Abstract
The development of Alzheimer's disease (AD) later in life may be reflective of environmental factors operating over the course of a lifetime. Educational and occupational attainments have been found to be protective against the development of the disease but participation in activities has received little attention. In a case-control study, we collected questionnaire data about 26 nonoccupational activities from ages 20 to 60. Participants included 193 people with probable or possible AD and 358 healthy control-group members. Activity patterns for intellectual, passive, and physical activities were classified by using an adaptation of a published scale in terms of “diversity” (total number of activities), “intensity” (hours per month), and “percentage intensity” (percentage of total activity hours devoted to each activity category). The control group was more active during midlife than the case group was for all three activity categories, even after controlling for age, gender, income adequacy, and education. The odds ratio for AD in those performing less than the mean value of activities was 3.85 (95% confidence interval: 2.65–5.58, P < 0.001). The increase in time devoted to intellectual activities from early adulthood (20–39) to middle adulthood (40–60) was associated with a significant decrease in the probability of membership in the case group. We conclude that diversity of activities and intensity of intellectual activities were reduced in patients with AD as compared with the control group. These findings may be because inactivity is a risk factor for the disease or because inactivity is a reflection of very early subclinical effects of the disease, or both.
Keywords: case-control study, dementia, epidemiology, leisure, recreation
Work in North America, Europe, Asia, and the Middle East has shown that the incidence and prevalence of Alzheimer's disease (AD) is lower in subjects with relatively higher levels of education (1–4). According to the East Boston study, each year of education reduced the risk of AD by 17% (3). Although the protection against development of AD provided by education could be an artifact produced by the ability of more highly educated persons to perform better on cognitive tests (4–7), many studies have used functional rather than psychometric measures for diagnosis and have documented the protective effect of education (1, 2, 4). Although the mechanisms of education protection remain unknown, Katzman (1) has proposed that the protective effects of education are related to neuronal reserve; individuals with higher levels of education are more resistant to the effects of the disease on cognition because of enhanced synaptic complexity. Occupational attainment also has been demonstrated to be protective against the disease (3, 8).
Educational protection also may be induced by lifelong patterns of neuronal activation associated with exposure to education (9–12). But education and occupation are not the only reflection of these lifelong patterns; recreational activities are also indications of the ways in which cognitive and other skills are used in daily life (13, 14). We have hypothesized that recreational tasks, in addition to education and occupation, are protective against the development of AD (10, 11). Leisure endeavors are reflective of the intrinsic value of an activity for an individual (14)—they may be more reflective of neurological factors than education or occupation, which are strongly influenced by socioeconomic determinants, especially in the earlier years of this century when economic, social, and military factors often determined who went to school and for how long. Recreational activities provide a reflection of neuronal reserve and activation that may be relatively independent of these economic, social, and military factors.
The pathological features of AD are most profound in the limbic system and temporal, frontal and association neocortices, and basal forebrain areas involved in learning, memory, emotion, judgement, abstraction, language, and executive functions (15). We therefore hypothesized that intellectual activities involving learning and memory would be most protective against the development of the disease.
Hultsch et al. (16) have reported that “favorable life experiences or conditions may forestall or attenuate the declines typically seen in a variety of cognitive processes in later adulthood.” Similarly, Schooler (17) has found that “environmental complexity” is associated with enhanced cognitive function throughout life. Because of the very chronic nature of AD (18) and its strong relation to age, it is likely that interactions between “favorable life experiences” (which may be associated with education and occupation) and cognitive decline will be operative for both healthy aging as well as neurodegenerative disorders such as AD.
We have evaluated the relationships between nonoccupational activities and AD in a case-control study. Activities from the ages of 20 to 60 years were studied. Information about activities after age 60 or 5 years before disease onset was not collected, because it is clear that the disease itself is associated with reduced activities (19, 20), a reduction that could very well occur in the premorbid period before the patient or family is aware of the onset of dementia.
Methods
Subjects.
