Abstract
Nonfermentative yeasts, such as Cryptococcus spp., have emerged as fungal pathogens during the last few years. However, standard methods to measure their antifungal susceptibility (antifungal susceptibility testing [AST]) are not completely reliable due to the impaired growth of these yeasts in standard media. In this work, we have compared the growth kinetics and the antifungal susceptibilities of representative species of nonfermentative yeasts such as Cryptococcus neoformans, Cryptococcus gattii, Cryptococcus albidus, Rhodotorula spp., Yarrowia lipolytica, Geotrichum spp., and Trichosporon spp. The effect of the growth medium (RPMI medium versus yeast nitrogen base [YNB]), glucose concentration (0.2% versus 2%), nitrogen source (ammonium sulfate), temperature (30°C versus 35°C), shaking, and inoculum size (103, 104, and 105 cells) were analyzed. The growth rate, lag phase, and maximum optical density were obtained from each growth experiment, and after multivariate analysis, YNB-based media demonstrated a significant improvement in the growth of yeasts. Shaking, an inoculum size of 105 CFU/ml, and incubation at 30°C also improved the growth kinetics of organisms. Supplementation with ammonium sulfate and with 2% glucose did not have any effect on growth. We also tested the antifungal susceptibilities of all the isolates by the reference methods of the CLSI and EUCAST, the EUCAST method with shaking, YNB under static conditions, and YNB with shaking. MIC values obtained under different conditions showed high percentages of agreement and significant correlation coefficient values between them. MIC value determinations according to CLSI and EUCAST standards were rather complicated, since more than half of isolates tested showed a limited growth index, hampering endpoint determinations. We conclude that AST conditions including YNB as an assay medium, agitation of the plates, reading after 48 h of incubation, an inoculum size of 105 CFU/ml, and incubation at 30°C made MIC determinations easier without an overestimation of MIC values.
Fungal pathogens pose an increasing problem in clinical practice, since their impact has significantly risen during the last years due to the increasing number of immunocompromised patients in our society. Classically, the fungi with higher prevalences among patients have been Aspergillus fumigatus and Candida albicans. However, in the last years, it has been observed that many other fungal species can also cause disseminated infection, so they are now considered emerging fungal pathogens. Of particular interest is the group composed of yeasts in which fermentation is absent, also known as nonfermentative yeasts, and their metabolism relies on respiration. The most prevalent species belonging to this group is Cryptococcus neoformans, which causes life-threatening meningitis in immunocompromised patients, especially HIV-positive individuals (2, 12, 22). Cryptococcus gattii has also gained relevance, particularly when it was shown that this yeast was the causative agent of an outbreak on Vancouver Island, British Columbia, Canada (1, 8, 11). Other examples of nonfermentative yeasts with clinical relevance are species from the genera Rhodotorula, Trichosporon, Geotrichum, Yarrowia, and Dipodascus. Many of these species exhibit decreased susceptibility and resistance to most of antifungal agents licensed for clinical therapy (5, 10). For example, most of them belong to the Basidiomycetes, which are intrinsically resistant to echinocandin antifungals (5-7). Moreover, some Trichosporon species, such as Trichosporon asahii, show a markedly reduced susceptibility to amphotericin B (14, 15).
Antifungal susceptibility testing (AST) has become a useful technique for the management of patients infected with pathogenic fungi. Several entities have proposed different methods to measure antimicrobial susceptibility, such as the Clinical and Laboratory Standards Institute (CLSI) (3, 4) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (19, 21). These methods are based on microdilution plates and RPMI medium. In the case of the CLSI method, the medium is supplemented with 0.2% glucose, and an initial inoculum of around 103 CFU per ml is used. In the case of the EUCAST protocol, glucose is added at 2%, and a higher inoculum is used (105 CFU). In both cases, the plates are incubated at 35°C without shaking. Other media based on minimal medium (yeast nitrogen base [YNB]) have also been tested (16, 18). In addition, a number of methods are commercially available, such as Sensititre-YeastOne or Etest strips.
However, susceptibility testing of nonfermentative yeasts has classically presented important technical problems. The lack of fermentation in these yeasts compromises growth in microdilution plates without shaking, especially when done according the protocols suggested by both the CLSI and EUCAST. For this reason, the MIC values obtained with regular standard methods are not completely reliable, and a standardization of the conditions for these groups of yeasts is still required (16).
In the present work, we have compared the growths of representative isolates of nonfermentative yeast species to determine the optimal growth conditions, with the aim of suggesting the best method to measure susceptibility testing. In addition, we have measured susceptibilities to nine different antifungal compounds in different media and under different conditions to test if they have any effect on MIC determinations.
MATERIALS AND METHODS
Yeast strains.
A total of 44 strains were used, which belong to the following species: C. neoformans (10 strains [CNM-CL4860, CNM-CL4959, CNM-CL4974, CNM-CL5507, CNM-CL5066, CNM-CL5154, CNM-CL5316, CNM-CL5387, CNM-CL5632, and CNM-CL5707]), C. gattii (5 strains [CNM-CL4998, CNM-CL5001, CNM-CL5004, CNM-CL5009, and CNM-CL5014]), Cryptococcus albidus (1 strain [CNM-CL6224]), Rhodotorula mucilaginosa (3 strains [CNM-CL5165, CNM-CL5315, and CNM-CL5532]), Rhodotorula glutinis (3 strains [CNM-CL5581, CNM-CL5852, and CNM-CL6144]), Yarrowia lipolytica (3 strains [CNM-CL5152, CNM-CL5187, and CNM-CL5531]), Dipodascus capitatus (4 strains [CNM-CL5573, CNM-CL5708, CNM-CL5725, and CNM-CL5807]), Trichosporon cutaneum (1 strain [CNM-CL4932]), Trichosporon mucoides (4 strains [CNM-CL5050, CNM-CL5113, CNM-CL5213, and CNM-CL5651]), Trichosporon inkin (3 strains [CNM-CL6021, CNM-CL6099, and CNM-CL5844]), Trichosporon ovoides (1 strain [CNM-CL5685]), Trichosporon asahii (3 strains [CNM-CL5793, CNM-CL5891, and CNM-CL6031]), and Geotrichum candidum (3 strains [CNM-CL5185, CNM-CL5342, and CNM-CL5913]). The strains were clinical isolates collected from deep sites and recovered from the collection of the Spanish Mycology Reference Laboratory of the National Centre for Microbiology. As quality control strains, we used C. albicans (ATCC 64548) for growth curves and Candida krusei ATCC 6258 and Candida parapsilosis ATCC 22019 for antifungal susceptibility testing. All the species and strains used in this work have been identified by molecular biology by sequencing of ribosomal internal transcribed spacer (ITS) genomic DNA and biochemical methods (13, 23). In the case of Trichosporon sp. identification, the intergenic spacer (IGS) sequence was sequenced (15).
