 5.7 × 104 CFU/well). After a 1-h incubation, the medium containing extracellular nonadherent bacteria was removed and the infected cells were washed once with the fresh test medium. In the case of the retention experiment, these cells were exposed to various concentrations of antimicrobial agents for 96 h. In the removal experiment, the cells were exposed to various concentrations of antimicrobial agents for 2 h, followed by removal of the agents and an additional 94-h incubation in the absence of the agents. These retention and removal experiments were run in parallel. The number of intracellular viable bacteria was determined on BCYEα agar at 2, 24, 48, and 96 h after initiation of exposure to the agent, as follows. The cells with intracellular bacteria were washed once with the fresh test medium and lysed with 0.1 ml of distilled water, followed by the determination of viable bacteria in the lysate.
Pulmonary infection model.
Specific-pathogen-free A/J mice (male, 6 weeks old) were obtained from Sankyo Labo Service Corporation (Tokyo, Japan). Mice were immunosuppressed by subcutaneous administration of a cortisone acetate (Sigma-Aldrich) suspension at 100 mg/kg of body weight for 3 consecutive days from 1 day before infection (5). The immunosuppressed mice were infected by intratracheal inoculation of a bacterial suspension (1.4 × 105 to 1.8 × 105 CFU/30 μl/mouse) while the mice were under anesthesia with a ketamine (Sankyo Yell Yakuhin Co., Ltd., Japan) and xylazine (Bayer Medical Ltd., Japan) mixture. Two experiments with different therapeutic schedules were performed. (i) The antimicrobial agents were orally administered 24, 48, 72, 96, and 120 h after infection. At 24 h after the last administration of the agent, the mice were killed and the numbers of viable bacteria in the lungs were determined by culture of tissue on BCYEα agar. (ii) The antimicrobial agents were orally administered 24 and 48 h after infection. At 24 and 72 h after the last administration of the agent, the mice were killed and the numbers of viable bacteria in the lungs were determined by culture of tissue on BCYEα agar. These BCYEα agars contained vancomycin (Sigma-Aldrich) at 5 μg/ml and polymyxin B (Sigma-Aldrich) at 100 units/ml to prevent the growth of other bacterial cells. The experimental protocols were approved by the Institutional Animal Care and Use Committee at Toho University School of Medicine.
Statistical analysis for efficacy in pulmonary infection model.
The differences in the number of viable bacteria between study groups were analyzed by Dunnett's multiple-comparison test or Welch's t test. Statistical significance was defined by a P value of <0.05.
RESULTS
Intracellular activities of antimicrobial agents.
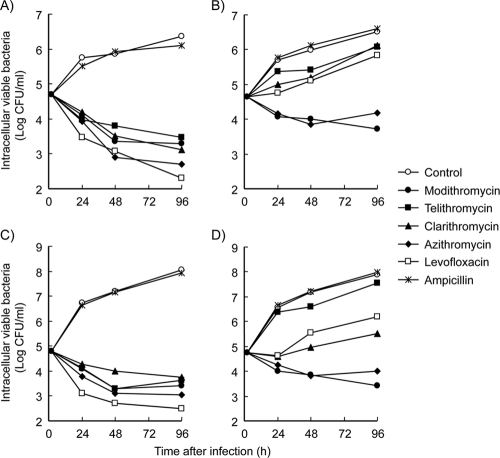
The intracellular antibacterial activities of modithromycin and the other antimicrobial agents against L. pneumophila were assessed by using two kinds of cells, bone marrow macrophages derived from an A/J mouse and A549 human alveolar epithelial cells, as the host cells to mimic actual sites of infection (Fig. 1). In the presence of these two kinds of cells, ampicillin did not decrease the number of intracellular bacteria. Almost all the β-lactam agents, including ampicillin, are ineffective against intracellular bacteria because of poor cellular penetration (9). These findings ascertained that the viable bacteria proliferate inside the cells and the intracellular activity of the agents could be determined.
FIG. 1.
