Abstract
Old World cutaneous leishmaniasis is a widespread and potentially disfiguring protozoal infection that is endemic in the Mediterranean basin, Africa, and parts of Asia. Human infection is caused by several species of Leishmania parasites, such as Leishmania infantum. Available systemic and topical treatments vary in efficacy and are often unjustified due to their toxicity. We report on a case that was treated with posaconazole, a drug typically considered an antifungal agent but which also targets specific metabolic pathways of the parasite.
Leishmania infantum is a known cause of Old World cutaneous leishmaniasis (OWCL). In acute OWCL, the lesion is usually limited to one or a few papules, plaques, or nodules that evolve over a period of months to the ulcerous form (4, 12). Treatment options for OWCL include topical paromomycin ointment, local infiltration with antimonials, thermotherapy, or miltefosine (5). However, except for thermotherapy, these treatments are not U.S. Food and Drug Administration (FDA) approved. Use of these therapies is also limited by variable efficacy and local and systemic side effects that affect tolerability and compliance (4, 5). We describe a patient with OWCL who is successfully treated with posaconazole, an oral drug typically considered an antifungal agent but which also targets specific metabolic pathways of the parasite.
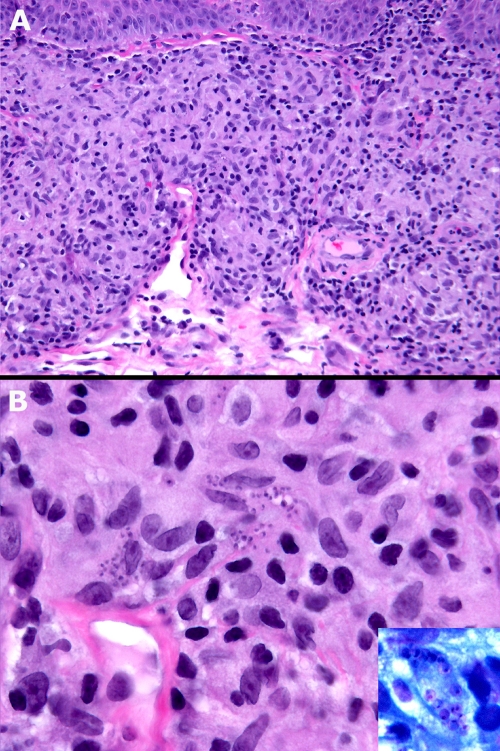
We report a 36-year-old Caucasian woman with a small, papular, and very itchy right wrist lesion that had been present for 1 month. Originally from Australia, she had been living in New York City for 12 years. She had traveled to Malta and Dubai 6 months earlier but was well during both trips. At presentation, she had no systemic complaints and appeared healthy. There was a small 1-cm by 1-cm, erythematous, keloid-like papule at the ulnar surface of the right wrist but no epitrochlear or axillary lymphadenopathy. The results of a complete blood count and chemistry and hepatic transaminase tests were within normal limits before and throughout the treatment period. Histologic examination of a shaved biopsy specimen of the lesion demonstrated Leishmania parasites (Fig. 1 A and B, inset).
FIG. 1.
(A) Diffuse mixed granulomatous dermal infiltrate, mainly lymphoid-histiocytic (hematoxylin and eosin stain; magnification, ×20). (B) Clusters of basophilic microorganisms (amastigotes) within the histiocytes (hematoxilin and eosin stain; magnification, ×60). Inset, amastigotes within a vacuolated histiocyte. The nuclei of L. infantum are clearly visible (Giemsa stain; magnification, ×100).
Biopsy specimens were also preserved in 70% ethanol for molecular testing. DNA was extracted using the phenol-chloroform method, suspended in Tris-EDTA (TE) buffer, and stored at 4°C until use. The Leishmania parasites were identified as L. infantum by the ribosomal DNA internal transcribed spacer 1 (ITS1) PCR using the primers LITSR and L5.8 (Fig. 2). The ITS1 PCR product (∼320 bp) was sequenced in the Department of Genetics of the Humboldt University of Berlin by employing the same primers used for PCR. The sequences obtained were processed and aligned against those of different Leishmania species deposited in GenBank, using the multiple alignment program BioEdit, and edited manually. The ITS1 sequence submitted to GenBank under accession number FR675940 demonstrated that the OWCL in our patient was due to L. infantum infection. Microsatellite analysis failed to reveal strain-specific results due to insufficient DNA source.