Subjects were participants in the Alzheimer's Disease Case-Control Study at Case Western Reserve University, University Hospitals of Cleveland, which was initiated in 1991. This project was approved by the Institutional Review Board of University Hospitals of Cleveland (09–92-210). Patients (N = 193) were recruited from clinical settings and the community and all were enrolled in the Research Registry of the University Alzheimer Center, University Hospitals of Cleveland. Patients were evaluated by neuropsychological, laboratory, and neurological exams and all had x-ray computed tomography or MRI scans of the brain. In all cases, patients had a probable (79%) or a possible AD (21%) diagnosis that was reached by consensus conference by using National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association criteria (21). Case-group patients were required to have had an onset of symptoms within 5 years of evaluation at the University Alzheimer Center, to minimize the contribution of premorbid features. The control-group members (N = 358) were the friends or neighbors of the case-group members or were members of the same organizations to which the case-group members belonged. Surrogates for case-group members were asked to identify friends, neighbors, or organizations to which the case-group members belonged. The control-group members were acquired by using frequency matching for age and gender. Spouse control-group members were not used to avoid overmatching. All case-group members had surrogates available who had known the case-group member for at least the last 10 years, and who had a close personal relationship with the case-group member. Surrogates were 62% spouses, 28% children, and 10% siblings or friends. Refusal rates for participation as control-group members were 65/346 (19%) for males and 106/537 (20%) for females (some subjects who agreed may have been refused participation because of exclusion criteria). Refusers were no different in comparison to responders in regard to gender, age, geographical location, or education. Control-group members were examined in the same way as case-group members and were determined to be free of neurological, psychiatric, or medical diseases affecting cognition. We compared control-group members obtained as friends or neighbors of case-group members with those acquired from organizations and found no differences in the two types of control-group members in regard to demographic variables, cognitive performance, or personality (neurotocism, extraversion, openness personality inventory; ref. 41). Subjects with a history of alcoholism, drug abuse, major head trauma, cancer, or other illnesses likely to impair cognition were not accepted into either the case or control groups. Control-group members were paid $30 for their participation in the study. After a complete description of the study was given to the subjects and their families, written informed consent was obtained.
Measures.
We studied 26 different types of activities, asking three questions about each one: (i) Did subjects participate in the activity at least once per month? If yes, (ii) how many hours per month in their 20s and 30s (i.e., early adulthood); and (iii) how many hours per month in their 40s and 50s (i.e., middle adulthood)? These data were referred to as the “ever/never” data, the “20s and 30s hours” data, and the “40s and 50s hours” data, respectively. We inquired about activities in the teen years but found these data to be unreliable because of missing data, as few appropriate informants could be found. We did not acquire data about the period following age 60, or less than 5 years before disease onset in case-group members, because of the confounding effect of the premorbid and morbid effects of illness on participation in activities. Questionnaire data included other possible risk or protective factors, including education, family history, medication use, medical history, diet, and smoking habits (22). Questionnaires were completed in the home by the control-group members themselves and by a surrogate for case-group members and mailed to the Alzheimer Center. Ninety-nine percent of questionnaires were returned. The activity questionnaire used is available from the authors upon request.
Data from the 26 activities were grouped into three general activity categories (passive, intellectual, and physical) adapted from empirical-theoretical work by Hultsch et al. (23). The 26 activities for the three activity categories also are available from the authors upon request. These activity types and categories were used to develop three major measures: diversity, intensity, and percentage intensity.
Diversity.
Diversity was defined as the sum of the total number of activities participated in at least once per month per category, divided by the total number of activities making up an activity category. (For example, for subjects who reported doing five physical activities, diversity scores equaled 0.56, because there were 9 possible physical activities variables and 5/9 = 0.56.)
Intensity.
Passive, intellectual, and physical intensity were defined as the sum of the total hours per month devoted to each activity type. For example, physical intensity was calculated by summing the hours per month devoted to baseball, football, basketball, soccer, hockey, working out in a gym, racquet sports, bike riding, golf, bowling, gardening, ice skating, roller skating, jogging, swimming, and walking for exercise. Separate intensity scores were calculated for early (ages 20 to 39) and middle adulthood (40 to 59).
Percentage intensity.