Intracellular antibacterial activities of modithromycin and other antimicrobial agents against the L. pneumophila Suzuki strain in A/J mouse-derived bone marrow macrophages (A and B) and A549 human alveolar epithelial cells (C and D). These monolayer cells were infected with a 1-h incubation. After removal of the nonadherent bacteria, the infected cell layers were exposed to the antimicrobial agents for 96 h (retention experiment; A and C) or were exposed for 2 h, followed by removal of the agents and an additional 94 h of incubation in the absence of the agents (removal experiment; B and D). The macrophage and epithelial cell systems were exposed to extracellular concentrations of 4× and 16× MICs of all test agents except ampicillin, respectively. The exposure to ampicillin was at 16× MIC in both cell systems. The numbers of viable bacteria in the cells were determined at 2, 24, 48, and 96 h after infection.
We performed two types of experiments, retention and removal experiments, as described in Materials and Methods. In the retention experiment, all test agents decreased the numbers of intracellular viable bacteria in both macrophages and epithelial cells when they were exposed at extracellular concentrations of 4× and 16× MIC, respectively, over 96 h of incubation. Levofloxacin showed rapid and the most bactericidal activity among the agents tested in both cell systems. On the other hand, in the removal experiment using the macrophages, rapid regrowth of intracellular bacteria was observed for up to 96 h when cells were exposed to telithromycin, clarithromycin, or levofloxacin at 4× MIC for 2 h. The regrowth of intracellular bacteria did not occur when cells were exposed to modithromycin and azithromycin at 4× MIC for 2 h. A similar result was observed when these antimicrobial agents were tested using the epithelial cell line A549, except that the test concentration was 16× MIC instead of 4× MIC.
Therapeutic efficacies of antimicrobial agents in pulmonary infections.
The in vivo efficacies of modithromycin and the other antimicrobial agents against L. pneumophila were assessed by using 5-day treatments and a pulmonary infection model in immunosuppressed A/J mice (Fig. 2). Once-daily administration of modithromycin at the two doses of 10 and 30 mg/kg led to significant decreases in the number of intrapulmonary viable bacteria to approximately 1 × 104 CFU/lung, whereas the number in nontreated mice reached 1.5 × 108 CFU/lung. At both doses, the therapeutic efficacy of modithromycin was significantly superior to the efficacies of telithromycin and clarithromycin (P < 0.05) and was comparable to the efficacies of azithromycin and levofloxacin.
FIG. 2.
In vivo efficacies of modithromycin and other antimicrobial agents against pulmonary infection with the L. pneumophila Suzuki strain in immunosuppressed A/J mice with 5-day treatments. The antimicrobial agents were orally administered at a dose of 10 or 30 mg/kg/day at 24, 48, 72, 96, and 120 h after infection. The numbers of viable bacteria in the lungs were determined 144 h after infection (n = 4 or 5). The differences in the numbers of viable bacteria between the groups treated with modithromycin and other antimicrobial agents at the same dose were analyzed by Dunnett's multiple-comparison tests. MOD, modithromycin; TEL, telithromycin; CLR, clarithromycin; AZM, azithromycin; LVX, levofloxacin.
The efficacies of modithromycin and the other antimicrobial agents were also assessed with different treatment periods. The antimicrobial agents were administered at a dose of 30 mg/kg once daily for 2 days, and then the intrapulmonary growth of viable bacteria was observed (Fig. 3). At 24 h after the last administration of modithromycin, the number of viable bacteria reached approximately 1 × 105 CFU/lung. After that, the increase in the number of viable bacteria in the modithromycin-treated group was suppressed for the next 48 h. These bactericidal and persistent efficacies were also observed in the azithromycin-treated group. On the other hand, the numbers of viable bacteria in the telithromycin- and levofloxacin-treated groups were significantly increased from 24 h to 72 h after the last treatment. Although levofloxacin was observed to have stronger bactericidal efficacy than modithromycin and azithromycin 24 h after the last treatment, an approximately 25-fold increase in the number of viable bacteria occurred during the next 48 h.
FIG. 3.
