Abstract
Adverse effects induced by HIV protease inhibitors (PIs) are a significant factor in limiting their clinical success. PIs directly contribute to peripheral insulin resistance and alterations in lipid metabolism. GS-8374 is a novel PI with potent antiretroviral activity and a favorable resistance profile. Here we report on the potential of GS-8374 to adversely affect glucose and lipid homeostasis. Acute effects of GS-8374 and control PIs on glucose uptake and lipid accumulation were assessed in vitro in mouse OP9 and primary human adipocytes, respectively. GS-8374 and atazanavir showed no effect on insulin-stimulated deoxyglucose uptake, whereas ritonavir and lopinavir caused significant reductions. Similarly, in vitro lipid accumulation was not significantly affected in adipocytes treated with either GS-8374 or atazanavir. In euglycemic-hyperinsulinemic clamp experiments performed in rats during acute infusion of therapeutic levels of PIs, sustained serum GS-8374 levels of 8 μM had no effect on peripheral glucose disposal (similar to the findings for atazanavir). Comparable serum levels of lopinavir and ritonavir produced acute 19% and 53% reductions in in vivo glucose disposal, respectively. In conclusion, similar to atazanavir, but unlike ritonavir and lopinavir, GS-8374 neither affects insulin-stimulated glucose uptake in adipocytes in culture nor acutely alters peripheral glucose disposal in a rodent model system. These results dissociate the antiretroviral activity of GS-8374 from adverse effects on insulin sensitivity observed with some of the first-generation PIs and provide further support for the use of these experimental systems in the preclinical evaluation of novel PIs.
With the development and clinical introduction of HIV protease inhibitors (PIs) over a decade ago, HIV infection has transitioned from a once fatal condition to a chronic, treatable disease. While HIV integrase and entry inhibitors have recently been added to antiretroviral therapeutic arsenals, PIs together with reverse transcriptase inhibitors remain a cornerstone of many highly active antiretroviral treatment (HAART) regimens. Despite the dramatic reduction in HIV-associated morbidity and mortality observed in the HAART era (20), enthusiasm for some of the currently available therapies is partly tempered by their propensity to induce adverse metabolic effects (7, 23) and/or by the development of viral resistance over time (15). Thus, there is a continuing need for the development of safer and more effective antiretroviral agents.
The complexity of HAART regimens, together with the confounding effects of disease-related comorbidities, has challenged efforts to determine the association of individual drugs with the various adverse effects observed in treated HIV-infected patients. However, significant progress has been made in understanding the acute effects of PIs on insulin sensitivity through both in vitro and in vivo studies. The identification of the insulin-responsive facilitative glucose transporter GLUT4 as a direct molecular target for acute effects of many of the first-generation PIs (17) has provided insight into the specific structural features of these antivirals that mediate this adverse effect (8). In addition to direct inhibition of GLUT4, it has been proposed that PIs may affect insulin sensitivity via modulating the insulin signaling pathways, including IRS-1 and -2 (24) and AKT (1). In contrast to the direct effects on GLUT4, insulin signaling alterations have usually been observed following chronic drug exposure. The relevance of the in vitro studies to acute changes in whole-body glucose disposal has been established by studying the acute effects of PIs in healthy HIV-negative volunteers (12-14). These effects directly correlate with the ability of PIs to influence peripheral glucose disposal under euglycemic-hyperinsulinemic clamp conditions in a healthy rodent model system (25). In this model, indinavir, ritonavir, and lopinavir have all been shown to acutely reduce peripheral glucose disposal. In contrast, atazanavir, which does not adversely alter GLUT4 activity in vitro (25), does not appear to acutely induce insulin resistance in the rodent model or in humans (18, 19). The use of atazanavir, however, is limited primarily to PI-naïve patients due to substantial cross-resistance with multiple other PIs (5).
Here we report on an in vitro and in vivo assessment of the ability of a novel PI, GS-8374, to affect glucose disposal and lipid metabolism. GS-8374 is a synthetic, peptidomimetic molecule containing a unique diethylphosphonate motif (Fig. 1). In a separate virologic profiling, we have shown that GS-8374 is a potent and selective PI and that the phosphonate moiety favorably affects its resistance profile (2, 3). In the present study, we have investigated the effects of GS-8374 on cultured differentiated adipocytes in terms of both lipid accumulation and insulin-stimulated glucose uptake in comparison to the effects of other PIs frequently used in the clinic. We have also studied the acute effects of GS-8374 and selected control PIs on peripheral insulin sensitivity in a healthy rodent model system.
