Abstract
Recent in vitro pharmacokinetic data suggest that the currently recommended dose of pyrazinamide may be suboptimal for killing intracellular bacilli in humans. We evaluated a range of pyrazinamide doses against intracellular and extracellular Mycobacterium tuberculosis in chronically infected mice and guinea pigs, respectively. Antibiotics were given five times weekly for 4 weeks beginning 28 days after infection. Human-equivalent doses of isoniazid reduced lung bacterial counts 10-fold in each species. Pyrazinamide given at 1/4 and 1/2 the human-equivalent dose was minimally active, while human-equivalent doses reduced lung bacterial counts by ∼1.0 log10 in each species. Doubling the human-equivalent dose of pyrazinamide reduced the lung bacillary burden by 1.7 and 3.0 log10 in mice and guinea pigs, respectively. As in humans and mice, pyrazinamide showed significant synergy with rifampin in guinea pigs. Clinical studies are warranted to investigate the sterilizing activity and tolerability of higher doses of pyrazinamide in combination tuberculosis regimens.
Although the antituberculous activity of pyrazinamide (Z) was recognized in the early 1950s, its mechanism of action remains poorly understood (25). After passive diffusion and active transport (35), Z accumulates intracellularly (52) and is converted to pyrazinoic acid by Mycobacterium tuberculosis pyrazinamidase (24, 39). Originally thought to act on mycobacterial fatty acid synthase I (54), pyrazinoic acid likely acts by disrupting the proton motive force required for essential cellular functions at acidic pH (53).
Although Z is poorly active against multiplying bacilli (22), the synergy of Z with rifampin (R) (27) has permitted the shortening of tuberculosis (TB) chemotherapy from 9 months to 6 months (41). Because of the strict requirement for acidic pH, Z is believed to target dormant bacilli residing within an acidic environment (30). Although Z-susceptible bacilli have been postulated to persist in the macrophage phagolysosome (37), recent studies suggest that the interior pH of these organelles may be only slightly acidic (11, 43), and Z lacks even bacteriostatic activity against intracellular M. tuberculosis in human monocyte-derived macrophages (20). However, activation of macrophages by gamma interferon (IFN-γ) leads to phagosome acidification to a pH range where Z is highly active (45). An alternative hypothesis is that Z kills bacilli residing in acidified lung compartments present during the early inflammatory stages of infection (30), which is consistent with clinical observations that Z activity is primarily limited to the first 2 months of therapy (5-7). In addition, hypoxia enhances the antituberculous activity of Z (48).
A recent study using an in vitro pharmacokinetic (PK) system found that that the area under the serum concentration-time curve (AUC)/MIC is the PK parameter most closely linked with M. tuberculosis killing by Z (18). Using Monte Carlo simulations of 10,000 patients, these investigators reasoned that the persistent bacilli targeted by Z are likely to be extracellular based on the unlikelihood of achieving bactericidal drug levels within pulmonary macrophages of most patients receiving standard Z doses, while effective exposures were achieved in the epithelial lining fluid of 80 to 90% of simulated patients treated with equal Z doses. Based on in vitro PK data and mathematical modeling, they suggested that increasing the currently recommended Z dose may improve treatment outcomes in patients with TB (18).
In order to determine whether Z is active against intracellular and/or extracellular bacilli in the mammalian host, we evaluated the bactericidal activity of a range of Z doses against chronic TB infection in mice and guinea pigs. Unlike mice, guinea pigs infected with M. tuberculosis form necrotic granulomas histologically resembling their human counterparts (29). Such lesions in humans (46) and guinea pigs (28) likely harbor persistent bacilli, which may encounter microenvironmental stress conditions including hypoxia (19, 28, 47) and acidic pH (16). On the other hand, TB infection in mice appears to be confined to the intraphagosomal compartment within cellular lesions lacking necrosis and associated microenvironmental conditions (14).
MATERIALS AND METHODS
M. tuberculosis strain.
The Johns Hopkins Center for Tuberculosis Research laboratory reference strain M. tuberculosis H37Rv (2) was passaged twice through mice and frozen in aliquots at −80°C before use. Aliquots were thawed and grown to logarithmic phase (optical density at 600 nm = 0.5) in Middlebrook 7H9 broth (Fisher, Pittsburgh, PA) with 10% oleic acid-albumin-dextrose-catalase (Difco, Detroit, MI) and 0.1% Tween 80 (Sigma, St. Louis, MO) prior to aerosol infections. The MICs of isoniazid and rifampin against this strain were determined to be 0.03 and 0.12 mg/liter, respectively. The H37Rv strain used for these studies was fully susceptible to Z. It tested positive for pyrazinamidase activity by the niacin test and was found to have an MIC of ≤100 μg/ml in 7H12 broth (pH 6.0) using Z concentrations of 100, 300, and 900 μg/ml. By the BACTEC 460 TB system (Becton Dickinson Microbiology Systems, Sparks, MD), the MIC was determined to be ≤100 μg/ml, and by MGIT 960 (Becton Dickinson) it was determined to be ≤900 μg/ml. Testing was performed by Leonid Heifets (National Jewish Medical Center, Denver, CO).