Percentage intensity was defined as the percent of total activity hours per month devoted to each activity category (passive, intellectual, and physical). Percentage intensity in early and middle adulthood was calculated by dividing intensity scores by the total number of hours devoted to all three activity categories. The result was then multiplied by 100. Separate percentage intensity scores were calculated for early and middle adulthood. (Because the percentage intensities of passive, intellectual, and physical activities were percent scores, by definition they always summed to 100%). Thus, for example, to calculate the percentage intellectual intensity in early adulthood, we summed the total hours devoted to “intellectual activities” in early adulthood (i.e., intellectual intensity), then divided by the sum of the hours devoted to all three activity categories in early adulthood, and then multiplied the result by 100.
Treatment of Missing Data.
Missing data were imputed in a two-step sequence. In the first step, missing values for the “ever/never” variables were imputed by using “hot-deck” procedures available in the SOLAS MISSING DATA ANALYSIS 1.0 statistical software (Statistical Solutions, Saugus, MA.). “Hot-deck imputation” sorts respondents and nonrespondents into imputation classes according to a user-specified set of auxiliary variables. Missing values are replaced with values taken from matching respondents (i.e., respondents similar with respect to the auxiliary variables). In the current study, sorting variables included: (i) year of birth, (ii) gender, and (iii) years of education. Imputed values were selected randomly from the imputation classes created by using these variables. The percentages of missing data across the 26 “ever/never” variables before imputation ranged from 0.5% to 3.8%.
In the second step, we imputed missing data for the “hours” variables. For subjects who reported that they had never completed an activity during their lives (as indicated by the “ever/never” variables), the corresponding missing “hours” data were coded automatically as 0. After this adjustment, missing data for the “hours” variables were imputed by using the “hot-deck” methods described above. Year of birth, gender, and years of education again were used as sorting variables. The percentages of missing data across the “hours” variables before imputation ranged from 0.5% to 11.6% in early adulthood and from 0.2% to 6.4% in middle adulthood.
Data Analysis.
Data analysis was accomplished in five steps:
1. Sociodemographic characteristics. Case- and control-group members were compared on the basis of basic sociodemographic characteristics by using t tests, χ2 tests, and the Wilcoxon sign-rank test, where appropriate.
2. Activity count. A t test was used to compare case- and control-group members on the basis of the overall raw count of activities in which subjects ever participated. All subjects were divided into two groups and were defined by the mean raw count of activities in which subjects ever participated, and the odds ratio for disease status in those having less than the mean raw count of activities was calculated.
3. Diversity. Separate one-way between-subjects ANOVAs were completed with case/control status as the independent variable and passive, intellectual, and physical “diversity” scores as dependent measures. To decrease the variance associated with sociodemographic characteristics, a series of one-way between-subjects analyses of covariance (ANCOVA) also were run, with year of birth, gender, years of education, and income adequacy as covariates. All subjects then were divided into two groups, defined by the mean diversity score for each diversity-dependent variable, and the odds ratio for disease status in those having less than the mean diversity scores was calculated.
4. Intensity. Case- and control-group members were compared in separate one-way ANOVAs on “intensity” scores in early adulthood and in middle adulthood. Again, to decrease variance associated with sociodemographic characteristics, a series of one-way ANCOVAs were run, with year of birth, gender, education, and income adequacy as covariates. All subjects were then divided into two groups, defined by the mean intensity score for each intensity variable, and the odds ratios for disease status in those having less than the mean intensity scores were calculated.
5. Percentage intensity. In a final set of analyses, the percentages of total hours per month devoted to intellectual, passive, and physical activities in early and middle adulthood were calculated. The percentages of intellectual and physical variables then were included as predictors in a logistic regression model, along with sociodemographic characteristics (year of birth, gender, years of education, and income adequacy) to predict membership in the case vs. control group.
Surrogate Substudy.
We examined the issue of the bias that may be introduced by the use of the surrogates caused by systematic under- or over-reporting types or hours of activities. The first 50 cognitively intact individuals who entered the case-control study to serve as control-group members were asked to fill out the Life History Questionnaire and also to have it filled out by a person whom they felt was well acquainted with their past and present activities. Because it would not have been possible to dictate the type of relationship between the case-group members with AD and their surrogate respondents, no attempt was made to influence choices regarding the relationship between the control-group members and their surrogates. By using the Hultsch classification scheme, intensity scores for passive, intellectual, and physical activities in the 20s and 30s and in the 40s and 50s were computed for the self and the surrogate responses. Paired t tests were run to test the null hypotheses of zero mean difference between self and surrogate responses.