Growth conditions and plate preparation.
Growth curves were performed with 96-well microdilution plates. A total of 48 different conditions were tested, as shown in Table 1. The assay media used were (i) RPMI medium with 0.2% glucose (Sigma-Aldrich Química SA, Madrid, Spain), (ii) RPMI medium supplemented with 2% glucose (Sigma-Aldrich), (iii) RPMI medium-2% glucose plus 0.5% ammonium sulfate (Merck, Darmstadt, Germany), and (iv) yeast nitrogen base (YNB; Difco, Sparks, MD) with 0.5% glucose, ammonium sulfate as the nitrogen source, and amino acids. For each medium, two different temperatures (30°C or 35°C), two different agitation conditions (shaking at 350 rpm or static), and three different inocula (103, 104, or 105 CFU/ml) were used. All the media were buffered to pH 7 using 165 mM (for RPMI medium) or 50 mM (for YNB) MOPS (morpholinepropanesulfonic acid) buffer (Sigma-Aldrich). All the media were prepared as a 2× stock, and 100 μl was added to each well of the microdilution plates. The plates were kept frozen at −80°C until they were used. To inoculate the plates, the yeast strains were grown in Sabouraud agar for 48 h at 30°C. An inoculum was then prepared in sterile distilled water spectrophotometrically, and suspensions of 106, 105, and 104 CFU/ml were prepared. Next, 100 μl of each inoculum was added to the plates in duplicate. To monitor the growth of the yeast, the plates were incubated in an iEMS Reader MF spectrophotometer (LabSystems; Thermo Fisher Scientific, Madrid, Spain), where the temperature (30°C or 35°C) and shaking could be regulated. The optical density (OD) of each well in the plate was monitored at 540 nm every 60 min during 72 h. The OD data were exported to Prism 4 for Windows software (GraphPad Software Inc., La Jolla, CA), which was used to calculate the latency, growth rate, and maximal OD.
TABLE 1.
Growth parameters for Cryptococcus neoformans under all the conditions used in the study
| Condition | Medium | % glucose | Concn of (NH4)2SO4 (%) | Temp (°C) | Shaking | Inoculum (cells/ml) | 95% CI for final OD | 95% CI for growth rate (OD increments/h) | 95% CI for lag phase (h) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | RPMI | 0.2 | None | 30 | Yes | 103 | 0.32-0.36 | 0.007-0.0086 | 15.1-18.2 |
| 2 | RPMI | 0.2 | None | 30 | Yes | 104 | 0.32-0.35 | 0.0076-0.009 | 9.3-12.67 |
| 3 | RPMI | 0.2 | None | 30 | Yes | 105 | 0.32-0.34 | 0.009-0.011 | 3.5-6.2 |
| 4 | RPMI | 0.2 | None | 30 | No | 103 | 0.26-0.28 | 0.009-0.13 | 11.8-16.2 |
| 5 | RPMI | 0.2 | None | 30 | No | 104 | 0.23-0.25 | 0.007-0.009 | 7.8-10.7 |
| 6 | RPMI | 0.2 | None | 30 | No | 105 | 0.23-0.24 | 0.007-0.009 | 2.1-4.8 |
| 7 | RPMI | 0.2 | None | 35 | Yes | 103 | 0.21-0.24 | 0.007-0.009 | 17.5-22.2 |
| 8 | RPMI | 0.2 | None | 35 | Yes | 104 | 0.23-0.25 | 0.006-0.008 | 12-15.7 |
| 9 | RPMI | 0.2 | None | 35 | Yes | 105 | 0.24-0.25 | 0.008-0.01 | 5.8-9.3 |
| 10 | RPMI | 0.2 | None | 35 | No | 103 | 0.17-0.18 | 0.0066-0.0084 | 11.9-14.7 |
| 11 | RPMI | 0.2 | None | 35 | No | 104 | 0.16-0.17 | 0.0064-0.0082 | 6-8.9 |
| 12 | RPMI | 0.2 | None | 35 | No | 105 | 0.16-0.17 | 0.0054-0.0072 | 0-3.5 |
| 13 | RPMI | 2 | None | 30 | Yes | 103 | 0.33-0.35 | 0.009-0.0105 | 16.2-18.8 |
| 14 | RPMI | 2 | None | 30 | Yes | 104 | 0.35-0.37 | 0.009-0.0106 | 9.7-12.4 |
| 15 | RPMI | 2 | None | 30 | Yes | 105 | 0.35-0.37 | 0.0089-0.0105 | 3.7-6.64 |
| 16 | RPMI | 2 | None | 30 | No | 103 | 0.24-0.25 | 0.0078-0.0093 | 13.3-15.6 |
| 17 | RPMI | 2 | None | 30 | No | 104 | 0.22-0.24 | 0.007-0.0086 | 7.3-10 |
| 18 | RPMI | 2 | None | 30 | No | 105 | 0.25-0.27 | 0.0069-0.009 | 0.38-4.6 |
| 19 | RPMI | 2 | None | 35 | Yes | 103 | 0.26-0.3 | 0.0065-0.0085 | 16-20.3 |