Antibiotics.
Isoniazid (H) and R were purchased from Sigma, and Z was obtained from Acros Organics (Morris Plains, NJ).
Animals.
Female outbred Hartley guinea pigs (250 to 300 g) and female BALB/c mice (6 to 8 weeks old) were purchased from Charles River (Wilmington, MA). All procedures were approved by the Johns Hopkins Animal Care and Use Committee.
Aerosol infections.
Log-phase cultures of M. tuberculosis H37Rv were diluted 50-fold (to ∼106 CFU/ml) for aerosol infection of mice and 500-fold (to ∼105 CFU/ml) in 1× phosphate-buffered saline for aerosol infection of guinea pigs. A group of 40 guinea pigs was aerosol-infected with a Madison chamber aerosol generation device (College of Engineering Shops, University of Wisconsin, Madison, WI) calibrated to deliver ∼100 bacilli into guinea pig lungs. A separate group of 50 mice were aerosol-infected by using an inhalation exposure system (Glas-Col, Terre Haute, IN) calibrated to deliver 100 bacilli to mouse lungs.
Antibiotic treatment.
Chemotherapy was initiated 28 days after aerosol infection (day 0) in each species. The dosing frequency was daily (5 days/week) for a total of 4 weeks. Guinea pigs received one of the following regimens: no treatment; H, 60 mg/kg; R, 50 mg/kg; Z, 75 mg/kg; Z, 150 mg/kg; Z, 300 mg/kg; Z, 600 mg/kg; or R (50 mg/kg) plus Z (300 mg/kg). To enhance palatability, doses of each drug were prepared in a homogeneous suspension of 40% sucrose in a final volume of 0.5 ml for each guinea pig, and delivered in the posterior oropharynx by automatic pipette with disposable tip (1). In the combination regimen RZ, the rifampin dose preceded the Z dose by at least 1 h to limit drug interactions (17). Mice received one of the following regimens by esophageal cannula: no treatment; H, 10 mg/kg; Z, 37.5 mg/kg; Z, 75 mg/kg; Z, 150 mg/kg; or Z, 300 mg/kg. Z doses in mice were selected to match those in guinea pigs, on the basis that Z at 150 mg/kg in mice and Z at 300 mg/kg in guinea pigs yield a similar AUC, which closely approximates that following standard dosing of Z in humans (2). The dose of H at 60 mg/kg in guinea pigs was chosen based on the observations that this dose yields an AUC of 34.1 ± 4.9 mg h/liter, which is well within the range of AUC for human slow and rapid acetylators (19.9 ± 6.1 and 48.2 ± 1.5, respectively) and closely matches the AUC of H at 25 mg/kg in the mouse (1). Although our prior work has suggested that R at 100 mg/kg yields an AUC that most closely approximates the AUC in humans after standard R dosing (2), a pilot experiment confirmed the results of previous studies showing excessive morbidity and mortality when guinea pigs are given such high doses of R on a daily basis (13). Since R at 50 mg/kg has been used successfully in combination regimens administered daily to M. tuberculosis-infected guinea pigs (21, 32, 40), this dose of R was chosen for the present study.
Study endpoints.
Mice and guinea pigs were sacrificed on the day after infection (day −27), at day 0, and at day 28 after treatment. At necropsy, lungs were harvested, weighed, examined for gross pathology, and photographed. One lung from each animal was fixed in 10% buffered formaldehyde for histopathology. The lungs were sectioned in a standardized fashion along the longitudinal axis (apex to lower lobe), traversing the maximum horizontal dimension (through the hilum). The tissue was embedded in paraffin wax, sectioned, and stained with both hematoxylin and eosin (H&E) and Kinyoun stain for acid-fast bacilli (AFB) detection. Each block had at least one H&E-stained slide and one AFB-stained slide. The tissue sections were evaluated by a single pathologist with expertise in pulmonary pathology (M.M.F.). Images were obtained by using an DP72 camera (Olympus, Center Valley, PA). The other lung from each animal was homogenized (23), and diluted and undiluted lung homogenates were plated on Middlebrook 7H11 plates (Becton Dickinson, Sparks, MD) for CFU enumeration.
Statistical analysis.
CFU data are derived from five mice and four guinea pigs per group. Log-transformed CFU were used to calculate means and standard deviations. A Student t test was used to assess the statistical significance between monotherapy groups. A linear regression model including single drug exposures, as well as an interaction term for the combination RZ, was used to determine the potential synergy for this drug combination relative to the contribution of R or Z given alone. A P value of 0.05 was considered significant for all statistical analyses.
RESULTS
Gross pathology and histopathology.
Treatment was initiated 28 days after infection in order to allow for the development of adaptive immune responses and species-specific differences in pathology prior to antibiotic treatment. At day 0 (start of treatment), gross examination of mouse lungs revealed discrete tubercle lesions (data not shown). Histological evaluation of mouse lungs revealed aggregates of mononuclear cells, predominantly lymphocytes admixed with few histiocytes and plasma cells, which were surrounded by areas of pulmonary edema in the intra-alveolar spaces (Fig. 1A and B). AFB were predominantly localized to foamy macrophages (Fig. 1B). Well-formed granulomas were not detected in any of the treated or untreated mice. Neither H nor Z (Fig. 1C and D) had a significant effect on the number or localization of the organisms or the pattern of inflammation.
