Abstract
A series of 9 quinolines and 18 styrylquinolines was evaluated for the drugs' in vitro antileishmanial activities and cytotoxicities. The 7-aroylstyrylquinoline scaffold appeared to be the most promising one, with the most interesting compound, no. 35, exhibiting a 50% inhibitory concentration (IC50) of 1.2 μM and a selectivity index value of 121.5. Compound 35 was 10-fold and 8-fold more active than miltefosine and sitamaquine, the reference compounds, with selectivity indexes 607-fold and 60-fold higher, respectively.
Leishmaniasis is a family of parasitic diseases that affect about 12 million people in tropical and subtropical areas in the form of three clinical expressions: visceral leishmaniasis, which is fatal in the absence of treatment; muco-cutaneous leishmaniasis; and cutaneous leishmaniasis, which is often self-curing. Classical drugs such as antimonials (Pentostam and Glucantime) are toxic, and drug resistance is increasing dangerously in the field (3). A liposomal amphotericin B formulation (AmBisome) less toxic than amphotericin B deoxycholate is gradually becoming the first-line therapy, especially in immunocompromised patients, but this drug must be administered by a parenteral route (11). Miltefosine (Impavido) was the first drug registered against visceral leishmaniasis in the last decade; however, its toxicity and the appearance of drug resistance justify the search for new chemical series in order to find an orally safe and active drug (8).
Quinolines substituted at the 2-position have shown in vivo activities against Leishmania donovani, and many compounds have been synthesized over the last decade (14). The Drug for Neglected Diseases Initiative (DNDi) has been considering this series for evaluation in preclinical development for about a year and a half. However, although promising, the series still requires improvements, and here we report the in vitro antileishmanial evaluation of new quinoline derivatives, including 2-[2-aryl(ethenyl)]-substituted quinoline (2-styrylquinolines) bearing additional aroyl/acyl groups at the C-7 position. In addition, some compounds within this series were recently shown to display substantial antiviral activity in HIV-infected cells (13, 22).
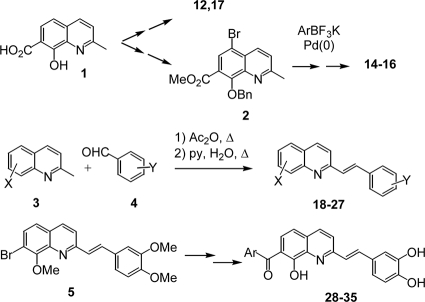
The synthesis of most of the compounds has been previously reported. Briefly, Kolbe carbonation of 8-hydroxyquinaldine afforded the pivotal hydroxyacid compound 1 (9), which was further elaborated into the 5-iodoquinaldine compound 12 and amide compound 17 (13). Similarly, bromination of the C-5 position and protection of the salicylic moiety provided 5-bromoquinaldine compound 2, which was engaged in a modified Suzuki cross-coupling reaction to give 5-arylated derivatives 14 to 16 (19). Styrylquinoline compounds 19 to 27 were prepared from the corresponding quinaldine compound 3 by Perkin-type condensation in refluxing acetic anhydride, followed by hydrolysis in a pyridine-water mixture (10, 16, 21, 22). Finally, the 7-aroyl-stryryquinoline derivatives 28 to 35 were obtained via the 7-bromostyrylquinoline compound 5 according to a three-step sequence involving lithiation followed by condensation with the required aldehyde, manganese dioxide oxidation, and deprotection (15) (Fig. 1).
FIG. 1.
General synthetic scheme for the quinoline and styrylquinoline derivatives evaluated in this study.