Results
Sociodemographic Characteristics.
The sociodemographic characteristics of our case- and control-group members are shown in Table 1. Case-group members had significantly lower levels of education than did control-group members, and the case group's median year of birth was slightly earlier than the control group's median year of birth.
Table 1.
Sociodemographic characteristics of case- and control-group members (total n = 551)
| Characteristic | Case-group members (n = 193) | Control-group members (n = 358) |
|---|---|---|
| Age*–[mean (SD)] | 72.5 (8.0) | 71.3 (6.0) |
| Male | 72.2 (7.3) | 71.7 (5.2) |
| Female | 72.7 (8.5) | 71.0 (6.4) |
| Year of birth–median (range)† | 1919 (1898–1944) | 1923 (1899–1936) |
| Male† | 1919 (1898–1940) | 1923 (1909–1934) |
| Female† | 1918 (1901–1944) | 1924 (1899–1936) |
| Gender–% (no.) | ||
| Male | 43.5% (84) | 39.7% (142) |
| Female | 56.5% (109) | 60.3% (216) |
| Education–[mean (SD)]†‡ | 13.0 (2.8) | 15.3 (2.8) |
| Income adequacy, lifetime average– [mean (SD)]§ | 1.7 (0.4) | 1.7 (0.4) |
| MMSE–[mean (SD)]†¶ | 17.8 (6.0) | 28.8 (1.0) |
| Number of activities ever performed†∥ | 12.9 (4.1) | 16.0 (3.4)∥ |
Age at entry into research registry.
P ≤ .001.
Indicates number of years completed.
Household income adequacy scores: 1 = “more than adequate”; 2 = “adequate”; 3 = “not at all adequate.”
Low scores on the Mini-Mental State Examination indicate greater cognitive impairment.
∥ Mean (Standard Deviation). These data are an average of early (20s and 30s) and adulthood (40s and 50s) ratings. There were 26 possible activities.
Activity Count.
Among the 26 activities we studied, control-group members reported performing more activities (mean = 16.0, SD = 3.4) than case-group members (mean = 12.9, SD = 4.1; P < 0.001). This result remained significant after controlling for the potential confounders, year of birth, sex, education, and income adequacy (P < .001). When all subjects were divided into two groups, defined by the mean of the raw count of activities, the odds ratio for disease status in those having less than the mean value of activities was 3.85 (95% confidence interval: 2.65–5.58, P < 0.001).
Diversity.
Passive, intellectual, and physical diversity scores were submitted to separate univariate ANOVAs and ANCOVAs. Results of diversity score ANOVAs and ANCOVAs are presented in Table 2. Control-group members participated in a greater diversity of passive, intellectual, and physical activities than did case-group members [all p values <0.001]. Results remained significant after controlling for covariates [all p values <0.001]. Thus, case- and control-group members differed in terms of the diversity of activities reported across the lifespan, with control-group members participating in a greater diversity of each class of activities than case-group members. The odds ratio for low passive diversity was 2.51 [95% confidence interval (CI): 1.75–3.59, P < 0.001], the ratio for low intellectual diversity was 2.43 (95% CI: 1.66–3.54, P < 0.001), and the ratio for low physical diversity was 2.67 (95% CI: 1.85–3.85, P < 0.001)
Table 2.
Comparisons between case- and control-group members on diversity scores
| Measure | Observed
mean, (SD)
|
ANOVA | Adjusted
mean*
|
ANCOVA | ||
|---|---|---|---|---|---|---|
| Case | Control | Case | Control | |||
| Passive diversity | 0.84 (0.18) | 0.91 (0.14) | F(1, 549) = 33.09† | 0.84 | 0.91 | F(1, 544) = 19.25† |
| Intellectual diversity | 0.43 (0.19) | 0.54 (0.16) | F(1, 549) = 45.95† | 0.44 | 0.54 | F(1, 544) = 33.33† |
| Physical diversity | 0.39 (0.22) | 0.55 (0.22) | F(1, 549) = 60.72† | 0.42 | 0.53 | F(1, 544) = 29.24† |
Adjusted means take into account the effects of covariates (year of birth, education, gender, and income adequacy) in the ANCOVAs.