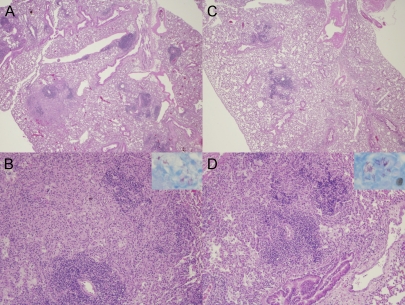
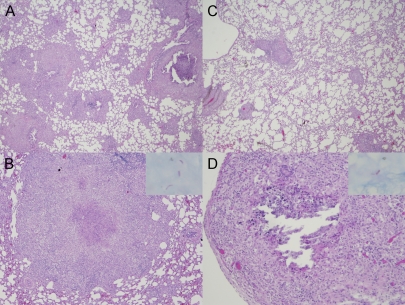
FIG. 1.
Lung histopathology in the murine model of chronic TB infection following pyrazinamide therapy. (A and B) Day 0 of treatment (28 days after aerosol infection). Magnifications: A, ×2; B, ×10. Interstitial fibrosis and marked pulmonary edema in the alveolar spaces. Cellular lesions are seen comprising mainly lymphocytes with few histiocytes and plasma cells (H&E stain). (Inset in panel B) AFB are detected within cells. (C and D) Day 28 after treatment with pyrazinamide at 150 mg/kg. Magnifications: C, ×2; D, ×10. Interstitial lymphocytic infiltrates surrounded by areas of pulmonary edema (H&E stain) can be seen. (Inset in panel D) AFB are confined to the intracellular compartment. Well-formed granulomas were not seen at either time point in any group.
Examination of guinea pig lungs at day 0 revealed grossly visible tubercles (data not shown), and histological evaluation revealed poorly formed granulomas comprising aggregates of epithelioid histiocytes admixed with a few lymphocytes and plasma cells with punctate necrosis (Fig. 2A and B). The organisms were more dispersed than those in mice and were located primarily within histiocytes (Fig. 2B, inset). At day 28, well-formed caseous granulomas were observed in each group, and organisms were localized extracellularly within necrotic areas in the center of the granuloma (Fig. 2D, inset). Z at 300 mg/kg led to a decrease in the number, as well as the size of granulomas, which tended to localize more peripherally (Fig. 2C and D). A similar effect was seen in the lungs of guinea pigs receiving H at 60 mg/kg and RZ (data not shown).
FIG. 2.
Lung histopathology in the guinea pig model of chronic TB infection after pyrazinamide therapy. (A and B) Day 0 of treatment (28 days after aerosol infection). Magnifications: A, ×2; B, ×10. Granulomas with punctuate necrosis and large areas of epithelioid histiocytes (H&E stain) are visible. (Inset in panel B) AFB are detected primarily within histiocytes. (C and D) Day 28 after treatment with pyrazinamide at 300 mg/kg. In panel C a decrease in the number and size of granulomas, which are localized more peripherally in the lungs (H&E stain, ×2 magnification), can be seen. Panel D shows a caseating granuloma with partially missing necrotic center, encircled by epithelioid histiocytes and lymphocytes (H&E stain, ×10 magnification). In the inset, AFB are extracellular and confined to areas of necrosis.
Dose-dependent activity of Z against chronic TB infection in mice.
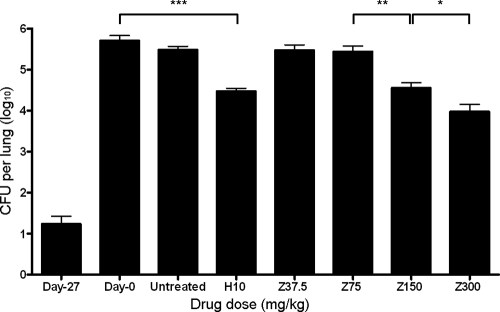
On the day after aerosol infection (day −27), 1.23 ± 0.43 log10 CFU were recovered from mouse lungs, and the lung bacillary burden increased to 5.7 ± 0.3 log10 CFU on the day of treatment initiation (day 0). Bacillary growth was contained by host immune responses in untreated mice, maintaining a stable lung census of 5.5 ± 0.2 log10 CFU at the completion of the experiment (Fig. 3). H at 10 mg/kg showed significant activity against M. tuberculosis in mice, reducing the lung CFU by ∼1.0 log10 to 4.5 ± 0.2 log10 CFU after 28 days of treatment (P = 0.00001). Z at 37.5 mg/kg and Z at 75 mg/kg did not exhibit significant bactericidal activity by day 28, since the lung CFU counts in these groups were 5.5 ± 0.3 log10 (P = 0.22) and 5.4 ± 0.3 log10 (P = 0.17), respectively. In contrast, by day 28, Z at 150 mg/kg (the human-equivalent dose) had significantly greater activity than Z at 75 mg/kg, reducing the bacterial burden in mouse lungs by ∼1.0 log10 to 4.6 ± 0.3 log10 CFU (P = 0.002). Doubling the Z dose to twice the human-equivalent dose (300 mg/kg) further significantly increased the bactericidal activity of Z relative to the 150-mg/kg dose, reducing lung CFU by 1.7 log10 to 4.0 ± 0.4 log10 CFU (P = 0.03; Fig. 3).