The antileishmanial evaluation of these compounds was then performed on Leishmania donovani amastigotes by using the luciferase-transfected Leishmania donovani (strain MHOM/IN/80/Dd8) promastigotes maintained in the laboratory of the Division of Parasitology, Central Drug Research Institute, Lucknow, India, since 2005 as described by Sunduru et al. (20). In order to assess the activity of compounds against the amastigote stage of the parasite, the mouse macrophage cell line J-774A.1, infected with promastigotes expressing the luciferase firefly reporter gene, was used. Cells were seeded in a 96-well plate at a density of 4 × 104 cells per ml in a final volume of 100 μl in RPMI 1640 containing 10% fetal calf serum, and the plates were incubated at 37°C in a CO2 incubator. After 24 h, the medium was replaced with fresh medium containing stationary-phase promastigotes (4 × 105/100 μl/well). Promastigotes were engulfed by the macrophage and transformed there into amastigotes. The test compounds were added at 2-fold dilutions in up to 7 points in fresh complete medium starting from a 100 μM concentration, and the plates were incubated at 37°C in a CO2 incubator for 72 h. After incubation, the drug-containing medium was decanted and 50 μl phosphate-buffered saline (PBS) was added in each well and mixed with an equal volume of Steady-Glo luciferase assay substrate dissolved in Steady-Glo luciferase assay buffer. After gentle shaking for 1 to 2 min, the readings were recorded in a luminometer (1, 4, 17). The values were expressed as relative luminescence units (RLU). Data were transformed into a graphic program (Excel). The 50% inhibitory concentration (IC50) for antileishmanial activity was calculated by nonlinear regression analysis of the concentration-response curve by using the four-parameter Hill equations. The in vitro Leishmania donovani intramacrophage amastigote system used to evaluate the antileishmanial activity of the compounds was the most relevant one, since it takes into account the pharmacokinetics barriers that a compound has to overcome before entering the parasite.
KB cells were used to evaluate the cytotoxicity of the compounds to mammalian cells, which allowed us to determine an in vitro selectivity index. The cell viability was determined with the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay (12). Exponentially growing KB cells at a density of 1 × 105 cells per ml in a 100-μl final volume were incubated in a 96-well plate with test drugs for 72 h. The test compounds were added at 3-fold dilutions for up to 7 points in complete medium starting from a 400 μM concentration and were incubated at 37°C in a humidified mixture of CO2 and 95% air in an incubator. Podophyllotoxin was used as a reference drug, and control wells containing dimethyl sulfoxide (DMSO) without drugs were also included in the experiment. Stock solutions of compounds were initially dissolved in DMSO and further diluted with fresh complete medium. After incubation, 25 μl of MTT reagent (5 mg/ml) in PBS medium, followed by syringe filtration, was added to each well and incubated at 37°C for 2 h. At the end of the incubation period, the supernatants were removed by inverting the plate completely without disturbing the cell layer, and 150 μl of pure DMSO was added to each well. After 15 min of shaking, the readings were recorded as absorbance at 544 nm on a microplate reader. The cytotoxic effects were expressed as 50% lethal dose (i.e., as the concentration of a compound which provoked a 50% reduction in cell viability compared to cells in culture medium alone). Fifty percent cytotoxic concentration (CC50) values were estimated as previously described (5, 12). The selectivity index (SI) for each compound was calculated as the ratio between cytotoxicity (CC50) and activity (IC50) against Leishmania amastigotes.
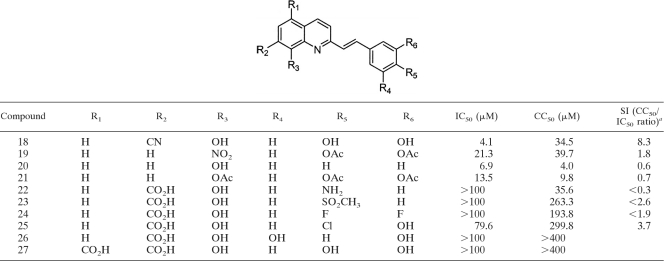
Among the simple quinolines (Table 1) and the styrylquinoline derivatives (Table 2), three compounds exhibited an IC50 for parasites of less than 10 μM (compounds 17, 18, and 20). The most interesting compound was compound 18, with an IC50 for L. donovani intramacrophage amastigotes of 4.1 μM and a selectivity index of 8.3. A clear-cut structure-activity relationship showed that the introduction of a carboxyl group at any position was responsible for both a dramatic decrease in the antileishmanial activity and a decrease in the cytotoxicity decrease. This observation was confirmed when two carboxyl groups were introduced into the same molecule, resulting in no activity and no cytotoxicity (compound 27). These results could be ascribed to an excessive hydrophilicity limiting the drug-parasite membrane interactions or a reaction between the carboxyl group with some compounds of the culture medium preventing the entry of the compound into the parasite.
TABLE 1.