| 20 | RPMI | 2 | None | 35 | Yes | 104 | 0.28-0.31 | 0.0074-0.0095 | 10.5-14.5 |
| 21 | RPMI | 2 | None | 35 | Yes | 105 | 0.33-0.35 | 0.0086-0.011 | 3.64-7.7 |
| 22 | RPMI | 2 | None | 35 | No | 103 | 0.20-0.22 | 0.0073-0.0091 | 11.9-14.7 |
| 23 | RPMI | 2 | None | 35 | No | 104 | 0.20-0.22 | 0.0073-0.009 | 5.8-8.8 |
| 24 | RPMI | 2 | None | 35 | No | 105 | 0.20-0.21 | 0.0068-0.0086 | 0.3-3.4 |
| 25 | RPMI | 2 | 0.5 | 30 | Yes | 103 | 0.20-0.21 | 0.0068-0.0086 | 19.4-22 |
| 26 | RPMI | 2 | 0.5 | 30 | Yes | 104 | 0.35-0.39 | 0.0089-0.01 | 12.2-14.8 |
| 27 | RPMI | 2 | 0.5 | 30 | Yes | 105 | 0.36-0.39 | 0.009-0.01 | 5.1-7.8 |
| 28 | RPMI | 2 | 0.5 | 30 | No | 103 | 0.21-0.23 | 0.0063-0.0079 | 14.5-17.9 |
| 29 | RPMI | 2 | 0.5 | 30 | No | 104 | 0.21-0.22 | 0.0064-0.008 | 8.6-11.7 |
| 30 | RPMI | 2 | 0.5 | 30 | No | 105 | 0.22-0.23 | 0.0068-0.0083 | 2.9-5.9 |
| 31 | RPMI | 2 | 0.5 | 35 | Yes | 103 | 0.24-0.27 | 0.0065-0.008 | 18.4-21.5 |
| 32 | RPMI | 2 | 0.5 | 35 | Yes | 104 | 0.25-0.28 | 0.0067-0.0084 | 10.9-14.5 |
| 33 | RPMI | 2 | 0.5 | 35 | Yes | 105 | 0.28-0.31 | 0.007-0.0091 | 3.6-7.9 |
| 34 | RPMI | 2 | 0.5 | 35 | No | 103 | 0.19-0.2 | 0.0065-0.0082 | 12.44-15.6 |
| 35 | RPMI | 2 | 0.5 | 35 | No | 104 | 0.18-0.19 | 0.0065-0.008 | 6.6-9.2 |
| 36 | RPMI | 2 | 0.5 | 35 | No | 105 | 0.18-0.19 | 0.0064-0.0077 | 0.9-0.35 |
| 37 | YNB | 0.5 | 0.5 | 30 | Yes | 103 | 0.46-0.52 | 0.0122-0.014 | 22.6-25.4 |
| 38 | YNB | 0.5 | 0.5 | 30 | Yes | 104 | 0.49-0.54 | 0.013-0.015 | 15.6-18.8 |
| 39 | YNB | 0.5 | 0.5 | 30 | Yes | 105 | 0.50-0.54 | 0.012-0.0151 | 7.8-11.3 |
| 40 | YNB | 0.5 | 0.5 | 30 | No | 103 | 0.20-0.22 | 0.006-0.0072 | 14.36-17.26 |
| 41 | YNB | 0.5 | 0.5 | 30 | No | 104 | 0.20-0.21 | 0.0058-0.007 | 7.6-10.7 |
| 42 | YNB | 0.5 | 0.5 | 30 | No | 105 | 0.21-0.22 | 0.0057-0.0069 | 1.5-4.6 |
| 43 | YNB | 0.5 | 0.5 | 35 | Yes | 103 | 0.32-0.37 | 0.0088-0.011 | 19.35-24.01 |
| 44 | YNB | 0.5 | 0.5 | 35 | Yes | 104 | 0.29-0.31 | 0.0088-0.011 | 14-17.4 |
| 45 | YNB | 0.5 | 0.5 | 35 | Yes | 105 | 0.3-0.32 | 0.0092-0.012 | 6.8-10.5 |
| 46 | YNB | 0.5 | 0.5 | 35 | No | 103 | 0.19-0.20 | 0.0059-0.0073 | 12-15.2 |
| 47 | YNB | 0.5 | 0.5 | 35 | No | 104 | 0.18-0.20 | 0.005-0.0061 | 4.9-8.2 |
| 48 | YNB | 0.5 | 0.5 | 35 | No | 105 | 0.19-0.20 | 0.0052-0.0063 | 0-1.9 |
Antifungals.
The following antifungal compounds were used: amphotericin B (range, 16.0 to 0.03 μg/ml) (Sigma-Aldrich), flucytosine (64.0 to 0.12 μg/ml) (Sigma-Aldrich), fluconazole (64.0 to 0.12 μg/ml) (Pfizer SA, Madrid, Spain), itraconazole (8.0 to 0.015 μg/ml) (Janssen SA, Madrid, Spain), voriconazole (8.0 to 0.015 μg/ml) (Pfizer SA), posaconazole (8.0 to 0.015 μg/ml) (Schering-Plough, Kenilworth, NJ), caspofungin (16.0 to 0.03 μg/ml) (Merck & Co., Inc., Rahway, NJ), micafungin (16.0 to 0.03 μg/ml) (Astellas Pharma, Inc., Tokyo, Japan), and anidulafungin (16.0 to 0.03 μg/ml) (Pfizer SA).
AST.
All the isolates were tested for antifungal susceptibility under five different sets of conditions: (i) the CLSI M27-A3 protocol (RPMI medium plus 0.2% glucose with an inoculum size of 103 CFU/ml at 35°C and static) (3, 4), (ii) the EUCAST document 7.1 protocol (RPMI medium plus 2% glucose with an inoculum size of 105 CFU/ml at 35°C and static) (19, 21), (iii) EUCAST conditions but with shaking and at 30°C, (iv) YNB static culture (YNB plus 0.5% glucose at 30°C with an inoculum size of 105 CFU/ml), and (v) YNB with shaking (YNB plus 0.5% glucose at 30°C with an inoculum size of 105 CFU/ml). For Cryptococcus spp., an additional condition was included in the experiments, the EUCAST protocol under static conditions but with a temperature of 30°C for incubation.