FIG. 3.
Dose-dependent activity of pyrazinamide (Z) against chronic murine TB. Mice were aerosol infected with M. tuberculosis H37Rv, and antibiotic treatment was initiated 28 days later (day 0). Mice were randomized to one of the following regimens: no treatment (untreated), isoniazid at 10 mg/kg (H10), Z at 37.5 mg/kg (Z37.5), Z at 75 mg/kg (Z75), Z at 150 mg/kg (Z150), or Z at 300 mg/kg (Z300). Mice were treated with antibiotics five times weekly for a total of 28 days. ***, P = 0.00001; **, P = 0.002; *, P = 0.03. The data represent mean CFU counts per lung ± the standard deviation. n = 5 mice per data point.
Dose-dependent activity of Z against chronic TB infection in guinea pigs.
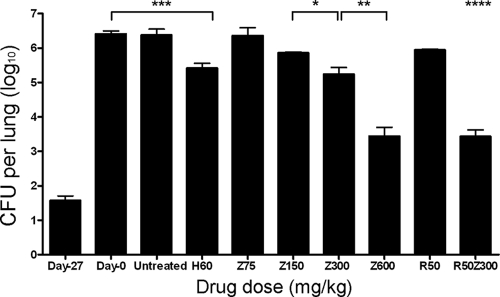
Guinea pig lungs were implanted with 1.6 ± 0.3 log10 bacilli on day −27, and the organisms multiplied to a peak lung burden of 6.4 ± 0.2 log10 CFU on day 0. As in mice, bacillary growth was controlled in the lungs of untreated guinea pigs, which had 6.4 ± 0.3 log10 CFU at the end of the study (Fig. 4). H at 60 mg/kg showed significant activity against M. tuberculosis in guinea pigs, reducing lung CFU by ∼1.0 log10 to 5.4 ± 0.3 log10 CFU after 28 days of treatment (P = 0.0009). Z at 75 mg/kg showed no bactericidal activity, since the lung bacillary burden of animals in this group at the end of treatment (6.4 ± 0.5 log10) was identical to that at treatment initiation (P = 0.997). Z at 150 mg/kg showed somewhat greater activity than Z at 75 mg/kg, reducing lung CFU by ∼0.4 log10 to 6.0 ± 0.1 log10, although this result was not statistically significant (P = 0.17). Guinea pigs receiving Z at 300 mg/kg (human-equivalent dose) had a statistically significant reduction in lung CFU relative to those receiving Z at 150 mg/kg, since CFU dropped by 1.1 log10 to 5.3 ± 0.4 log10 (P = 0.02). Relative to Z at 300 mg/kg, doubling the human-equivalent dose to Z at 600 mg/kg markedly increased its bactericidal activity against M. tuberculosis in guinea pigs, since lung CFU counts were reduced by 3 log10 to 3.4 ± 0.5 log10 CFU (P = 0.001; Fig. 4).
FIG. 4.
Dose-dependent activity of pyrazinamide (Z) and synergistic activity of Z and rifampin (R) against chronic guinea pig TB. Guinea pigs were aerosol infected with M. tuberculosis H37Rv, and antibiotic treatment was initiated 28 days later (Day 0). Guinea pigs were randomized to one of the following regimens: no treatment (untreated), isoniazid at 60 mg/kg (H60); Z at 75 mg/kg (Z75), Z at 150 mg/kg (Z150), Z at 300 mg/kg (Z300), Z at 600 mg/kg (Z600), R at 50 mg/kg (R50), or R (50 mg/kg) plus Z (300 mg/kg) (R50Z300). Guinea pigs were treated with antibiotics five times weekly for a total of 28 days. ***, P = 0.0009; **, P = 0.001; *, P = 0.02; ****, P = 0.001 for multiplicative as opposed to additive interaction of R and Z by linear regression model. The data represent mean CFU counts per lung ± the standard deviation. n = 4 guinea pigs per data point.
Synergistic bactericidal activity of RZ combination against chronic TB in guinea pigs.
The synergistic activity of R and Z against M. tuberculosis has been well documented in mice (26) and humans (50). We evaluated the potential synergy of the RZ combination administered at human-equivalent doses in guinea pigs. Although treatment with R at 50 mg/kg alone and Z at 300 mg/kg alone resulted in bacillary kills of ∼0.5 and ∼1.0 log10, respectively, the combination RZ showed synergy, reducing the lung bacillary burden of guinea pigs by 3 log10 to 3.4 ± 0.4 log10 (Fig. 4). In a linear regression model, there was a statistically significant interaction between R and Z, indicating a multiplicative (as opposed to simply additive) effect of both drugs when used in combination compared to use of either drugs alone, no drugs, or isoniazid alone (P = 0.001 for interaction term).