FIG. 1.
Structures of GS-8374, a novel HIV-1 protease inhibitor containing a diethylphosphonate motif, and comparative PIs used in the glucose and lipid metabolism studies.
MATERIALS AND METHODS
Materials.
2-Deoxy-1-[3H]glucose was purchased from Sigma (St. Louis, MO). Reference standards for lopinavir, ritonavir, and atazanavir were obtained from the U.S. National Institutes of Health (NIH) AIDS Reference and Reagents Program (Germantown, MD). GS-8374 was synthesized at Gilead Sciences (Foster City, CA). PIs used in the in vitro studies have been isolated from their commercial formulations. Insulin (Humulin) was purchased from Eli Lilly (Indianapolis, IN). Primary human adipocytes and AdipoRed reagent were obtained from Cambrex (East Rutherford, NJ). OP9 stromal cells were obtained from ATCC (Manassas, VA). Male Wistar rats (weight, 125 to 150 g) were purchased from Charles River (Wilmington, MA). MicroRenathane tubing for venous catheters was obtained from Braintree Scientific (Braintree, MA). PE-50 tubing for arterial catheters was obtained from Becton Dickinson (Franklin Lakes, NJ). Blood glucose levels were determined using a Glucometer Elite XL device (Bayer, Tarrytown, NY). The assay kit for rat insulin determinations was purchased from Linco (St. Charles, MO).
Lipid accumulation in primary human adipocytes.
Human preadipocytes isolated from subcutaneous adipose tissue (Cambrex) were grown in a preadipocyte growth medium (PGM; PT 800; Cambrex) supplemented with 10% fetal bovine serum (FBS) for a maximum of two passages, according to the vendor's protocol. Preadipocytes were seeded in PGM into 96-well plates at a density of 10,000 cells per well. Cell differentiation was induced 24 h later by switching the cells into differentiation medium (PGM supplemented with 10 μg/ml insulin, 1 μM dexamethasone, 200 μM indomethacin, 500 μM isobutyl-methylxanthine). Drugs to be tested were added at the same time. The cells were maintained in the presence of drugs for 8 days, with the drug-containing medium being replaced after 4 days. At the end of the incubation period, the cytotoxicity of the drugs used was evaluated by visual inspection of the cells under a light microscope. No changes in cell morphology or density were observed for any of the tested concentrations. The cells were then washed with phosphate-buffered saline (PBS), and intracellular lipid accumulation was measured using Nile Red dye, specific for fluorescent staining of lipids (AdipoRed; Cambrex). After 10 min, fluorescence (excitation at 485 nm, emission at 572 nm) was measured on a fluorescence plate reader, and the effective concentration of each drug reducing the fluorescence signal from Nile Red by 50% (EC50) was calculated (100% and 0% were defined from the fluorescence signals in vehicle-treated cells and undifferentiated cells, respectively).
Glucose uptake in cultured mouse adipocytes.
Mouse OP9 preadipocytes (catalog no. CRL 2749; ATCC) were maintained in basal medium (BM; minimal essential alpha medium supplemented with 1.5 g/liter sodium bicarbonate, 20% FBS, and 1% antibiotic solution). OP9 cells were passaged twice a week and kept below 70% confluence. For testing of PIs, the cells were seeded in basal medium into 24-well plates at a density of 70,000 cells per well. Cell differentiation was induced 24 h later by switching the cells into a differentiation medium (KnockOut SR; Invitrogen) supplemented with 5 ng/ml of bone morphogenic protein-4 (BMP-4; R&D Systems, Minneapolis, MN). After 4 days, the differentiating cells were switched back to regular growth medium and incubated for an additional 3 days, at which point the degree of cell differentiation was visually assessed by the formation of lipid droplets in the cytoplasm. These conditions were found to provide the lowest background uptake and least variability, thus being optimal for the detection of specific glucose uptake upon insulin stimulation. At the time of insulin response testing, the medium was removed; cells were washed and exposed for 60 min to 10 μM tested drugs in a Krebs Ringer HEPES buffer (121 mM NaCl, 4.9 mM KCl, 1.2 mM MgSO4, 0.33 mM CaCl2, 12 mM HEPES) with or without 1 μM insulin. Cells were then exposed to 2-deoxy-1-[3H]glucose for 10 min, washed three times with ice-cold PBS, and lysed in PBS with 0.3% Triton X-100. Samples were analyzed by liquid scintillation counting. Inhibition of insulin-stimulated glucose uptake was calculated as a percentage of the difference between the basal and insulin-stimulated deoxy-d-glucose uptake relative to that for the untreated control.