In all cases, flat-bottom 96-well microdilution plates were used, and both visual and spectrophotometric readings were performed at 24 and 48 h. For visual readings, the MIC value was estimated as total growth inhibition (for amphotericin B and echinocandins) or prominent decrease of growth (flucytosine, azoles, and echinocandins). In the case of spectrophotometric readings, the MIC90 (of amphotericin B and echinocandins) or MIC50 (of flucytosine, azoles, and echinocandins) was calculated.
Statistical analysis.
The results were processed using different software. Growth curves were obtained by using Excel. The growth curves were exported to GraphPad software, and the lag phase (hours), growth rate (OD units/hour), and maximum OD (arbitrary units) were calculated by using Gompertz modeling. For each curve, an R2 for the adjustment of the curve to the model was obtained. The lag phase of the growth cycle was defined as the time during which the optical density (A540) does not increase. The beginning of the growth phase was defined as a change in the A540 of >0.015 U (the lower detectable optical density). Exponential-phase broth cultures were marked by a specific growth rate of >0.02 U per h. Stationary-phase broth cultures were marked by a lack of a continued exponential increase in the A540.
The data were transferred to a PASW database (PASW Statistics 18; PASW Statistics, Madrid, Spain), where the presence or absence of a clear logarithmic phase was included. The significance of the differences between methodologies was determined by analysis of variance (ANOVA) (Bonferroni post hoc) or nonparametric tests. When the effect of one variable was studied, the others were fixed as constants. Differences in proportions were determined by Fisher's exact test or by chi-square analysis. In addition, a multivariate analysis was done as well, including discriminant analysis and logistic regression analysis (data expressed as odds ratios [ORs] with 95% confidence intervals [CIs]). A P value of <0.01 was considered significant.
For MIC analyses, all the values were processed with PASW statistical software. The OD of the drug-free well must be >0.2 to calculate the spectrophotometric MICs. Both on-scale and off-scale results obtained by AST procedures were included in the analysis. The low off-scale MICs were left unchanged, and the high off-scale MICs were converted to the next highest concentration. The reproducibility of the results obtained under different AST conditions was calculated by determining the percentage of agreement between MIC values. Agreement was defined as discrepancies in MIC results of no more than ±2 2-fold dilutions. In addition, the correlation between the results was evaluated by using the intraclass correlation coefficient (ICC), which was expressed to a maximum value of 1 and with a 95% CI. In order to approximate a normal distribution, the MICs were transformed to log2 values. A P value of <0.01 was considered statistically significant. The ICC is a reverse measurement of the variability of the counting values. The ICC was calculated using the formula ICC = (group mean square − error mean square)/(group mean square + error mean square) and thus has a maximum value of 1 if there is a perfect correlation and a minimum value of −1 if there is a complete absence of correlation. The ICC evaluates the correlation between values offering statistical significance, since it takes into account the number of cases and absolute value of the counting. The ICC is a scales analysis and exhibits the highest statistical power for correlation studies (17).
RESULTS
Effect of the growth medium on nonfermentative yeasts.
We performed growth curves for 44 different isolates from nonfermentative yeasts in different assay media. In addition, we also tested the effect of the addition of a nitrogen source (ammonium sulfate) to the media. The effects of temperature (30°C or 35°C), inoculum (103, 104, or 105 CFU/ml), and shaking were also analyzed. Table 1 summarizes all the conditions tested in this study.
We first analyzed the influence of the different conditions on the appearance of significant growth (final OD higher than 0.2) and on the presence of a classical exponential growth curve with defined logarithmic growth. When growth kinetics were analyzed by the ANOVA test, the use of YNB as an assay medium, an inoculum size of 105 CFU/ml, the agitation of trays, and an incubation temperature of 30°C had a significant influence (P < 0.01) on the OD value and on the presence of exponential growth. The addition of glucose or ammonium sulfate had no significant effect on growth kinetics.
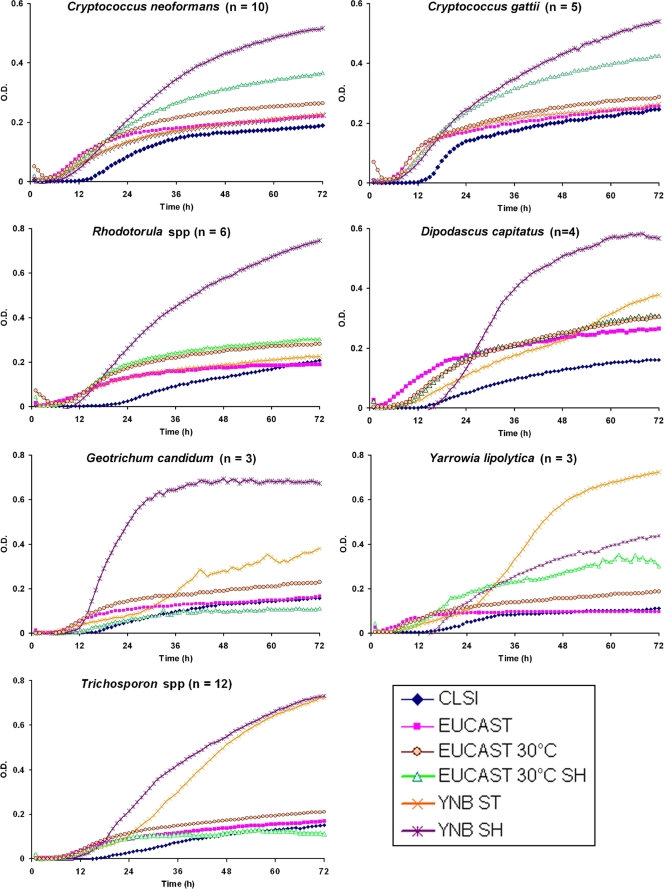
When a multivariate analysis was done, we found that the most relevant factor to obtain higher OD values and exponential growth was the use of YNB medium, which increased the average growth of the isolates tested by 4-fold (OR, 4.5; 95% CI, 1.9 to 7.4; P < 0.01). Concerning the inoculum, a positive correlation was also found between the use of a higher inoculum and growth (OR, 3.0; 95% CI, 1.2 to 5.6; P < 0.01). The shaking of the trays also had a positive effect on exponential growth (OR, 1.7; 95% CI, 1.2 to 1.9; P < 0.01). Incubation at 35°C was inversely correlated with the optical densities reached (OR, 0.5; 95% CI, 0.2 to 0.7; P < 0.01). Supplementation of the medium with glucose or ammonium sulfate did not improve the growth of the yeast. Figure 1 displays the average growth kinetics of the different species under different incubation conditions, which were chosen for further studies. As shown in Fig. 1, the incubation of organisms according both CLSI and EUCAST standard procedures made longer latency phases and lower maximum ODs (many of them <0.2). The growth kinetics hardly ever reached the exponential phase. However, incubation conditions, including YNB, shaking, 0.5% glucose at 30°C, and an inoculum size of 105 CFU/ml, increased OD values. In addition, it made the latency phase shorter, and an exponential phase was observed for most of the species tested. Conditions including shaking and incubation at 30°C also showed better growth parameters than both CLSI and EUCAST standard conditions.