DISCUSSION
Our study presents three major findings. (i) In contrast to previous studies (12, 42), Z is highly active in the guinea pig model of chronic TB infection. (ii) Z given at the currently recommended dose in humans exhibits equivalent activity against tubercle bacilli in both predominantly intracellular and predominantly extracellular animal models of TB infection. (iii) Finally, the activity of Z increases against both intracellular as well as extracellular bacilli by doubling the standard clinical dose of the drug.
Our findings appear to conflict with those of older studies, which showed limited or no activity of Z in M. tuberculosis-infected guinea pigs (12, 42). Dessau et al. showed that, when initiated 14 days after intraperitoneal inoculation with M. tuberculosis and continued for 3 weeks, Z at 50 mg given orally once daily was slightly more effective than p-aminosalicylic acid at 300 mg in the diet and nicotinamide at 200 mg orally but was slightly less effective than 4,4′-diaminodiphenylsulfone at 50 mg given orally and streptomycin at 15 mg given subcutaneously, as assessed by gross pathology of the spleen, omentum, and lymph nodes (12). These investigators were not able to establish a dose-response curve for Z because of significant variability in response to the same drug dosage with different batches of the drug. Moreover, Z did not appear to be associated with any significant survival benefit in M. tuberculosis-infected guinea pigs relative to untreated controls in two separate survival experiments (12). Steenken and Wolinsky showed that Z at 50 mg given orally twice daily, when initiated 21 days after subcutaneous infection with M. tuberculosis H37Rv, had no significant effect on organ gross pathology 75 days after infection (42). In a second experiment, in which treatment was initiated 30 days after infection and gross pathology was assessed 100 days after infection, Z at 55 mg/kg given orally twice daily showed very modest activity relative to daily H at 5 mg given intramuscularly (42). These early findings have led authorities to conclude that Z lacks activity in the guinea pig model of TB infection (50). However, recent studies have found that the PK parameter most closely linked with M. tuberculosis killing by Z is the AUC/MIC (18), and it should be noted that the doses of Z used in the earlier studies yield an AUC well below that achieved by the currently recommended dose of 1 to 2 g (15 to 30 mg/kg) a day for adult humans (34). On the other hand, the Z dose used to treat guinea pigs in our study (300 mg/kg) more closely approximates the plasma AUC after standard Z dosing in humans (2), and our data indicate that the subtherapeutic doses used in the earlier studies show minimal antituberculous activity. In addition, the early studies used only gross pathology and survival as study outcome measures. Our findings show that even at a dose twice the human-equivalent dose, Z had no detectable effect on gross pathology and histology.
Our data differ from clinical observations showing that Z monotherapy leads to the emergence of drug resistance in some patients within 2 to 3 weeks, particularly in those with large cavities or high sputum bacillary content (49), thus terminating the dose effect of the drug. Although Z-resistant mutants were not enumerated at the conclusion of our study due to the technically challenging and nonstandardized nature of Z resistance testing, it is important to note that our animal models of chronic TB infection differ significantly from humans with pulmonary TB. The bacillary burden at the start of treatment in mice and guinea pigs in our study was ∼106 CFU, whereas in most cases of human cavitary TB, the number of bacilli exceeds 109 (8). Since the spontaneous mutations associated with Z resistance occur at a frequency of 10−6, it is possible that there were no Z-resistant mutants present at the start of therapy in our study. Alternatively, Z-resistant mutants may have reduced virulence in mice and/or guinea pigs, as in the case of H-resistant mutants in guinea pigs (1).
A recent study using an in vitro pharmacokinetic (PK) system and Monte Carlo simulations of 10,000 patients concluded that the population of persisters targeted by Z must be localized to an extracellular compartment in the lungs (18). Our findings corroborate the hypothesis that Z has activity against extracellular bacilli in mammalian lungs. However, our observations that human-equivalent doses of Z have similar bactericidal activity in the mouse model of TB infection indicate that the drug may also target intracellular populations of slowly replicating bacilli. There are several potential explanations for the apparent discrepancy between our findings and those of Gumbo et al. Previous studies have shown that Z is more active against “old,” nongrowing tubercle bacilli than against “young,” actively replicating organisms (51). Thus, it is possible that the killing activity of Z since may not be best represented in the in vitro hollow fiber system, in which the organisms are not senescent and are actively multiplying (18). This hypothesis is consistent with our observations that Z lacks even bacteriostatic activity against M. tuberculosis in mice if initiated immediately after infection, when the organisms are multiplying exponentially prior to immune containment (3, 36). In addition, the Monte Carlo simulations in the study by Gumbo et al. were based on measurements of Z concentration in plasma, as well as in epithelial lining fluid and alveolar cells recovered by bronchoalveolar lavage from 40 normal volunteers and subjects with AIDS following administration of 1 g of Z once daily for 5 days (10). First, drug concentrations within alveolar cells accessible by bronchoscopy may not be representative of those within activated macrophages in the lung parenchyma. Second, even if drug concentrations are significantly lower in IFN-γ-activated lung macrophages relative to those in plasma and epithelial lining fluid (i.e., lower AUC), it is possible that acidification of the intraphagosomal compartment pH to ∼4.5 within activated macrophages may render the slowly replicating bacilli contained therein more susceptible to Z (44). Drug concentrations in mouse and guinea pig epithelial lining fluid and alveolar macrophages were not measured directly in our study. However, since IFN-γ is critical for immune containment of M. tuberculosis infection in mice (15), it is possible that bacilli contained within the phagosomes of IFN-γ-activated macrophages in chronically infected mice may be more susceptible to Z. Although its precise role has not been established, IFN-γ is induced and appears to be functional in patients with TB (4, 9, 38) and may be important in human immune protection against mycobacterial infection (31). Thus, Z may target at least two different populations of M. tuberculosis: bacilli contained within the phagosome of activated lung macrophages, as represented in the mouse model of TB infection, and extracellular organisms residing within acidic compartments of necrotic granulomas, as represented by the guinea pig model of TB infection.