Animal procedures.
All animal procedures were approved by the Animal Studies Committee at Washington University School of Medicine. Male Wistar rats were housed in the animal facility at Washington University and maintained in a controlled room with a 12-h light and 12-h dark cycle. The animals were supplied with a standard rat chow diet and water ad libitum. Catheters were inserted into the left internal carotid artery and right jugular vein while the rats were under methohexital anesthesia as previously described (9). Rats were allowed to recover from the stress of surgery at least 5 days before experiments were performed. All animals weighed between 205 and 220 g at the time that experiments were performed. Rats were fasted overnight before each experiment.
Drug preparation and determination of plasma PI levels.
Stock solutions of ritonavir, atazanavir, and GS-8374 were prepared in 50% ethanol-50% normal saline. Lopinavir stock solutions were prepared in 50% polyethylene glycol. Serum PI levels were determined by the high-performance liquid chromatography (HPLC) method of Foisy and Sommadossi (6) using a Waters 626 HPLC system with a Microsorb C8 column. Samples were run in 50 μl serum. Standard curves were generated by adding serially diluted PIs directly to control rat serum.
Euglycemic-hyperinsulinemic clamp experiments.
Catheters were flushed with normal saline, and heparin (40 units/kg) was administered to maintain catheter patency. After determination of fasting blood glucose levels, a constant infusion of the tested PIs or vehicle alone was started through the venous catheter at a rate of 10 μl/min using a Harvard 11 apparatus pump and was continued for the duration of the clamp experiment. The total amount of ethanol or polyethylene glycol infused was normalized to 5 μl/kg of body weight/min and 2.8 μl/kg/min, respectively. After 30 min of drug infusion, insulin (10 mU/kg/min) in normal saline containing 0.3% bovine serum albumin (BSA) was infused through the venous catheter. At 10-min intervals, ∼500 μl of blood was removed from the arterial catheter into a syringe. Blood (5 μl) was then sampled directly from the catheter to determine blood glucose levels. The dead-space blood was then reinfused into the animal. Next, dextrose (50%) was infused through the venous catheter at a rate sufficient to maintain a plasma glucose level of 100 to 110 mg/dl. Insulin sensitivity was determined by the average infusion rate during the final 30 min of each 90-min clamp experiment.
Statistical analysis.
Data are presented as means ± standard deviations. Statistical differences among groups in the in vivo studies were tested using analysis of variance (ANOVA) with the post hoc Bonferroni test. Differences were assumed to be significant in each case if P was <0.05, unless indicated otherwise; nonsignificant differences are not indicated.
RESULTS
In vitro effect of PIs on lipid accumulation in adipocytes.
Previously, we have demonstrated that the effect of PIs on adipocyte function in vitro accurately predicts their metabolic actions in vivo (25). To determine the effect of GS-8374 on lipid homeostasis, primary human adipocytes were cultured for 8 days in the presence or absence of selected PIs, after which lipid accumulation was assessed (see Materials and Methods). As shown in Table 1, GS-8374, along with atazanavir, amprenavir, indinavir, and darunavir, had a negligible effect on lipid accumulation compared to that for the vehicle-treated control, with the predicted EC50 being greater than 30 μM, a value significantly higher than the therapeutic concentrations of the drugs (21). Conversely, nelfinavir, saquinavir, lopinavir, and ritonavir all significantly inhibited lipid accumulation, with calculated EC50s being 8 to 17 μM, representing levels approaching their maximum plasma therapeutic concentration range.
TABLE 1.
Effects of GS-8374 and marketed PIs on lipid accumulation in primary human adipocytes in vitro
| Protease inhibitor | EC50 (μM) for lipid accumulationa |
|---|---|
| GS-8374 | >30 |
| Darunavir | >30 |
| Lopinavir | 16 ± 5 |
| Atazanavir | >30 |
| Ritonavir | 17 ± 8 |
| Amprenavir | >30 |
| Indinavir | >30 |
| Nelfinavir | 8 ± 3 |
Data shown represent means ± standard deviations from at least four independent experiments. DMSO was used as a vehicle control; no effect on lipid accumulation was observed in the presence of DMSO.