FIG. 1.
Growth curves of different species of nonfermentative yeast in different media. Fungal growth was recorded during 72 h as described in Materials and Methods. The graph represents the average optical densities of the stains used for each species (n) under the following growth conditions: the CLSI protocol (RPMI medium with 0.2% glucose at 35°C with 0.5 × 103 to 2.5 × 103 CFU/ml and no shaking), the EUCAST protocol (RPMI medium plus 2% glucose at 35°C with 1 × 105 to 5 × 105 CFU/ml and no shaking), the EUCAST protocol at 30°C, the EUCAST protocol at 30°C with shaking (SH), YNB under static (ST) conditions (YNB medium plus 0.5% glucose with 1 × 105 to 5 × 105 CFU/ml at 30°C under static conditions), and YNB under shaking conditions (YNB medium plus 0.5% glucose with 1 × 105 to 5 × 105 CFU/ml at 30°C with shaking during the incubation).
Since C. neoformans is the most prevalent pathogen among nonfermentative yeasts, we performed the multivariate analysis for this pathogen independently. In this case, we found that the most important factor with statistical significance to obtain a clear logarithmic growth phase was incubation with shaking of the media (OR, 2.3; 95% CI, 1.5 to 3.1; P < 0.01), followed by incubation at 30°C (OR, 1.9; 95% CI, 1.3 to 2.6; P < 0.01). Table 1 shows different growth parameters obtained for C. neoformans. Although the inoculum size did not influence the appearance of logarithmic growth, it had a strong influence on the lag phase, being significantly shorter when a higher inoculum was used (Table 1).
Antifungal susceptibility testing.
Based on the initial analysis of the growth of the yeasts, we selected different media to measure susceptibility to nine different antifungal agents. We chose two standard reference methods used for antifungal susceptibility testing: the CLSI and EUCAST methods. In addition, we used a modified EUCAST method in which the plates were incubated at 30°C with shaking at 350 rpm and, in the case of the Cryptococcus spp. only, also without shaking. Finally, we included YNB-based medium incubated at 30°C and inoculated with an initial dose of 105 CFU/ml.
All the MIC values were obtained both visually and spectrophotometrically. Using the method recommended by the CLSI, we could obtain MIC values only at 24 h in 38.4% of the cases, which were distributed as follows: T. asahii (100% of the cases), T. inkin (100%), T. mucoides (75%), T. ovoides (100%), Y. lipolytica (100%), and D. capitatus (100%). For the rest of the species and strains, we could not determine MIC values at 24 h using CLSI recommendations due to the lack of growth. With EUCAST and YNB-based methods, most of isolates showed discernible growth after 24 h of incubation (OD of >0.2). However, as shown in Fig. 1, the end of the exponential growth phase and stationary phase of growth kinetics were reached on average after 36 to 48 h of incubation. Subsequently, we performed a descriptive analysis of the MIC values obtained after 48 h of incubation, which are shown in Table 2 for C. neoformans, Table 3 for C. gattii, and Table 4 for the rest of the species. It should be noted that MIC value determinations by CLSI and EUCAST methods were rather complicated, since more than half of isolates tested showed a limited growth index, with a final OD of 0.2 to 0.3. This made the endpoint determination to set MIC values particularly difficult for the visual reading.
TABLE 2.
MICs for Cryptococcus neoformans obtained with each mediuma
| Antifungal | CLSI method |
EUCAST method |
EUCAST method at 30°C with shaking |
EUCAST method at 30°C |
YNB shaken |
YNB static |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GM | Min-max | GM | Min-max | GM | Min-max | GM | Min-max | GM | Min-max | GM | Min-max | |
| AmB | 0.13 | 0.06-0.25 | 0.35 | 0.25-0.50 | 0.31 | 0.12-0.50 | 0.06 | 0.03-0.12 | 0.44 | 0.25-1 | 0.41 | 0.25-0.50 |
| Flucytosine | 3.73 | 2-4 | 6.50 | 1-16 | 9.85 | 8-16 | 6.50 | 2-16 | 16 | 8-32 | 7.46 | 1-16 |
| Fluconazole | 2.46 | 0.25-8 | 4.59 | 0.50-16 | 8 | 0.50-16 | 4.92 | 1-32 | 5.66 | 0.50-16 | 2.46 | 0.50-8 |
| Itraconazole | 0.09 | 0.015-0.25 | 0.07 | 0.015-0.25 | 0.05 | 0.015-0.25 | 0.08 | 0.015-0.50 | 0.18 | 0.03-4 | 0.08 | 0.015-0.25 |
| Voriconazole | 0.07 | 0.015-0.25 | 0.06 | 0.015-0.25 | 0.15 | 0.015-0.50 | 0.07 | 0.015-0.25 | 0.07 | 0.015-0.25 | 0.06 | 0.015-0.12 |
| Posaconazole | 0.16 | 0.015-0.25 | 0.11 | 0.03-0.25 | 0.17 | 0.03-0.50 | 0.05 | 0.03-0.25 | 0.21 | 0.015-8 | 0.10 | 0.03-0.25 |
| Caspofungin | >16 | 16-32 | 16 | 8->16 | >16 | 16->16 | >16 | 16->16 | >16 | 16->16 | >16 | 8->16 |
| Micafungin | >16 | 16-32 | >16 | >16->16 | >16 | >16->16 | >16 | >16->16 | >16 | >16->16 | >16 | >16->16 |
| Anidulafungin | >16 | 32-32 | >16 | 0.06->16 | >16 | >16->16 | >16 | 4->16 | >16 | 16->16 | >16 | >16->16 |
AmB, amphotericin B; GM, geometric mean.