Although the experimental design of our study did not allow us to identify the dose associated with the maximum possible bacterial killing in each model (Emax), our data demonstrate increased antituberculous activity of Z in mice and guinea pigs when the human-equivalent dose is doubled. In particular, this effect was most pronounced in guinea pig lungs, in which the bacilli are located primarily extracellularly within necrotic granulomas resembling human TB lesions (28, 29). Our findings support the hypothesis that higher doses of Z than those currently used in clinical practice could result in better treatment outcomes (18), which may be particularly important in the setting of multidrug-resistant TB, for which the treatment is longer and cure rates lower than for drug-susceptible TB. Recent data suggest that doubling the currently recommended dose of Z to 60 mg/kg daily may not be associated with an increased risk of hepatotoxicity (33). Clinical studies designed to investigate the potential benefit versus toxicity of higher doses of Z in combination regimens are warranted.
Acknowledgments
We thank Michael L. Pinn for technical assistance with guinea pig experiments and Sandeep Tyagi and Austin Minkowski for technical assistance with mouse experiments.
This study was supported by The Bill and Melinda Gates Foundation (TB Accelerator grant 42851 to J.H.G., E.L.N., and P.C.K.) and the National Institutes of Health (AI064229 and AI083125 to P.C.K.).
Footnotes
Published ahead of print on 31 January 2011.
REFERENCES
- 1.Ahmad, Z., et al. 2009. Biphasic kill curve of isoniazid reveals the presence of drug-tolerant, not drug-resistant, Mycobacterium tuberculosis in the guinea pig. J. Infect. Dis. 200:1136-1143. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad, Z., et al. 2010. Comparison of the “Denver regimen” against acute tuberculosis in the mouse and guinea pig. J. Antimicrob. Chemother. 65:729-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almeida, D., et al. 2009. Paradoxical effect of isoniazid on the activity of rifampin-pyrazinamide combination in a mouse model of tuberculosis. Antimicrob. Agents Chemother. 53:4178-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes, P. F., et al. 1990. Local production of tumor necrosis factor and IFN-gamma in tuberculous pleuritis. J. Immunol. 145:149-154. [PubMed] [Google Scholar]
- 5.British Medical Research Council. 1986. Controlled clinical trial of 4 short-course regimens of chemotherapy (three 6-month and one 8-month) for pulmonary tuberculosis: final report. East and Central African/British Medical Research Council Fifth Collaborative Study. Tubercle 67:5-15. [DOI] [PubMed] [Google Scholar]
- 6.British Medical Research Council. 1991. Controlled trial of 2, 4, and 6 months of pyrazinamide in 6-month, three-times-weekly regimens for smear-positive pulmonary tuberculosis, including an assessment of a combined preparation of isoniazid, rifampin, and pyrazinamide: results at 30 months. Hong Kong Chest Service/British Medical Research Council. Am. Rev. Respir. Dis. 143:700-706. [DOI] [PubMed] [Google Scholar]
- 7.British Medical Research Council. 1986. Long-term follow-up of a clinical trial of six-month and four-month regimens of chemotherapy in the treatment of pulmonary tuberculosis. Singapore Tuberculosis Service/British Medical Research Council. Am. Rev. Respir. Dis. 133:779-783. [PubMed] [Google Scholar]
- 8.Canetti, G. 1965. Present aspects of bacterial resistance in tuberculosis. Am. Rev. Respir. Dis. 92:687-703. [DOI] [PubMed] [Google Scholar]
- 9.Choi, H. S., P. R. Rai, H. W. Chu, C. Cool, and E. D. Chan. 2002. Analysis of nitric oxide synthase and nitrotyrosine expression in human pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 166:178-186. [DOI] [PubMed] [Google Scholar]
- 10.Conte, J. E., Jr., J. A. Golden, S. Duncan, E. McKenna, and E. Zurlinden. 1999. Intrapulmonary concentrations of pyrazinamide. Antimicrob. Agents Chemother. 43:1329-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crowle, A. J., R. Dahl, E. Ross, and M. H. May. 1991. Evidence that vesicles containing living, virulent Mycobacterium tuberculosis or Mycobacterium avium in cultured human macrophages are not acidic. Infect. Immun. 59:1823-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dessau, F. I., R. L. Yeager, F. J. Burger, and J. H. Williams. 1952. Pyrazinamide (aldinamide) in experimental tuberculosis of the guinea pig. Am. Rev. Tuberc. 65:519-522. [PubMed] [Google Scholar]
- 13.Dickinson, J. M., and D. A. Mitchison. 1970. Suitability of rifampicin for intermittent administration in the treatment of tuberculosis. Tubercle 51:82-94. [DOI] [PubMed] [Google Scholar]
- 14.Flynn, J., and J. Chan. 2004. Animal models of tuberculosis, p. 237-250. In W. Rom and S. Garay (ed.), Tuberculosis, 2nd ed. Lippincott/The Williams & Wilkins Co., Philadelphia, PA.