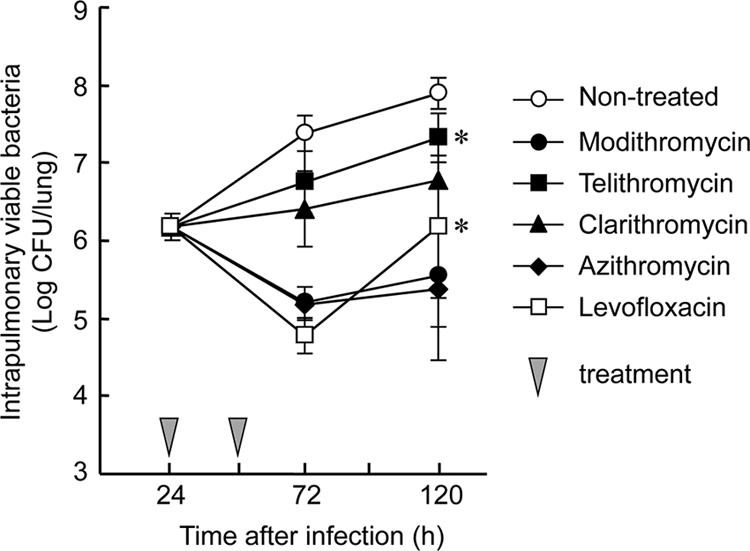
In vivo efficacies of modithromycin and other antimicrobial agents with 2-day treatments against pulmonary infection with the L. pneumophila Suzuki strain in immunosuppressed A/J mice. The antimicrobial agents were orally administered at a dose of 30 mg/kg/day 24 and 48 h after infection (n = 5). *, the differences between the numbers of viable bacteria 72 and 120 h after infection in each antimicrobial agent were analyzed by Welch's t tests (P < 0.05).
DISCUSSION
Recently, to assess the intracellular anti-Legionella activity of antimicrobial agents, infection models using various kinds of cells derived from humans, mice, and guinea pigs have been developed (2, 10, 11, 14, 22). In this study, we used the A/J mouse strain for determination of both in vitro intracellular antibacterial activity and in vivo efficacy in pulmonary infection models, because it has been reported that this strain permits growth of Legionella as a feature of its genetic background (8, 27).
The present study has demonstrated that modithromycin had excellent intracellular bactericidal activities against L. pneumophila in murine macrophages and human alveolar epithelial cells. In particular, the removal experiments have shown that modithromycin as well as azithromycin inhibited the intracellular growth of bacteria for 94 h after only a 2-h exposure, while telithromycin, clarithromycin, and levofloxacin did not. The favorable intracellular activity of azithromycin was considered to be caused by its long-lasting cellular pharmacokinetic features. It is well-known that macrolide agents generally possess the ability to be highly concentrated in cells. Bosnar et al. have reported on the characterization of these classes of macrolide agents, including azalide and ketolide agents, with respect to cellular uptake and efflux using several types of cells (4). Azithromycin showed rapid and nonsaturable uptake into the cells and was slowly released from the preloaded cells. On the other hand, clarithromycin and telithromycin displayed rapid and saturable uptake into the cells and were rapidly released from the preloaded cells. In addition, both influx of levofloxacin into cells and its efflux from cells are rapid (9). These findings showed that azithromycin might accumulate in cells at high concentrations for longer periods, which prevented the growth of intracellular bacteria for longer periods with a short exposure to azithromycin than the time of growth inhibition achieved with telithromycin, clarithromycin, and levofloxacin. The results of the removal experiment suggested that modithromycin might also have cellular pharmacokinetics like those of azithromycin, although the possibility that modithromycin has a good postantibiotic effect against Legionella spp. was not excluded. The differences in the efficacious concentrations of antimicrobials between these cell systems were unclear. However, several factors can be considered, such as the growth rate of intracellular bacteria and differences in the penetration rates of antimicrobial agents into the cells.