FIG. 2.
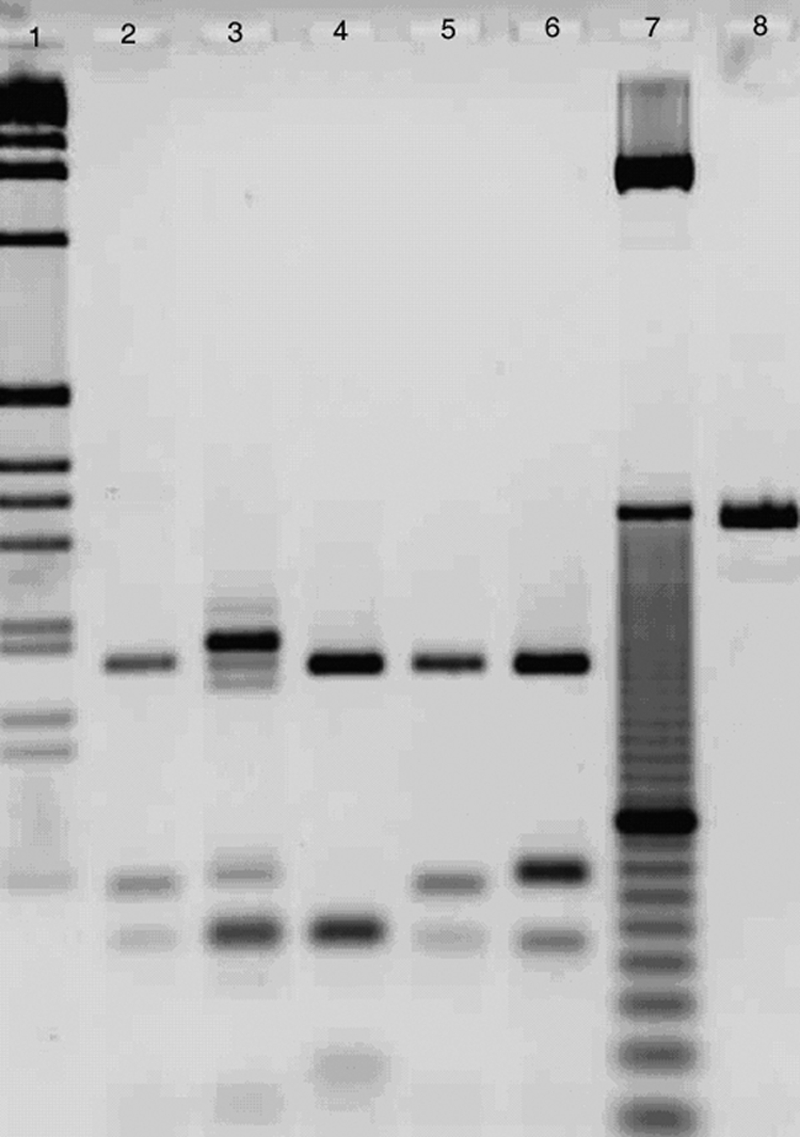
Species identification by ITS1 PCR-restriction fragment length polymorphism analysis with HaeIII enzyme digestion. Lanes: 1 and 7, 1-kb and 10-bp DNA ladders, respectively; 8, undigested ITS1 PCR product; 2, strain MHOM/US/2010/SLRCHCL isolated from the patient with CL; 3 to 6, reference strains belonging to different Leishmania species (L. aethiopica [MHOM/ET/1994/Gere], L. tropica [MHOM/PS/2001/ISL590], L. infantum [MHOM/ES/1993/PM1], and L. donovani [MHOM/SD/1993/LEM338], respectively).
Our patient was reluctant to undergo treatment requiring daily parenteral administration and was concerned about potential local and systemic adverse effects. Consequently, we offered her a trial of posaconazole, 400 mg orally twice daily, assuming that a prolonged course might be required but without a preset duration. However, the lesion resolved rapidly and the patient discontinued therapy after 14 days, requesting observation before additional therapy was prescribed. Despite the short course of treatment, there have been no signs of primary recurrence or visceral involvement for more than 15 months following completion of treatment.