P ≤ .001.
Intensity.
Passive, intellectual, and physical “intensity” scores in early and middle adulthood were submitted to separate univariate ANOVAs. Results are presented in Table 3. The data show that control-group members participated in a higher mean total hours per month of passive and intellectual activities in early adulthood than did case-group members. Control-group members also participated in a higher mean total hours per month of intellectual activities in late adulthood than did case-group members. However, after controlling for covariates, the “passive hour” difference in early adulthood was no longer statistically significant. Thus, in both early and middle adulthood, control-group members participated in a higher mean total hours per month of intellectual activities than did case-group members.
Table 3.
Comparisons between case- and control-group members on “intensity” scores
| Measure | Observed mean,
(SD)
|
ANOVA | Adjusted mean*
|
ANCOVA | ||
|---|---|---|---|---|---|---|
| Case | Control | Case | Control | |||
| Early adulthood | ||||||
| Passive intensity | 59.28 (50.27) | 69.88 (55.71) | F(1, 549) = 4.85† | 62.23 | 68.43 | F(1, 544) = 1.48 |
| Intellectual intensity | 55.63 (40.36) | 68.79 (52.23) | F(1, 549) = 9.27‡ | 57.45 | 67.94 | F(1, 544) = 4.79† |
| Physical intensity | 32.06 (33.66) | 33.97 (34.58) | F(1, 549) = 0.39 | 31.18 | 34.50 | F(1, 544) = 0.99 |
| Middle adulthood | ||||||
| Passive intensity | 98.90 (68.82) | 101.89 (61.31) | F(1, 549) = 0.27 | 99.33 | 101.84 | F(1, 544) = 0.16 |
| Intellectual intensity | 68.06 (59.80) | 79.14 (55.66) | F(1, 549) = 4.71† | 68.15 | 79.21 | F(1, 544) = 3.82† |
| Physical intensity | 38.12 (38.13) | 40.80 (33.43) | F(1, 549) = 0.73 | 37.74 | 41.09 | F(1, 544) = 0.96 |
Adjusted means take into account the effects of covariates (year of birth, education, gender, and income adequacy) in the ANCOVAs.
P ≤ 0.05.
P ≤ 0.01.
Percentage Intensity and Logistic Regression Results.
The percentage of total hours per month devoted to passive, intellectual, and physical activities in early and in middle adulthood were calculated. Means and standard deviations, stratified by group and gender, are shown in Table 4. Changes (i.e., difference scores) from early to middle adulthood in the percentage of total hours per month devoted to passive, intellectual, and physical activities were also calculated. The data show that many of the subjects in our sample neither increased nor decreased the time devoted to passive, intellectual, or physical activities from early to middle adulthood, with modal scores of 0 for each of the three difference scores (data not shown). However, there were some subjects who increased their percent passive, intellectual, or physical activities from early to middle adulthood, whereas others decreased their percentage of activities in one or more categories. On average, for both case and control groups, passive activities increased, whereas intellectual and physical activities decreased with age.
Table 4.
Mean percent total hours per month devoted to passive, intellectual, and physical activities, by group and gender
| Group | Gender | Percent
total hours per month
|
|||||
|---|---|---|---|---|---|---|---|
| Passive
|
Intellectual
|
Physical
|
|||||
| Mean | SD | Mean | SD | Mean | SD | ||
| Early adulthood | |||||||
| Case | Male | 38.6 | 20.3 | 36.6 | 19.0 | 24.8 | 17.7 |
| Female | 41.4 | 20.4 | 40.6 | 17.6 | 18.1 | 15.3 | |
| Control | Male | 38.0 | 17.4 | 38.3 | 16.0 | 23.7 | 15.7 |
| Female | 42.2 | 18.4 | 40.7 | 17.1 | 17.1 | 14.2 | |
| Middle adulthood | |||||||
| Case | Male | 46.8 | 19.1 | 31.6 | 16.6 | 21.6 | 14.8 |
| Female | 52.4 | 18.6 | 32.4 | 15.6 | 15.2 | 13.4 | |
| Control | Male | 44.0 | 15.1 | 35.0 | 14.5 | 21.1 | 12.2 |
| Female | 47.3 | 15.8 | 35.4 | 13.2 | 17.3 | 11.3 | |
Percent passive, intellectual, and physical may not always sum to 100% because of rounding error.