In vitro antileishmanial activity and cytotoxicity results for compounds 6 and 10 to 17
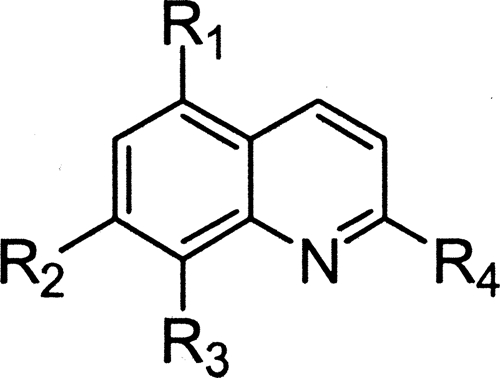 |
a The selectivity index (SI) is defined as the ratio of CC50 on KB cells to IC50 on L. donovani intramacrophage amastigotes.
TABLE 2.
In vitro antileishmanial activity and cytotoxicity results for styrylquinolines 18 to 27
a The selectivity index (SI) is defined as the ratio of CC50 on KB cells to IC50 on L. donovani intramacrophage amastigotes.
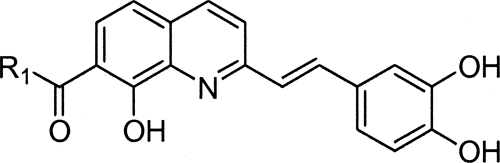
Among the 7-aroylstyrylquinolines (Table 3), the most interesting compound was compound 35, which exhibited an IC50 of 1.2 μM and a selectivity index of 121.5. Compound 35 was 10-fold and 8-fold more active than miltefosine and sitamaquine, the reference compounds, with selectivity indexes 607-fold and 60-fold higher, respectively.
TABLE 3.
In vitro antileishmanial activity and cytotoxicity results for 7-aroylstyrylquinoline compounds 28 to 35
a The selectivity index (SI) is defined as the ratio of CC50 on KB cells to IC50 on L. donovani intramacrophage amastigotes.
Compound 34 had an IC50 of 2.1 μM and a selectivity index of 27.3. These compounds exhibited the best selectivity indexes in their series, despite the presence of a nitro group. The presence of the nitro group at the meta position greatly increased the selectivity index, as evidenced by the much lower selectivity index of the parent compound, no. 28.
Recent work has confirmed the interesting antileishmanial properties of other quinoline series (2, 6, 18). In addition, quinolines have recently been found to inhibit leishmanial GDP-mannose-pyrophosphorylase, an enzyme system producing a range of mannose-rich glycoconjugates that are essential for parasite survival and virulence (7). This potential for selective action against a Leishmania-specific target makes quinolines a promising series of antileishmanial drugs. Moreover, we have tried to select quinoline-resistant L. donovani promastigotes in the lab by in vitro drug pressure and have only obtained a slight decrease in sensitivity since the IC50s were no more than twice those of the wild-type line (data not shown). This encouraging result is an additive justification for further studies of 2-substituted quinolines.
In conclusion, compound 35, due to its high in vitro antileishmanial activity and low toxicity, is the most interesting compound to emerge from more than 150 derivatives of 2-substituted quinolines that have now been synthesized and evaluated. It has now been selected as a candidate for evaluation in vivo with L. donovani mouse or hamster models via the DNDi pipeline.
Acknowledgments
This work was supported by the Drug for Neglected Disease Initiative (DNDi).
The transgenic L. donovani promastigotes were originally procured from Neena Goyal, Division of Biochemistry, Central Drug Research Institute, Lucknow, India.
Footnotes
Published ahead of print on 10 January 2011.