TABLE 3.
MICs for Cryptococcus gattii obtained with each mediuma
| Antifungal | CLSI method |
EUCAST method |
EUCAST method at 30°C with shaking |
EUCAST method at 30°C |
YNB shaken |
YNB static |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GM | Min-max | GM | Min-max | GM | Min-max | GM | Min-max | GM | Min-max | GM | Min-max | |
| AmB | 0.14 | 0.12-0.25 | 0.29 | 0.25-0.50 | 0.25 | 0.12-0.50 | 0.06 | 0.06-0.06 | 0.44 | 0.25-0.50 | 0.50 | 0.50-0.50 |
| Flucytosine | 1.52 | 0.50-4 | 3.03 | 1-8 | 3.03 | 2-8 | 6.06 | 4-16 | 6.06 | 4-16 | 6.96 | 4-16 |
| Fluconazole | 8.00 | 2.00-16 | 18.38 | 8-32 | 24.25 | 8-64 | 21.11 | 4-64 | 13.93 | 2-32 | 13.93 | 4-32 |
| Itraconazole | 0.25 | 0.12-0.5 | 0.38 | 0.12-0.50 | 0.25 | 0.12-0.50 | 0.25 | 0.12-0.50 | 0.50 | 0.25-1 | 0.50 | 0.25-1 |
| Voriconazole | 0.12 | 0.06-0.25 | 0.28 | 0.12-0.50 | 0.38 | 0.12-1 | 0.19 | 0.06-0.50 | 0.33 | 012-1 | 0.33 | 0.12-0.50 |
| Posaconazole | 0.28 | 0.12-0.5 | 0.38 | 0.12-0.50 | 0.33 | 0.12-0.50 | 0.14 | 0.03-0.50 | 0.50 | 0.25-1 | 0.44 | 0.25-0.50 |
| Caspofungin | 13.93 | 8-16 | >16 | 16->16 | 16 | 16-16 | >16 | 16->16 | >16 | 16->16 | >16 | 16->16 |
| Micafungin | >16 | >16->16 | >16 | >16->16 | >16 | >16->16 | >16 | 2->16 | >16 | >16->16 | >16 | >16->16 |
| Anidulafungin | >16 | 4->16 | >16 | 4->16 | >16 | 4->16 | >16 | 4->16 | >16 | 4->16 | >16 | 4->16 |
GM, geometric mean.
TABLE 4.
MICs for Rhodotorula mucilaginosa, R. glutinis, Yarrowia lipolytica, Geotrichum candidum, Trichosporon cutaneum, T. asahii, T. ovoides, T. mucoides, T. inkin, Dipodascus capitatus, and Cryptococcus albidus obtained with each mediuma
| Antifungal | CLSI method |
EUCAST method |
EUCAST method at 30°C with shaking |
EUCAST method at 30°C |
YNB shaken |
YNB static |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GM | Min-max | GM | Min-max | GM | Min-max | GM | Min-max | GM | Min-max | GM | Min-max | |
| AmB | 0.72 | 0.12-16 | 1.03 | 0.25-32 | 0.90 | 0.12-32 | ND | ND | 0.95 | 0.25-8 | 1.15 | 0.12-32 |
| Flucytosine | 2.9 | 0.12->64 | 4.51 | 0.12->64 | 8.59 | 0.12->64 | ND | ND | 2.44 | 0.12->64 | 2.93 | 0.12->64 |
| Fluconazole | 8.2 | 0.50->64 | 8.00 | 0.25->64 | 9.75 | 0.25->64 | ND | ND | 6.03 | 0.25->64 | 10.61 | 0.25->64 |
| Itraconazole | 0.47 | 0.03->8 | 0.41 | 0.015-32 | 0.47 | 0.015-16 | ND | ND | 0.27 | 0.015-16 | 0.31 | 0.015-16 |
| Voriconazole | 0.28 | 0.015-8 | 0.26 | 0.015-16 | 0.33 | 0.015-16 | ND | ND | 0.12 | 0.015-2 | 0.17 | 0.015-8 |
| Posaconazole | 0.6 | 0.12->8 | 0.56 | 0.03-16 | 0.49 | 0.015-16 | ND | ND | 0.24 | 0.03-2 | 0.22 | 0.015-16 |
| Caspofungin | 12.4 | 2-16 | 13.37 | 2->16 | 9.74 | 0.03->16 | ND | ND | 9.33 | 1->16 | 10.34 | 1->16 |
| Micafungin | >16 | 1->16 | >16 | 1->16 | 15.98 | 0.12->16 | ND | ND | 10.33 | 0.06->16 | 10.31 | 0.06->16 |
| Anidulafungin | 13.7 | 1->16 | 13.72 | 0.50->16 | 10.75 | 0.03->16 | ND | ND | 6.01 | 0.03->16 | 5.27 | 0.03->16 |
GM, geometric mean; ND, not determined.
To establish the degree of correlation between the MIC values obtained with different media, we performed two different types of analysis. First, we calculated the intraclass correlation coefficients (ICCs) for all the comparisons. The ICC values were in most cases above 0.8, with good 95% CIs, and were statistically significant (P < 0.01), which indicated that there were no significant differences between the MICs obtained with the different methods. In addition, we calculated the agreement between the MIC values, considering that the MIC values that differed in less than two 2-fold dilutions were the same. The MIC values obtained with the different media showed an agreement above 90%, confirming that the MIC value was highly reproducible independent of the medium used. The use of YNB, agitation of plates, incubation at 30°C, and an inoculum size of 105 CFU/ml did not have a significant effect on MIC determinations, and MIC values were not falsely high.