Human CL includes a spectrum of diseases ranging from self-limiting (localized cutaneous) to severely disfiguring (diffuse cutaneous) or destructive and debilitating forms, such as borderline disseminated and mucocutaneous leishmaniasis (12), depending on the parasite species involved and the patient's immune response (4, 12, 13). It is endemic in 88 countries, with an overall prevalence of 12 million cases and approximately 2 million new cases reported annually (4, 13), and is one of the 10 leading infections in travelers returning from tropical countries (7). Due to the recent armed conflicts in the Middle East, over 500 cases of CL and five cases of visceral leishmaniasis (VL) have been reported among U.S. military personnel (13). In the New World, CL usually involves the Leishmania mexicana species complex and parasites belonging to the Viannia subgenus; OWCL classically involves Leishmania major, Leishmania tropica, Leishmania donovani, Leishmania aethiopica, and L. infantum (4, 12, 13). In L. infantum-associated disease, relapse with visceral involvement occurs frequently in AIDS patients and in immunocompetent patients treated with antimonials (7), typically manifesting several months after cure of the primary lesion (4).
To date, there have been no randomized, double-blind, placebo-controlled clinical studies for treatment of L. infantum (4). In addition, resistance to the pentavalent antimonials and to second-line agents such as amphotericin and pentamidine is increasing throughout regions where the disease is endemic (4, 10, 12, 13). Failure of pentavalent antimonial treatment was initially described in dogs infected with L. infantum (11) and later confirmed in humans (10). Numerous cases of primary and secondary unresponsiveness have now been reported (6).
Ergosterol is the major sterol in trypanosomatid parasites as well as in fungi; thus, antifungal agents that target the biosynthesis of ergosterol also have activity against infections caused by Leishmania species and Trypanosoma cruzi. In CL, the efficacy of azole treatment varies, depending on the Leishmania species involved (16), reflecting biochemical and molecular differences among species (9). The imidazoles and the structurally related triazoles have potent but selective activity in vitro and in vivo against Leishmania (1, 16). The efficacy of these compounds ranges from high activity against L. mexicana and L. major infections to little or none against Leishmania braziliensis and L. donovani infections (15, 16). As a consequence, success rates in patients are variable and in vitro studies have sometimes produced contradictory data (2, 15).
In contrast, the new antifungal triazole posaconazole (Noxafil; Schering-Plough, Kenilworth, NJ) has been shown to have broad activity against trypanosomatid parasites, including several Leishmania species and T. cruzi (1, 9). Posaconazole has been effective in both animal models and humans affected by Chagas' disease (14, 17). In vitro studies have shown that posaconazole is 30 to 100 times more potent than ketoconazole as an antiproliferative agent and sterol biosynthesis inhibitor in epimastigotes and amastigotes of T. cruzi (16) and retains activity against chemoresistant strains (3). Its efficacy against Leishmania amazonensis and L. donovani has been demonstrated in experimental murine models of CL and VL (1). The enhanced potency of posaconazole in vivo has been postulated to be due to structural and conformational properties influencing its pharmacokinetic efficacy (8), excellent oral bioavailability, and ability to disrupt intracellular calcium homeostasis, probably due to structural alterations of the parasite's mitochondrion (3).
To our knowledge, this is the first reported case of a patient with CL successfully treated with the triazole antifungal posaconazole. The rapid resolution of the lesion without evidence of relapse in a period of more than 1 year suggests that the orally administered triazole antifungal posaconazole is a promising therapeutic alternative for the treatment of OWCL and might constitute a rational therapeutic approach for the treatment of viscerotropic strains of L. infantum causing VL.
Nucleotide sequence accession number.
The ITS1 sequence described here was submitted to GenBank under accession number FR675940.
Footnotes
Published ahead of print on 31 January 2011.