In vitro effect of PIs on insulin-stimulated glucose uptake.
Next, the effects of GS-8374 and selected PIs on glucose uptake were assessed in cultured mouse OP9 differentiated adipocytes. In general, there was a trend for a correlation between the effects of the respective PI on lipid accumulation and glucose uptake (Table 2). Those PIs that had negligible effects on lipid accumulation in primary human adipocytes (GS-8374, darunavir, atazanavir, and amprenavir; EC50s > 30 μM) also did not appreciably block glucose uptake in the OP9 adipocytes (<8.1% inhibition at 10 μM versus that for the vehicle-treated control). Notably, indinavir showed no effect on lipid accumulation but inhibited the glucose uptake, thus being an exception to this trend. However, it should be noted that the effect of indinavir on the insulin-stimulated glucose uptake was somewhat variable, which is in contrast to the reproducible results obtained with all the other PIs. Conversely, PIs such as ritonavir, lopinavir, and saquinavir that showed a measurable inhibition of lipid accumulation (EC50s < 20 μM) also blocked glucose uptake to a greater extent. These data suggest that for some PIs the effect on glucose uptake may in part contribute to reduced lipid accumulation. Notably, GS-8374 had no discernible effect on glucose uptake relative to that for the control, a result similar to the results for atazanavir, amprenavir, and darunavir. On the basis of our previous biochemical studies of PI action, we conclude that the lack of an effect of GS-8374 on acute glucose uptake reflects its inability to directly target and block the insulin-sensitive GLUT4 transporters in the cultured adipocytes.
TABLE 2.
Effects of GS-8374 and marketed PIs on insulin-stimulated glucose uptake in mouse adipocytes in vitro
| Protease inhibitor | % inhibition of glucose uptake at 10 μMa |
|---|---|
| GS-8374 | −0.1 ± 0.1 |
| Darunavir | −0.7 ± 1.2 |
| Lopinavir | 44.0 ± 1.7 |
| Atazanavir | 0.3 ± 0.6 |
| Ritonavir | 60.7 ± 1.2 |
| Amprenavir | 8.1 ± 1.6 |
| Indinavir | 14.0 ± 12.5 |
| Nelfinavir | 28.4 ± 8.2 |
Data shown represent means ± standard deviations from three independent experiments. DMSO was used as a vehicle control; no effect on glucose uptake was observed in the presence of DMSO.
Acute in vivo effects of PIs on peripheral glucose disposal.
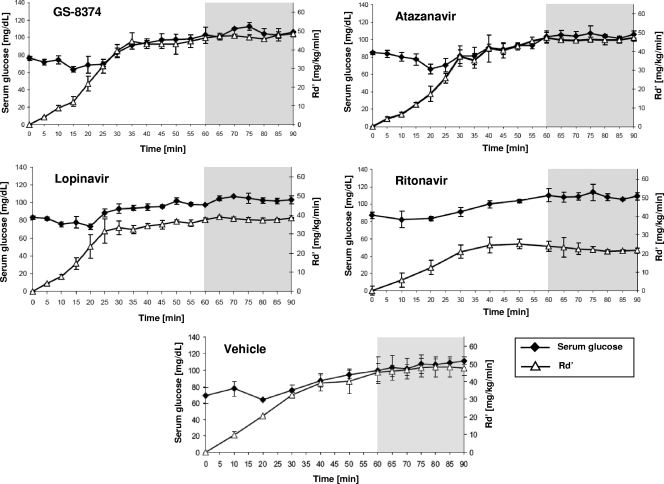
The effects of PIs on glucose uptake in vitro should also be evident in vivo and reflected by a concomitant change in peripheral insulin sensitivity. To assess the effect of GS-8374 on glucose disposal, euglycemic-hyperinsulinemic clamps (4) were performed on healthy, PI-naïve rats. In these experiments, insulin (10 mU/kg/min) and PIs were administered by constant intravenous infusion in order to maintain serum PI levels of 8 to 10 μM. This is within the therapeutic range for peak serum drug levels achieved in treated human patients for currently marketed PIs (21). Together with constant insulin administration, glucose was continuously infused at a variable rate in order to maintain the serum glucose level at 110 mg/dl (Table 3). Under steady-state conditions, peripheral insulin sensitivity directly correlates with the rate of glucose infusion necessary to maintain the target serum glucose level. As a negative control, rat clamps were performed using atazanavir, as this PI has previously been shown not to affect peripheral insulin sensitivity in this rodent model system (25). Conversely, ritonavir, which does alter insulin sensitivity, was used as a positive control. Serum insulin levels achieved during the euglycemic clamp experiments, as well as serum glucose levels, were not significantly different from those in vehicle-treated animals (Table 3). As shown in Fig. 2, the rates of glucose infusion in GS-8374-treated rats over the 90-min experiment approximated those observed for vehicle- or atazanavir-treated rats. Conversely, the infusion rates were significantly lower in the ritonavir- and lopinavir-treated rats, consistent with a drug-induced reduction in peripheral insulin sensitivity. The average glucose disposal rates (Rd′; mg/kg/min) under steady-state conditions (i.e., during the final 30 min of the clamp) are summarized in Table 3. Importantly, the average serum drug levels were similar for all three drugs (Table 3). In summary, the clamp data corroborate the in vitro data and indicate that GS-8374 does not alter in vivo glucose disposal in peripheral tissues.