DISCUSSION
Although antifungal susceptibility testing has become a powerful tool that helps in the management of treatment of patients in clinical practice, it has not yet been fully standardized for many microorganisms. The protocols suggested by the CLSI and EUCAST, based on RPMI medium, have been standardized for fermentative yeasts and filamentous molds (3, 4, 19, 20). In contrast, no protocol has been fully tested for nonfermentative yeasts. Among this group of microorganisms, Cryptococcus spp. are the most prevalent pathogens found in clinical practice, so the few studies that focused on this subject used this pathogen as a model. The growth of nonfermentative yeasts with these protocols is largely compromised with the conventional methods used for AST, due to the oxygen-limited environment found in the microdilution plates. Several modifications of standard conditions have been suggested to improve the growth of the yeasts under these conditions. A previously reported study demonstrated that the use of YNB-based media (pH 5.4) produced a higher growth yield, which was slightly decreased when the medium was buffered to pH 7 (9). Although nonbuffered YNB medium increased the growth yield, this condition has not been considered for antifungal susceptibility testing, since an acidic pH could influence the activities of some antifungal compounds. The use of YNB for Cryptococcus spp. was studied previously (16, 18). In addition to the use of YNB, it was shown that the introduction of shaking with the incubation significantly improves the growth of the yeasts (16).
In addition to Cryptococcus species, other nonfermentative yeasts have been described in the last few years as opportunistic pathogens among immunosuppressed patients, and no standardization for AST has been performed for these species. For these reasons, in this work we have compared different protocols for the performance of AST with the aim of providing an optimal protocol for nonfermentative yeasts. We studied the effects of different variables (medium, temperature, shaking, supplementation with ammonium as a nitrogen source, and initial inoculum) on different strains belonging to different nonfermentative yeast species. In general, our results are in agreement with previous findings, and we obtained optimal growth in YNB-based medium. In some cases, such as the cases of Cryptococcus spp., Rhodotorula spp., D. capitatus, and G. candidum, the improvement in YNB-based medium was particularly clear with agitation, indicating that for these species the main factor limiting growth was the oxygen availability in the well. In contrast, for some other species, YNB significantly improved growth even under static conditions (i.e., Trichosporon spp.). This clearly indicates that nonfermentative yeasts present other metabolic differences, such as the capacity to metabolize amino acids or other carbon sources that determine their growth under AST conditions. We found that nitrogen was not a limited nutrient in the RPMI-based media, since supplementation with ammonium sulfate did not improve the growth of the yeast. In all cases, growth was significantly improved when a lower temperature (30°C) was used. Although this temperature is not the physiological one during infection, it increased the growth rate of most of the isolates, so it guaranteed a more reliable MIC determination.
We decided to compare the antifungal susceptibility tests under the conditions recommended by the CLSI and EUCAST and to compare them with YNB-based media (with and without agitation) and EUCAST medium at 30°C, with and without agitation. We observed that when the MIC values were obtained after 48 h, there was a high agreement between the MICs measured with the different media, even compared with the CLSI method. The latter conditions were not suitable for most of the species after 24 h, since most of the yeasts did not grow after this time. Our results indicate that the MIC was not significantly affected by the growth medium, which indicates that YNB-based media, the introduction of agitation, and incubation at lower temperatures are factors that could be introduced under standard conditions to perform AST of nonfermentative yeast. In those cases in which the shaking of the plates might not be feasible, the use of YNB-based medium might result in better growth and in a reliable and reproducible measurement of the MIC values. Although we observed that there was a good correlation in the MIC values obtained with different media, we believe that the use of YNB has several advantages, such as improving the growth of the yeasts and the possibility of obtaining reliable readings after 24 h of incubation, compared with the 72 h suggested by the CLSI protocol. At present, our work does not imply an imminent change in the general recommendations given by the EUCAST or CLSI, but we believe that our findings provide reliable conditions to perform AST in cases where the growth of the isolate is suspected to be compromised under EUCAST- or CLSI-recommended conditions and should be considered feasible alternatives for reference laboratories where the number of nonfermentative yeasts analyzed is presumably higher than that found in regular practice in single hospitals. We also hope that our work will contribute to the modification of the current standards to improve the AST of nonfermentative yeasts.
Our data demonstrate that the growth of nonfermentative yeasts can be improved by introducing different new factors to the well-established protocols suggested by the CLSI and EUCAST. For Cryptococcus spp., the use of shaking is highly recommended. Incubation at 30°C should also be considered. In addition, the use of YNB medium might help to obtain the MIC value for a set of nonfermentative yeasts, even in the absence of shaking. Since AST and MIC determinations are a tool that has become extremely useful for the management of patients infected with fungal pathogens, we believe that our data contribute to the development of new standards that will provide MIC values for nonfermentative yeasts in a timely and reliable manner.