Next, the intellectual and physical percentages for intensity variables in early and middle adulthood and the sociodemographic variables were included as predictors in a logistic regression model with case vs. control status as the dependent measure. Preliminary analyses, which included interaction variables with gender as predictors, did not reveal significant effects [P values >0.05]. Therefore, interaction terms with gender were not included in the equation.
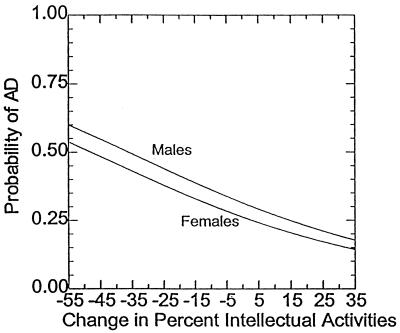
Results from a logistic regression model indicate that, when holding constant sociodemographic characteristics, percentage intensity of intellectual and physical activities in early adulthood, and physical activities in middle adulthood, the percentage of intellectual intensity during middle adulthood was a significant predictor of membership in the case vs. the control group (P < .05). As a graphical presentation of these results, we plotted separately for men and women the probability of membership in the case vs. the control group as a function of change in the percentage of total hours per month devoted to intellectual activities in middle adulthood and the means of the other independent variables (Fig. 1). These results mean that increases from early to middle adulthood in the percentage of intensity of intellectual activities is associated with a statistically significant decrease in the probability of membership in the case vs. the control group when also controlling for covariates. The model fit was good, with a Nagelkerke pseudo R2 of 0.26 (24). In this analysis, we held constant the early-adulthood activities measures so that an increase in the middle-adulthood activities measures can be interpreted as a change from early to middle adulthood.
Figure 1.
Probability of membership in the case group for men and women as a function of changes from early to middle adulthood in percentage of total hours per month devoted to intellectual activities (means of other independent variables included in the logistic regression model).
Fig. 1 represents predicted case- vs. control-group membership results for the “average” male and for the “average” female in our sample in terms of sociodemographic features—passive, intellectual, and physical activities in early adulthood and passive and physical activities in middle adulthood. The graph shows that, assuming these average values, for those subjects who increased their percentages of intellectual activities from early to middle adulthood (and thus decreased their passive activities by a corresponding amount), the probability of membership in the case group decreased. However, for those subjects who decreased their percentages of intellectual activities from early to middle adulthood (and thus increased their passive activities by a corresponding amount), the probability of membership in the case group increased.
Surrogate Substudy.
Forty-nine pairs were available for analysis. The null hypotheses of zero mean differences were not rejected. The test values obtained were as follows: passive intensity 20s/30s: t(29) = 1.271, P = 0.214; passive intensity: 40s/50s t(39) = 0.736, P = 0.466; intellectual intensity 20s/30s: t(29) = 0.232, P = 0.818; intellectual intensity 40s/50s: t(38) = 0.408, P = 0.686; physical intensity 20s/30s: t(26) = 0.418, P = 0.679; and physical intensity 40s/50s: t(35) = −1.125, P = 0.268. Thus, on average, surrogates do not appear to under- or over-report events. The different degrees of freedom of the test statistics reflect missing data, largely from the surrogate respondents. As expected, this occurs more frequently when recalling the earlier periods of life.