TABLE 3.
Comparison of glucose disposal in rats infused with GS-8374 or control PIs
| Treatment | Glucose concn in blooda (mg/dl) | Insulin concn (ng/ml) | Avg plasma exposurea (μM) | Glucose disposal rateb (mg/kg/min) | Glucose disposal rate (% of control) |
|---|---|---|---|---|---|
| Vehicle | 105.3 ± 7.4 | 4.62 ± 1.03 | NA | 47.1 ± 1.6 | 100 |
| GS-8374 | 105.6 ± 1.3 | 4.07 ± 2.19 | 7.7 ± 0.4 | 47.0 ± 0.2 | 99.7 |
| Atazanavir | 104.4 ± 3.3 | 5.21 ± 0.69 | 9.4 ± 1.5 | 46.6 ± 0.9 | 99.1 |
| Lopinavir | 103.3 ± 1.7 | 4.12 ± 2.40 | 10.9 ± 1.1 | 38.0 ± 0.6c | 80.8 |
| Ritonavir | 109.2 ± 1.6 | 3.65 ± 0.87 | 8.7 ± 0.4 | 22.3 ± 1.6c | 47.4 |
All values represent the means ± standard deviations from three and four different vehicle- and drug-treated animals, respectively. NA, not applicable.
Rate of glucose disposal (mg/kg/min) was averaged from measurements from the last 30 min of each experiment.
Statistically significant difference from vehicle-treated animals, as determined by ANOVA with the Bonferonni post hoc test (P < 0.01).
FIG. 2.
Acute effects of GS-8374 and other PIs on peripheral insulin sensitivity. Catheters were inserted into the carotid arteries and jugular veins of 200- to 300-g male Wistar rats at least 4 days prior to experiments. Euglycemic-hyperinsulinemic clamps were performed using 40 mU/kg/min regular human insulin. The rate of infusion of 50% dextrose was adjusted to maintain plasma glucose levels at 110 mg/dl (Table 2). Insulin sensitivity was determined by the average rate of glucose infusion (Rd′) over the final 30 min (steady-state phase) of each 2-hour experiment (gray-shaded region). Data represent means ± standard deviations, with n = 3 and 4 for vehicle and each of the drug-treated animals, respectively.
DISCUSSION
The long-term successful use of PIs as a part of increasingly effective antiretroviral combination therapy requires potent drugs with favorable viral resistance and metabolic profiles. In virological studies presented in parallel with this report, we have characterized the unique viral resistance properties of a novel, potent PI, GS-8374 (2). In the present study, we demonstrate that GS-8374 does not adversely affect glucose or lipid metabolism either in vitro or in vivo. This conclusion is based on several lines of evidence. First, treatment of human primary adipocytes for 8 days with GS-8374 or atazanavir at concentrations up to 30 μM showed no effect on normal lipid accumulation. Under the same conditions, ritonavir and lopinavir affected lipid accumulation with EC50s of 17 and 16 μM, respectively. Second, acute treatment of differentiated OP9 mouse adipocytes with GS-8374 or atazanavir showed no effect on insulin-stimulated 2-deoxyglucose uptake, whereas ritonavir and lopinavir caused 60 and 44% inhibition, respectively, under the same assay conditions. Finally, in a rat model system, sustained serum levels of GS-8374 reaching approximately 8 μM had no acute effect on peripheral glucose disposal compared to the effect of vehicle or atazanavir treatment. Consistent with previous studies (25), comparable serum levels of ritonavir or lopinavir produced an acute reduction of glucose disposal. We hypothesize that, similar to atazanavir (5, 25), the lack of acute effects of GS-8374 on insulin sensitivity in vivo reflects its inability to directly target and block insulin-sensitive GLUT4 in skeletal muscle and fat tissues. On the basis of the antiviral potency, favorable resistance profile, and moderate serum protein binding (2), we expect that GS-8374 serum levels lower than those achieved in the in vivo insulin sensitivity study would be sufficient for a potent clinical antiviral effect in both treatment-naïve and PI-experienced patients.