REFERENCES
- 1.Bhandari, K., et al. 2010. Synthesis of substituted aryloxy alkyl and aryloxy aryl alkyl imidazoles as antileishmanial agents. Bioorg. Med. Chem. Lett. 20:291-293. [DOI] [PubMed] [Google Scholar]
- 2.Ferreira, M. E., et al. 2010. Antileishmanial activity of furoquinolines and coumarins from Helietta apiculata. Phytomedicine 17:375-378. [DOI] [PubMed] [Google Scholar]
- 3.Frézard, F., C. Demicheli, and R. R. Ribeiro. 2009. Pentavalent antimonials: new perspective for old drugs. Molecules 14:2317-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta, L., A. Talwar, Nishi, S. Palne, S. Gupta, and P. M. Chauhan. 2007. Synthesis of marine alkaloid: 8,9-dihydrocoscinamide B and its analogues as novel class of antileishmanial agents. Bioorg. Med. Chem. Lett. 17:4075-4079. [DOI] [PubMed] [Google Scholar]
- 5.Huber, W., and J. C. Koella. 1993. A comparison of three methods of estimating EC50 in studies of drug resistance of malaria parasites. Acta Trop. 55:257-261. [DOI] [PubMed] [Google Scholar]
- 6.Isaac-Márquez, A. P., J. D. McChesney, N. P. Nanayakara, A. R. Satoskar, and C. M. Lezama-Dávila. 2010. Leishmanicidal activity of racemic +/− 8-[(4-amino-1-methylbutyl)amino]-6-methoxy-4-methyl-5-[3,4-dichlorophenoxy]quinoline. Nat. Prod. Commun. 3:387-390. [PubMed] [Google Scholar]
- 7.Lackovic, K., et al. 2010. Inhibitors of Leishmania GDP-mannose pyrophosphorylase identified by high-throughput screening of small-molecule chemical library. Antimicrob. Agents Chemother. 54:1712-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maltezou, H. C. 2010. Drug resistance in visceral leishmaniasis. J. Biomed. Biotechnol. 2010:617521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meek, W. H., and C. H. Fuschman. 1969. Carboxylation of substituted phenols in N,N-dimethylamide solvents at atmospheric presssure. J. Chem. Eng. Data 14:388-391. [Google Scholar]
- 10.Mekouar, K., et al. 1998. Styrylquinoline derivatives: a new class of potent HIV-1-integrase inhibitors that block HIV-1 replication in CEM cells. J. Med. Chem. 41:2846-2857. [DOI] [PubMed] [Google Scholar]
- 11.Moore, E. M., and D. N. Lockwood. 2010. Treatment of visceral leishmaniasis. J. Global Infect. Dis. 2:151-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mosmann, T. J. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65:55-63. [DOI] [PubMed] [Google Scholar]
- 13.Mouscadet, J. F., and D. Desmaële. 2010. Chemistry and structure-activity relationship of the styrylquinoline-type HIV integrase inhibitors. Molecules 15:3048-3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakayama, H., et al. 2005. Efficacy of orally administered 2-substituted quinolines in experimental murine cutaneous and visceral leishmaniases. Antimicrob. Agents Chemother. 49:4950-4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Normand-Bayle, M., et al. 2005. New HIV-1 replication inhibitors of the styryquinoline class bearing aroyl/acyl groups at the C-7 position: synthesis and biological activity. Bioorg. Med. Chem. Lett. 15:4019-4022. [DOI] [PubMed] [Google Scholar]
- 16.Polanski, J., et al. 2002. Use of the Kohonen neural network for rapid screening of ex vivo anti-HIV activity of styrylquinolines. J. Med. Chem. 45:4647-4654. [DOI] [PubMed] [Google Scholar]
- 17.Porwal, S., et al. 2009. Discovery of novel antileishmanial agents in an attempt to synthesize pentamidine-aplysinopsin hybrid molecule. J. Med. Chem. 52:5793-5802. [DOI] [PubMed] [Google Scholar]
- 18.Rizvi, S. U., et al. 2010. Antimicrobial and antileishmanial studies of novel (2E)-3-(2-chloro-6-methyl/methoxyquinolin-3-yl)-1-(aryl)prop-2-en-1-ones. Chem. Pharm. Bull. 58:301-306. [DOI] [PubMed] [Google Scholar]
- 19.Sliman, F., and D. Desmaële. 2010. Synthesis of 5-aryl- and 5-heteroaryl-7-carboxyl-8-hydroxyquinaldines through Suzuki cross-coupling reaction with potassium organotrifluoroborates. Synthesis 2010:619-630. [Google Scholar]
- 20.Sunduru, N., Nishi, S. Palne, P. M. Chauhan, and S. Gupta. 2009. Synthesis and antileishmanial activity of novel 2,4,6-trisubstituted pyrimidines and 1,3,5-triazines. Eur. J. Med. Chem. 44:2473-2481. [DOI] [PubMed] [Google Scholar]
- 21.Zouhiri, F., et al. 2005. HIV-1 replication inhibitors of the styrylquinoline class: introduction of an additional carboxyl group at the C-5 position of the quinoline. Tetrahedron Lett. 46:2201-2205. [Google Scholar]
- 22.Zouhiri, F., et al. 2000. Structure-activity relationships and binding mode of styrylquinolines as potent inhibitors of HIV-1-integrase and replication of HIV-1 in cell culture. J. Med. Chem. 43:1533-1540. [DOI] [PubMed] [Google Scholar]