Discussion
Our results indicate that patients with AD are less active in midlife (early and middle adulthood) in terms of intellectual, passive, and physical activities than control-group members. The lower premorbid activity levels in patients with AD persisted in measures of intellectual, passive, and physical activities, calculated by using an independently developed scale following statistical correction for year of birth, sex, education, and income adequacy. These differences were not explained by differing educational levels in the two groups. We minimized the influence of early disease on participation in activities by collecting data only concerning the period of midlife ending at age 60 or ending 5 years before disease onset (whichever was earlier). Our results indicate that low participation in activities in midlife (in addition to low levels of educational and occupational achievement) is a risk factor for the disease.
We found that diversity of intellectual, passive, and physical activities were all protective against the development of AD. Our hypothesis that intellectual activities were protective was confirmed, but the effects were seen for passive and physical activities as well. However, the differences between case- and control-group members were greatest in regard to intellectual activities. Odds ratios showed that people who were relatively inactive (for intellectual, passive, or physical activities) had about a 250% increased risk of developing AD. Physical exercise has been reported by our group to be protective against development of the disease.** There are many beneficial effects of physical activity that may be related to reduced risk of AD: lower body weight, improved diet (including increased consumption of antioxidants and lower fat intake), improved blood pressure and cardiovascular health, as well as beneficial effects on blood clotting (25).
We cannot exclude the possibility that our data reflect the very early effects of the disease, several decades before symptom onset. Snowdon and colleagues (18) in the Nun Study have demonstrated a remarkable relationship between early-life linguistic abilities and the late risk of developing AD, suggesting that the disease may have early effects on performance several decades before symptom onset. Positron emission tomographic (PET) studies have also shown that the metabolic effects of AD in apolipoprotein (apo) E-ɛ4 homozygotes may begin 10–20 years before diagnosis (26, 27). Also, ApoE-ɛ4 homozygotes have been reported to have preclinical memory decline in immediate and delayed recall (28). A prospective study of the Framingham cohort also has shown preclinical decline in verbal memory in people who were to eventually develop AD (29). Pathological studies of Down's syndrome brain have shown that diffuse plaques of β-amyloid 1–42 develop as early as 12 years of age (30), even though loss of function is not seen until after age 35. It is possible that the presymptomatic cognitive effects demonstrated in the Nun Study (18) and the early metabolic changes documented with PET (26, 27) also represent risk factors for AD. Both explanations may very well be concurrently correct. It may be possible to analyze the relative contributions of early disease and risk/protective effects in longitudinal studies of transgenic mice having AD genes.
Fig. 1 demonstrates that for the average subject, reduction in intellectual pursuits over the four decades from early to middle adulthood increases the probability of AD. This relationship between disease and intellectual activity may be interpreted as evidence of the progressive effects of the disease on participation or may also represent a protective effect of intellectual activities.
We used an “asymmetrical” method of data collection, obtaining information from surrogates for case-group members and obtaining information from control-group members from themselves, because case-group members cannot self report and control-group members have the most accurate information about their own lives. We did not use surrogates for control-group members because that would not have created a genuinely “symmetrical” method, as the caregiving relationship between case-group members and their surrogates is not the same as the noncaregiving relationship between control-group members and their surrogates in that the surrogates of control-group members are not as well informed. Our surrogate substudy demonstrates that the use of surrogate responders for control-group members would have added imprecision to the data without altering average values, and that there was no systematic under- or over-reporting introduced by the use of control-group members responding for themselves. Our choice of respondents is also supported by a study of alcohol consumption in which primary and proxy respondents have been found to provide similar information (31). Kondo et al. (32) also compared direct and indirect answers in a study on AD, and found that all responses agreed 70% or more (κ = 1, P < 0.05). Proxy respondents have also been found to be a reliable source of information for dementia studies in observations from the MIRAGE project (42).
Previous reports of relationships between activities and AD did not account for the effects of early disease in the several years preceding symptoms. Certainly premorbid decline can cause reduction in activities in years preceding onset of clinical dementia, as demonstrated by Fabrigoule et al. (20) in an incidence study of dementia in subjects who were followed for 3 years. This premorbid effect could influence relationships with physical (33), mental (32, 34), and social activities (19). A study by Zabar and colleagues‡‡ found no difference in activities between case- and highly selected control-group members participating in the Baltimore Longitudinal Study of aging. Again, premorbid activities were not addressed.