In the murine pulmonary infection model, a severe infection in an immunosuppressed host was used to easily evaluate the efficacies of modithromycin and the other antimicrobial agents tested (5, 17). The results of 5-day treatments demonstrated that telithromycin and clarithromycin have weak activity against the pulmonary growth of L. pneumophila in the immunosuppressed mice. However, modithromycin caused a significant decrease in the numbers of viable bacteria in the lungs, as did azithromycin and levofloxacin. In addition, modithromycin and azithromycin showed persistent efficacy that prevented the intrapulmonary regrowth of bacteria even after the last treatment, while levofloxacin did not. These results seem to agree well with the excellent intracellular activity of modithromycin and azithromycin in the removal experiments. It should be noted that the in vitro intracellular activity and in vivo efficacy of modithromycin against L. pneumophila were as potent as those of azithromycin, which is recommended for use for the treatment of L. pneumophila pneumonia because of its features of a half-life longer than that of other agents, good lung distribution, and nonsaturable uptake into phagocytes and epithelial cells (4, 24, 25, 28). Modithromycin appears to have an excellent intracellular pharmacodynamic profile, as described above, and has also been reported to have a larger lung-to-plasma ratio of distribution in rat (23). These features would lead to the excellent therapeutic efficacy of modithromycin against L. pneumophila infections, comparable to that of azithromycin.
In summary, modithromycin has not only excellent intracellular bactericidal activity but also persistent effects against intracellular and intrapulmonary growth of L. pneumophila in experimental infections in vitro and in vivo. This persistent efficacy of modithromycin against the intrapulmonary growth of bacteria is an excellent advantage for the treatment of pulmonary infections. Therefore, modithromycin should be a promising antimicrobial agent for the treatment of respiratory infections caused by intracellular pathogens.
Acknowledgments
We thank A. Ohno, S. Miyazaki, K. Sugihara, H. Maki, M. Tsuji, H. Miwa, and Y. Yamano for experimental technical support and/or helpful discussion.
T. Sato is an employee of Shionogi & Co., Ltd., but does not have stock or options. We have no other possible conflicts of interest to report.
This work was supported by Shionogi & Co., Ltd.
Footnotes
Published ahead of print on 10 January 2011.
REFERENCES
- 1.Amsden, G. W. 1993. Treatment of Legionnaires' disease. Drugs 65:605-614. [DOI] [PubMed] [Google Scholar]
- 2.Baltch, A. L., R. P. Smith, W. J. Ritz, M. A. Franke, and P. B. Michelsen. 2000. Antibacterial effect of telithromycin (HMR 3647) and comparative antibiotics against intracellular Legionella pneumophila. J. Antimicrob. Chemother. 46:51-55. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett, J. G., et al. 2000. Practice guidelines for the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 31:347-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosnar, M., Z. Kelneric, V. Munic, V. Erakovic, and M. J. Parnham. 2005. Cellular uptake and efflux of azithromycin, erythromycin, clarithromycin, telithromycin, and cethromycin. Antimicrob. Agents Chemother. 49:2372-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brieland, J. K., D. Loebenberg, F. Menzel, and R. S. Hare. 2000. Efficacy of SCH27899 in an animal model of Legionnaires' disease using immunocompromised A/J. mice. Antimicrob. Agents Chemother. 44:1333-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrne, B., and M. S. Swanson. 1998. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect. Immun. 66:3029-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carratalà, J., and C. Garcia-Vidal. 2010. An update on Legionella. Curr. Opin. Infect. Dis. 23:152-157. [DOI] [PubMed] [Google Scholar]
- 8.Diez, E., Z. Yaraghi, A. MacKenzie, and P. Gros. 2000. The neuronal apoptosis inhibitory protein (Naip) is expressed in macrophages and is modulated after phagocytosis and during intracellular infection with Legionella pneumophila. J. Immunol. 164:1470-1477. [DOI] [PubMed] [Google Scholar]