Our earlier demonstration that aromatic peptides mimicking the core peptidic structure found in most PIs also block glucose uptake suggested that the aromatic peptide backbone is important for the ability of PIs to interact with GLUT4 (8). Furthermore, the demonstration that the dihydropyrone-based PI tipranavir does not acutely influence peripheral glucose disposal further supported the importance of the peptidomimetic structure in mediating the inhibition of GLUT4 activity (10). PIs such as indinavir and ritonavir have been shown to directly affect GLUT4 activity through noncompetitive inhibition (16, 17). In contrast, atazanavir has been shown to be devoid of direct inhibitory effects on GLUT4 transport activity, possibly due to steric interference preventing GLUT4 binding because of the presence of a pyridine ring and/or a reduced overall hydrophobicity of atazanavir compared to that of most of the first-generation PIs. Interestingly, our data demonstrated that darunavir, despite the presence of the signature aromatic peptidomimetic motif in its structure (11), does not appear to adversely affect the metabolic status of differentiated adipocytes. This indicates that more complex interactions are likely to affect the binding of peptidomimetic PIs to GLUT4. Importantly, the unique diethylphosphonate moiety present within GS-8374, which itself is a close structural analog of darunavir, does not appear to increase its interaction with GLUT4 relative to that of darunavir.
While the development of potent PIs has been strongly influenced by rational structure-based design (22), the binding site of PIs to GLUT4 remains to be established, and an X-ray crystal structure for this transporter is not yet available. Thus, molecular modeling similar to that used in addressing the problem of viral resistance has not been possible for predicting the effects of PIs on glucose metabolism and homeostasis. As the development of newer generations of PIs and other antiretroviral agents continues, the use of effective strategies to screen for potential drug-related toxicities will become increasingly important.
In summary, the current studies suggest that GS-8374 has a low potential for metabolic adverse effects compared to the potentials of some other PIs and confirm that these effects are not inherently linked to the antiretroviral activity of PIs. Importantly, these data provide further support for the use of in vitro cultured adipocyte and in vivo rodent clamp systems in the preclinical evaluation of the safety of novel PIs. Further progression of GS-8374 development should allow the first prospective analysis and confirmation of this approach.
Acknowledgments
This research was supported in part by NIH grant DK64572.
Footnotes
Published ahead of print on 18 January 2011.
REFERENCES
- 1.Ben-Romano, R., et al. 2004. Nelfinavir-induced insulin resistance is associated with impaired plasma membrane recruitment of the PI 3-kinase effectors Akt/PKB and PKC-zeta. Diabetologia 47:1107-1117. [DOI] [PubMed] [Google Scholar]
- 2.Callebaut, C., et al. 2011. In vitro characterization of GS-8374, a novel phosphonate-containing inhibitor of HIV-1 protease with a favorable resistance profile. Antimicrob. Agents Chemother. 55:1366-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cihlar, T., et al. 2006. Suppression of HIV-1 protease inhibitor resistance by phosphonate-mediated solvent anchoring. J. Mol. Biol. 363:635-647. [DOI] [PubMed] [Google Scholar]
- 4.Defronzo, R., J. Tobin, and R. Andres. 1979. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am. J. Physiol. 237:E214-E223. [DOI] [PubMed] [Google Scholar]
- 5.Fernández-Montero, J., P. Barreiro, and V. Soriano. 2009. HIV protease inhibitors: recent clinical trials and recommendations on use. Expert Opin. Pharmacother. 10:1615-1629. [DOI] [PubMed] [Google Scholar]