- 15.Flynn, J. L., et al. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomez, J. E., and J. D. McKinney. 2004. Persistence and drug tolerance, p. 101-114. In W. N. Rom and S. M. Garay (ed.), Tuberculosis, 2nd ed. Lippincott/The Williams & Wilkins Co., Philadelphia, PA.
- 17.Grosset, J., C. Truffot-Pernot, C. Lacroix, and B. Ji. 1992. Antagonism between isoniazid and the combination pyrazinamide-rifampin against tuberculosis infection in mice. Antimicrob. Agents Chemother. 36:548-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gumbo, T., C. S. Dona, C. Meek, and R. Leff. 2009. Pharmacokinetics-pharmacodynamics of pyrazinamide in a novel in vitro model of tuberculosis for sterilizing effect: a paradigm for faster assessment of new antituberculosis drugs. Antimicrob. Agents Chemother. 53:3197-3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haapanen, J. H., I. Kass, G. Gensini, and G. Middlebrook. 1959. Studies on the gaseous content of tuberculous cavities. Am. Rev. Respir. Dis. 80:1-5. [DOI] [PubMed] [Google Scholar]
- 20.Heifets, L., M. Higgins, and B. Simon. 2000. Pyrazinamide is not active against Mycobacterium tuberculosis residing in cultured human monocyte-derived macrophages. Int. J. Tuberc. Lung Dis. 4:491-495. [PubMed] [Google Scholar]
- 21.Hoff, D. R., et al. 2008. Metronidazole lacks antibacterial activity in guinea pigs infected with Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 52:4137-4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jindani, A., V. R. Aber, E. A. Edwards, and D. A. Mitchison. 1980. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am. Rev. Respir. Dis. 121:939-949. [DOI] [PubMed] [Google Scholar]
- 23.Klinkenberg, L. G., L. A. Sutherland, W. R. Bishai, and P. C. Karakousis. 2008. Metronidazole lacks activity against Mycobacterium tuberculosis in an in vivo hypoxic granuloma model of latency. J. Infect. Dis. 198:275-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konno, K., F. M. Feldmann, and W. McDermott. 1967. Pyrazinamide susceptibility and amidase activity of tubercle bacilli. Am. Rev. Respir. Dis. 95:461-469. [DOI] [PubMed] [Google Scholar]
- 25.Kushner, S., et al. 1952. Experimental chemotherapy of tuberculosis. II. The synthesis of pyrazinamides and related compounds. J. Am. Chem. Soc. 74:3617-3621. [Google Scholar]
- 26.Lecoeur, H. F., P. H. Lagrange, C. Truffot-Pernot, M. Gheorghiu, and J. Grosset. 1989. Relapses after stopping chemotherapy for experimental tuberculosis in genetically resistant and susceptible strains of mice. Clin. Exp. Immunol. 76:458-462. [PMC free article] [PubMed] [Google Scholar]
- 27.Lecoeur, H. F., C. Truffot-Pernot, and J. H. Grosset. 1989. Experimental short-course preventive therapy of tuberculosis with rifampin and pyrazinamide. Am. Rev. Respir. Dis. 140:1189-1193. [DOI] [PubMed] [Google Scholar]
- 28.Lenaerts, A. J., et al. 2007. Location of persisting mycobacteria in a Guinea pig model of tuberculosis revealed by r207910. Antimicrob. Agents Chemother. 51:3338-3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMurray, D. N. 1994. Guinea pig model of tuberculosis, p. 135-147. In B. Bloom (ed.), Tuberculosis: pathogenesis, protection, and control. American Society for Microbiology, Washington, DC.