- 9.Donowitz, G. R. 1994. Tissue-directed antibiotics and intracellular parasites: complex interaction of phagocytes, pathogens, and drugs. Clin. Infect. Dis. 19:926-930. [DOI] [PubMed] [Google Scholar]
- 10.Edelstein, P. H., and M. A. Edelstein. 1989. WIN 57273 is bactericidal for Legionella pneumophila grown in alveolar macrophages. Antimicrob. Agents Chemother. 33:2132-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higa, F., et al. 2005. In vitro activity of pazufloxacin, tosufloxacin and other quinolones against Legionella species. J. Antimicrob. Chemother. 56:1053-1057. [DOI] [PubMed] [Google Scholar]
- 12.Homma, T., et al. 2010. In vitro antibacterial activities of S-013420, a novel bicyclolide, against respiratory tract pathogens. J. Antimicrob. Chemother. 65:1433-1440. [DOI] [PubMed] [Google Scholar]
- 13.Horwitz, M. A., and S. C. Silverstein. 1980. Legionnaires' disease bacterium (Legionella pneumophila) multiplies intracellularly in human monocytes. J. Clin. Invest. 66:441-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung, R., L. H. Danziger, and S. L. Pendland. 2002. Intracellular activity of ABT-773 and other antimicrobial agents against Legionella pneumophila. J. Antimicrob. Chemother. 49:857-861. [DOI] [PubMed] [Google Scholar]
- 15.Mandell, L. A., et al. 2000. Canadian guidelines for the initial management of community-acquired pneumonia: an evidence-based update by the Canadian Infectious Diseases Society and the Canadian Thoracic Society. Clin. Infect. Dis. 31:383-421. [DOI] [PubMed] [Google Scholar]
- 16.Niederman, M. S., et al. 2001. Guidelines for the management of adults with community-acquired pneumonia. Am. J. Respir. Crit. Care Med. 163:1730-1754. [DOI] [PubMed] [Google Scholar]
- 17.North, R. J. 1971. The action of cortisone acetate on cell-mediated immunity to infection. J. Exp. Med. 134:1485-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pedro-Botet, M. L., and V. L. Yu. 2009. Treatment strategies for Legionella infection. Expert Opin. Pharmacother. 10:1109-1121. [DOI] [PubMed] [Google Scholar]
- 19.Roig, J., and J. Rello. 2003. Legionnaires' disease: a rational approach to therapy. J. Antimicrob. Chemother. 51:1119-1129. [DOI] [PubMed] [Google Scholar]
- 20.Roig, J., J. Casal, P. Gispert, and E. Gea. 2006. 13—Antibiotic therapy of community-acquired pneumonia (CAP) caused by atypical agents. Med. Mal. Infect. 36:680-689. [DOI] [PubMed] [Google Scholar]
- 21.Sato, T., et al. 2011. In vitro antibacterial activity of modithromycin, a novel 6,11-bridged bicyclolide, against respiratory pathogens, including macrolide-resistant gram-positive cocci. Antimicrob. Agents Chemother. 55:1588-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tano, E., O. Cars, and E. Löwdin. 2005. Pharmacodynamic studies of moxifloxacin and erythromycin against intracellular Legionella pneumophila in an in vitro kinetic model. J. Antimicrob. Chemother. 56:240-242. [DOI] [PubMed] [Google Scholar]
- 23.Tsuji, M., et al. 2005. S-013420, a new bridged bicyclic ketolide. II. In vivo activity against experimental animal infection models, abstr. F-2035. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 24.Vallée, E., E. Azoulay-Dupuis, J. J. Pocidalo, and E. Bergogne-Bérézin. 1992. Activity and local delivery of azithromycin in a mouse model of Haemophilus influenzae lung infection. Antimicrob. Agents Chemother. 36:1412-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Bambeke, F., and P. M. Tulkens. 2001. Macrolides: pharmacokinetics and pharmacodynamics. Int. J. Antimicrob. Agents 18(Suppl. 1):S17-S23. [DOI] [PubMed] [Google Scholar]
- 26.Yoshizawa, S., et al. 2005. Legionella pneumophila evades gamma interferon-mediated growth suppression through interleukin-10 induction in bone marrow-derived macrophages. Infect. Immun. 73:2709-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zamboni, D. S., et al. 2006. The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat. Immunol. 7:318-325. [DOI] [PubMed] [Google Scholar]
- 28.Zhanel, G. G., et al. 2001. Review of macrolides and ketolides: focus on respiratory tract infections. Drugs 61:443-498. [DOI] [PubMed] [Google Scholar]