- 6.Foisy, M., and J. Sommadossi. 1999. Rapid quantification of indinavir in human plasma by high-performance liquid chromatography with ultraviolet detection. J. Chromatogr. 721:239-247. [DOI] [PubMed] [Google Scholar]
- 7.Hadigan, C., et al. 2001. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin. Infect. Dis. 32:130-139. [DOI] [PubMed] [Google Scholar]
- 8.Hertel, J., H. Struthers, C. Baird Horj, and P. Hruz. 2004. A structural basis for the acute effects of HIV protease inhibitors on GLUT4 intrinsic activity. J. Biol. Chem. 279:55147-55152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hruz, P., H. Murata, H. Qiu, and M. Mueckler. 2002. Indinavir induces acute and reversible peripheral insulin resistance in rats. Diabetes 51:937-942. [DOI] [PubMed] [Google Scholar]
- 10.Hruz, P., and Q. Yan. 2006. Tipranavir without ritonavir does not acutely induce peripheral insulin resistance in a rodent mode. J. Acquir. Immune Defic. Syndr. 43:624-625. [DOI] [PubMed] [Google Scholar]
- 11.Koh, Y., et al. 2003. Novel bis-tetrahydrofuranylurethane-containing nonpeptidic protease inhibitor (PI) UIC-94017 (TMC114) with potent activity against multi-PI-resistant human immunodeficiency virus in vitro. Antimicrob. Agents Chemother. 47:3123-3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee, G., et al. 2006. Single-dose lopinavir-ritonavir acutely inhibits insulin-mediated glucose disposal in healthy volunteers. Clin. Infect. Dis. 43:658-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee, G., et al. 2004. Ritonavir acutely induces insulin resistance in healthy normal human volunteers. Antivir. Ther. 9:L6. [Google Scholar]
- 14.Lee, G., et al. 2007. Effects of ritonavir and amprenavir on insulin sensitivity in healthy volunteers. AIDS 21:2183-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez-Cajas, J., and M. A. Wainberg. 2007. Protease inhibitor resistance in HIV-infected patients: molecular and clinical perspectives. Antiviral Res. 76:203-221. [DOI] [PubMed] [Google Scholar]
- 16.Murata, H., P. Hruz, and M. Mueckler. 2002. Indinavir inhibits the glucose transporter isoform Glut4 at physiologic concentrations. AIDS 16:859-863. [DOI] [PubMed] [Google Scholar]
- 17.Murata, H., P. W. Kruz, and M. Mueckler. 2000. The mechanism of insulin resistance caused by HIV protease inhibitor therapy. J. Biol. Chem. 275:20251-20254. [DOI] [PubMed] [Google Scholar]
- 18.Noor, M. A., O. P. Flint, J. F. Maa, and R. A. Parker. 2006. Effects of atazanavir/ritonavir and lopinavir/ritonavir on glucose uptake and insulin sensitivity: demonstrable differences in vitro and clinically. AIDS 20:1813-1821. [DOI] [PubMed] [Google Scholar]
- 19.Noor, M. A., J. F. Maa, M. F. Giordano, and S. L. Hodder. 2004. Abstr. XV Int. AIDS Conf., abstr. WePeB5874.
- 20.Palella, F. J., Jr, et al. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N. Engl. J. Med. 338:853-860. [DOI] [PubMed] [Google Scholar]
- 21.Physicians’ Desk Reference. 2008. Physicians’ desk reference. Medical Economics Company, Montvale, NJ.
- 22.Reddy, M., and M. Erion. 1998. Structure-based drug design approaches for predicting binding affinities of HIV1 protease inhibitors. J. Enzyme Inhib. 14:1-14. [DOI] [PubMed] [Google Scholar]
- 23.Sadler, B. M., et al. 2001. Pharmacokinetics and safety of amprenavir and ritonavir following multiple-dose, co-administration to healthy volunteers. AIDS 15:1009-1018. [DOI] [PubMed] [Google Scholar]
- 24.Schütt, M., J. Zhou, M. Meier, and H. Klein. 2004. Long-term effects of HIV-1 protease inhibitors on insulin secretion and insulin signaling in INS-1 beta cells. J. Endocrinol. 183:445-454. [DOI] [PubMed] [Google Scholar]
- 25.Yan, Q., and P. Hruz. 2005. Direct comparison of the acute in vivo effects of HIV protease inhibitors on peripheral glucose disposal. J. Acquir. Immune Defic. Syndr. 40:398-403. [DOI] [PMC free article] [PubMed] [Google Scholar]