Acknowledgments
A.C.M.-A. has been funded by a fellowship from the AlBan Asociacion Grupo Santander program from European Union (2007) and is currently funded by the Fundación Carolina. L.B.-M. has a research contract from REIPI (Red Española de Investigación en Patología Infecciosa, Proyect MPY 1022/07_1). O.Z. is funded by grants PCI2006-A7-0606 and SAF2008-03761 from the Spanish Ministry of Science and Innovation. In the past 5 years, M.C.-E. has received grant support from Astellas Pharma, bioMerieux, Gilead Sciences, Merck Sharp and Dohme, Pfizer, Schering-Plough, Soria Melguizo SA, the European Union, the ALBAN program, the Spanish Agency for International Cooperation, the Spanish Ministry of Culture and Education, the Spanish Health Research Fund, the Instituto de Salud Carlos III, the Ramón Areces Foundation, and the Mutua Madrileña Foundation. He has been an advisor/consultant to the Panamerican Health Organization, Gilead Sciences, Merck Sharp and Dohme, Pfizer, and Schering-Plough. He has been paid for talks on behalf of Gilead Sciences, Merck Sharp and Dohme, Pfizer, and Schering-Plough. In the past 5 years, J.L.R.-T. has received grant support from Astellas Pharma, Gilead Sciences, Merck Sharp and Dohme, Pfizer, Schering-Plough, Soria Melguizo SA, the European Union, the Spanish Agency for International Cooperation, the Spanish Ministry of Culture and Education, the Spanish Health Research Fund, the Instituto de Salud Carlos III, the Ramon Areces Foundation, and the Mutua Madrileña Foundation. He has been an advisor/consultant to the Panamerican Health Organization, Gilead Sciences, Merck Sharp and Dohme, Mycognostica, Pfizer, and Schering-Plough. He has been paid for talks on behalf of Gilead Sciences, Merck Sharp and Dohme, Pfizer, and Schering-Plough.
Footnotes
Published ahead of print on 18 January 2011.
REFERENCES
- 1.Byrnes, E. J., et al. 2010. Emergence and pathogenicity of highly virulent Cryptococcus gattii genotypes in the northwest United States. PLoS Pathog. 6:e1000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casadevall, A., and J. R. Perfect. 1998. Cryptococcus neoformans. ASM Press, Washington, DC.
- 3.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard, 2nd ed. M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA.
- 4.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeast. Approved standard, 3rd ed. M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA.
- 5.Cuenca-Estrella, M., et al. 2006. Head-to-head comparison of the activities of currently available antifungal agents against 3,378 Spanish clinical isolates of yeasts and filamentous fungi. Antimicrob. Agents Chemother. 50:917-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denning, D. W. 2003. Echinocandin antifungal drugs. Lancet 362:1142-1151. [DOI] [PubMed] [Google Scholar]
- 7.Fera, M. T., E. La Camera, and A. De Sarro. 2009. New triazoles and echinocandins: mode of action, in vitro activity and mechanisms of resistance. Expert Rev. Anti Infect. Ther. 7:981-998. [DOI] [PubMed] [Google Scholar]
- 8.Galanis, E., L. Hoang, P. Kibsey, M. Morshed, and P. Phillips. 2009. Clinical presentation, diagnosis and management of Cryptococcus gattii cases: lessons learned from British Columbia. Can. J. Infect. Dis. Med. Microbiol. 20:23-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghannoum, M. A., et al. 1992. Susceptibility testing of Cryptococcus neoformans: a microdilution technique. J. Clin. Microbiol. 30:2881-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomez-Lopez, A., O. Zaragoza, J. L. Rodriguez-Tudela, and M. Cuenca-Estrella. 2008. Pharmacotherapy of yeast infections. Expert Opin. Pharmacother. 9:2801-2816. [DOI] [PubMed] [Google Scholar]
- 11.Hoang, L. M., J. A. Maguire, P. Doyle, M. Fyfe, and D. L. Roscoe. 2004. Cryptococcus neoformans infections at Vancouver Hospital and Health Sciences Centre (1997-2002): epidemiology, microbiology and histopathology. J. Med. Microbiol. 53:935-940. [DOI] [PubMed] [Google Scholar]
- 12.Kerkering, T. M., R. J. Duma, and S. Shadomy. 1981. The evolution of pulmonary cryptococcosis: clinical implications from a study of 41 patients with and without compromising host factors. Ann. Intern. Med. 94:611-616. [DOI] [PubMed] [Google Scholar]
- 13.Kurtzman, C. P., and J. W. Fell. 1998. The yeasts. A taxonomic study. Elsevier Science BV, Amsterdam, Netherlands.
- 14.Paphitou, N. I., et al. 2002. In vitro antifungal susceptibilities of Trichosporon species. Antimicrob. Agents Chemother. 46:1144-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez-Tudela, J. L., et al. 2005. Susceptibility patterns and molecular identification of Trichosporon species. Antimicrob. Agents Chemother. 49:4026-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez-Tudela, J. L., et al. 2000. Influence of shaking on antifungal susceptibility testing of Cryptococcus neoformans: a comparison of the NCCLS standard M27A medium, buffered yeast nitrogen base, and RPMI-2% glucose. Antimicrob. Agents Chemother. 44:400-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.SPSS, Inc. 2009. Statistical analysis and advanced statistical analysis. SPSS, Inc., Chicago, IL.
- 18.St. Germain, G., and C. Dion. 1996. Effect of media on growth rate and susceptibility testing of Cryptococcus neoformans. Mycoses 39:201-206. [DOI] [PubMed] [Google Scholar]
- 19.Subcommittee on Antifungal Susceptibility Testing of the ESCMID European Committee for Antimicrobial Susceptibility Testing. 2008. EUCAST definitive document EDef 7.1: method for the determination of broth dilution MICs of antifungal agents for fermentative yeasts. Clin. Microbiol. Infect. 14:398-405. [DOI] [PubMed] [Google Scholar]
- 20.Subcommittee on Antifungal Susceptibility Testing of the ESCMID European Committee for Antimicrobial Susceptibility Testing. 2008. EUCAST technical note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia-forming moulds. Clin. Microbiol. Infect. 14:982-984. [DOI] [PubMed] [Google Scholar]
- 21.Subcommittee on Antifungal Susceptibility Testing of the ESCMID European Committee for Antimicrobial Susceptibility Testing. 2008. EUCAST technical note on voriconazole. Clin. Microbiol. Infect. 14:985-987. [DOI] [PubMed] [Google Scholar]
- 22.Sugar, A. M. 1991. Cryptococcosis in the patient with AIDS. Mycopathologia 114:153-157. [DOI] [PubMed] [Google Scholar]
- 23.White, T. J., T. Bruns, S. Lee, and J. Taylor. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics, p. 315-322. In G. H. Gelfand, J. J. Sninsky, T. J. White, and M. A. Innis (ed.), PCR protocols: a guide to methods and applications. Academic Press, San Diego, CA.