REFERENCES
- 1.Al-Abdely, H. M., J. R. Graybill, D. Loebenberg, and P. C. Melby. 1999. Efficacy of the triazole SCH 56592 against Leishmania amazonensis and Leishmania donovani in experimental murine cutaneous and visceral leishmaniases. Antimicrob. Agents Chemother. 43:2910-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beach, D. H., L. J. Goad, and G. G. Holz, Jr. 1988. Effects of antimycotic azoles on growth and sterol biosynthesis of Leishmania promastigotes. Mol. Biochem. Parasitol. 31:149-162. [DOI] [PubMed] [Google Scholar]
- 3.Benaim, G., et al. 2006. Amiodarone has intrinsic anti-Trypanosoma cruzi activity and acts synergistically with posaconazole. J. Med. Chem. 49:892-899. [DOI] [PubMed] [Google Scholar]
- 4.Blum, J., P. Desjeux, E. Schwartz, B. Beck, and C. Hatz. 2004. Treatment of cutaneous leishmaniasis among travellers. J. Antimicrob. Chemother. 53:158-166. [DOI] [PubMed] [Google Scholar]
- 5.Blum, J. A., and C. F. Hatz. 2009. Treatment of cutaneous leishmaniasis in travelers 2009. J. Travel Med. 16:123-131. [DOI] [PubMed] [Google Scholar]
- 6.Bryceson, A. D. M., et al. 1985. Visceral leishmaniasis unresponsive to antimonial drugs. I. Clinical and immunological studies. Trans. R. Soc. Trop. Med. Hyg. 79:700-704. [DOI] [PubMed] [Google Scholar]
- 7.Caumes, E., et al. 1995. Dermatoses associated with travel to tropical countries: a prospective study of the diagnosis and management of 269 patients presenting to a tropical disease unit. Clin. Infect. Dis. 20:542-548. [DOI] [PubMed] [Google Scholar]
- 8.Chen, C. K., et al. 2010. Structural characterization of CYP51 from Trypanosoma cruzi and Trypanosoma brucei bound to the antifungal drugs posaconazole and fluconazole. PLoS Negl. Trop. Dis. 4:e651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Croft, S. L., S. Sundar, and A. H. Fairlamb. 2006. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 19:111-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faraut-Gambarelli, F., et al. 1997. In vitro and in vivo resistance of Leishmania infantum to meglumine antimoniate: a study of 37 strains collected from patients with visceral leishmaniasis. Antimicrob. Agents Chemother. 41:827-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gramiccia, M., L. Gradoni, and S. Orsini. 1992. Decreased sensitivity to meglumine antimoniate (Glucantime) of Leishmania infantum isolated from dogs after several courses of drug treatment. Ann. Trop. Med. Parasitol. 86:613-620. [DOI] [PubMed] [Google Scholar]
- 12.Machado-Pinto, J., and D. Azulay. 2006. Leishmaniasis, p. 41-48. In S. K. Tyring, O. Lupi, and U. R. Hengge (ed.), Tropical dermatology, 1st ed. Elsevier Churchill Livingstone, Philadelphia, PA.
- 13.Ouellette, M., et al. 2008. Drug resistance in Leishmania, p. 159-176. In P. J. Myler and N. Fasel (ed.), Leishmania after the genome, 1st ed. Caister Academic Press, Norfolk, United Kingdom.
- 14.Pinazo, M., et al. 2010. Successful treatment with posaconazole of a patient with chronic Chagas disease and systemic lupus erythematosus. Am. J. Trop. Med. Hyg. 82:583-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rangel, H., F. Dagger, A. Hernandez, A. Liendo, and J. A. Urbina. 1996. Naturally azole-resistant Leishmania braziliensis promastigotes are rendered susceptible in the presence of terbinafine: comparative study with azole-susceptible Leishmania mexicana promastigotes. Antimicrob. Agents Chemother. 40:2785-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Urbina, J. A. 1997. Lipid biosynthesis pathways as chemotherapeutic targets in kinetoplastid parasites. Parasitology 114:S91-S99. [PubMed] [Google Scholar]
- 17.Urbina, J. A., et al. 1998. Antiproliferative effects and mechanism of action of SCH 56592 against Trypanosoma (Schizotrypanum) cruzi: in vitro and in vivo studies. Antimicrob. Agents Chemother. 42:1771-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]