- 30.Mitchison, D. A. 1985. The action of antituberculosis drugs in short-course chemotherapy. Tubercle 66:219-225. [DOI] [PubMed] [Google Scholar]
- 31.Newport, M. J., et al. 1996. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N. Engl. J. Med. 335:1941-1949. [DOI] [PubMed] [Google Scholar]
- 32.Ordway, D. J., et al. 2010. Evaluation of standard chemotherapy in the guinea pig model of tuberculosis. Antimicrob. Agents Chemother. 54:1820-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pasipanodya, J. G., and T. Gumbo. 2010. Clinical and toxicodynamic evidence that high-dose pyrazinamide is not more hepatotoxic than the low doses currently used. Antimicrob. Agents Chemother. 54:2847-2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peloquin, C. A., et al. 1997. Population pharmacokinetic modeling of isoniazid, rifampin, and pyrazinamide. Antimicrob. Agents Chemother. 41:2670-2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raynaud, C., et al. 1999. Mechanisms of pyrazinamide resistance in mycobacteria: importance of lack of uptake in addition to lack of pyrazinamidase activity. Microbiology 145(Pt. 6):1359-1367. [DOI] [PubMed] [Google Scholar]
- 36.Rullas, J., et al. 2010. Fast standardized therapeutic-efficacy assay for drug discovery against tuberculosis. Antimicrob. Agents Chemother. 54:2262-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salfinger, M., A. J. Crowle, and L. B. Reller. 1990. Pyrazinamide and pyrazinoic acid activity against tubercle bacilli in cultured human macrophages and in the BACTEC system. J. Infect. Dis. 162:201-207. [DOI] [PubMed] [Google Scholar]
- 38.Schon, T., et al. 2004. Local production of nitric oxide in patients with tuberculosis. Int. J. Tuberc. Lung Dis. 8:1134-1137. [PubMed] [Google Scholar]
- 39.Scorpio, A., and Y. Zhang. 1996. Mutations in pncA, a gene encoding pyrazinamidase/nicotinamidase, cause resistance to the antituberculous drug pyrazinamide in tubercle bacillus. Nat. Med. 2:662-667. [DOI] [PubMed] [Google Scholar]
- 40.Shang, S., et al. 2011. Activities of TMC207, rifampin, and pyrazinamide against Mycobacterium tuberculosis infection in guinea pigs. Antimicrob. Agents Chemother. 55:124-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steele, M. A., and R. M. Des Prez. 1988. The role of pyrazinamide in tuberculosis chemotherapy. Chest 94:845-850. [DOI] [PubMed] [Google Scholar]
- 42.Steenken, W., Jr., and E. Wolinsky. 1954. The antituberculous activity of pyrazinamide in vitro and in the guinea pig. Am. Rev. Tuberc. 70:367-369. [DOI] [PubMed] [Google Scholar]
- 43.Sturgill-Koszycki, S., et al. 1994. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science 263:678-681. [DOI] [PubMed] [Google Scholar]
- 44.Vandal, O. H., C. F. Nathan, and S. Ehrt. 2009. Acid resistance in Mycobacterium tuberculosis. J. Bacteriol. 191:4714-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vandal, O. H., L. M. Pierini, D. Schnappinger, C. F. Nathan, and S. Ehrt. 2008. A membrane protein preserves intrabacterial pH in intraphagosomal Mycobacterium tuberculosis. Nat. Med. 14:849-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vandiviere, H. M., W. E. Loring, I. Melvin, and S. Willis. 1956. The treated pulmonary lesion and its tubercle bacillus. II. The death and resurrection. Am. J. Med. Sci. 232:30-37. [DOI] [PubMed] [Google Scholar]
- 47.Via, L. E., et al. 2008. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect. Immun. 76:2333-2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wade, M. M., and Y. Zhang. 2004. Anaerobic incubation conditions enhance pyrazinamide activity against Mycobacterium tuberculosis. J. Med. Microbiol. 53:769-773. [DOI] [PubMed] [Google Scholar]
- 49.Yeager, R. L., W. G. Munroe, and F. I. Dessau. 1952. Pyrazinamide (aldinamide) in the treatment of pulmonary tuberculosis. Am. Rev. Tuberc. 65:523-546. [PubMed] [Google Scholar]
- 50.Zhang, Y., and D. Mitchison. 2003. The curious characteristics of pyrazinamide: a review. Int. J. Tuberc. Lung Dis. 7:6-21. [PubMed] [Google Scholar]
- 51.Zhang, Y., S. Permar, and Z. Sun. 2002. Conditions that may affect the results of susceptibility testing of Mycobacterium tuberculosis to pyrazinamide. J. Med. Microbiol. 51:42-49. [DOI] [PubMed] [Google Scholar]
- 52.Zhang, Y., A. Scorpio, H. Nikaido, and Z. Sun. 1999. Role of acid pH and deficient efflux of pyrazinoic acid in unique susceptibility of Mycobacterium tuberculosis to pyrazinamide. J. Bacteriol. 181:2044-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang, Y., M. M. Wade, A. Scorpio, H. Zhang, and Z. Sun. 2003. Mode of action of pyrazinamide: disruption of Mycobacterium tuberculosis membrane transport and energetics by pyrazinoic acid. J. Antimicrob. Chemother. 52:790-795. [DOI] [PubMed] [Google Scholar]
- 54.Zimhony, O., J. S. Cox, J. T. Welch, C. Vilcheze, and W. R. Jacobs, Jr. 2000. Pyrazinamide inhibits the eukaryotic-like fatty acid synthetase I (FASI) of Mycobacterium tuberculosis. Nat. Med. 6:1043-1047. [DOI] [PubMed] [Google Scholar]