Our study has important limitations. Case-group members were recruited from our Alzheimer Center and, along with our control-group members, were not population-based. However, the demographic features of our case- and control-group members (Table 1) are similar to other studies on AD in this country. The use of friends as control-group members and the use of control-group members from the same organizations as case-group members is likely to produce some overmatching, suggesting that our main effects may have been stronger if population-based control-group members were used. Activity participation reflects a complex constellation of economic, occupational, and other factors, and hours of participation do not necessarily reflect quality of participation (i.e., some people may do a task more effectively and spend less time doing it). We have been able to record only whether an activity was done and for how long. We were able to statistically account for some possible confounders (age, sex, education, income adequacy) but did not consider others, such as apoE genotype. Also deserving consideration are possible confounders, which may be independently related to both AD risk and activity levels, including early-life environment, level of medical care, heart disease, personality, stress, occupation, and socioeconomic status (9, 40). Another important confounder, education, was controlled for in the analysis. The classification of activities as intellectual, passive, or physical is arbitrary but supported by the work of Hultsch et al. (23).
Retrospective assessment of participation in activities is likely to contain inaccuracies. However, the only other way to obtain the data required is with a very long-term prospective study. Data from short-term (3–5 years) prospective studies may be influenced by the premorbid and morbid effects of disease—“high-ability individuals lead intellectually active lives until cognitive decline in old age limits their activities” (16).
Donald Hebb had predicted that use contributes to the establishment and maintenance of synapses (12). It may also be that neuronal activation, associated with functional activity, spares the brain from the Alzheimer process through beneficial effects on membranes and amyloid β protein production, degradation, and aggregation (9, 10, 15).
Our results are in accord with those of Hultsch et al. (16), who reported recently that “intellectually engaging activities buffer against longitudinally measured cognitive decline” in a study of 214 persons aged 55 to 86 at time of first assessment. Wilson and colleagues (36) evaluated 6,162 persons in a geographically defined biracial population and also found that cognitive function was related to “composite measures of the frequency and intensity of cognitive activity.” Our results are compatible also with the view that environmental complexity is associated with enhanced cognitive functioning (17) and that underactivity is a risk factor for the development of AD, as proposed in Swaab's (12, 37) “use it or lose it” scenario.
Humans remain genetically equipped for life as Paleolithic hunter–gatherers (35). Activity levels consistent with human survival were certainly higher for all of human history than they are now in the 21st century. A protective relationship between high activity levels and AD may explain partially the low prevalence of the disease in rural India (38) or urban Nigeria [despite high apoE-e4 allele frequency (39)]. Activity levels in developing countries are certainly very high. Relationships between recreational activities and the development of neurodegenerative disorders has received relatively little attention. We believe that the interactions reported here are important because of their significance for public policy.
Acknowledgments
We are grateful for the early guidance of A. R. Feinstein, A. A. Rimm, and J. Guralnik. The contributions of M. McClendon and C. Esteban-Santillian are acknowledged gratefully. This work was supported in part by grants from the National Institute on Aging (PO 263-MO-818915 and VO1 AG1713-01A1), the Alzheimer's Disease Research Center Program (P50 AG 08012), the Mandel Foundation, the Nickman family, the Institute for the Study of Aging (New York), and Philip Morris USA. The sponsors had no role in the design, conduct, interpretation, or analysis of the study. Preliminary accounts of this work have been presented (11).
Abbreviations
- AD
Alzheimer's disease
- ANCOVA
analyses of covariance
Footnotes
Present address: National Institute on Aging/National Institutes of Health, Bethesda, MD 20892.
Zabar, Y. U., Corrada, M., Fozard, J., Cosa, P. & Kawas, C. (1996) Neurology 46, A435 (abstr.).
References
- 1.Katzman R. Neurology. 1993;43:13–20. doi: 10.1212/wnl.43.1_part_1.13. [DOI] [PubMed] [Google Scholar]
- 2.Zhang M Y, Katzman R, Salmon D, Jin H, Cai G J, Wang Z Y, Qu G Y, Grant I, Yu E, Levy P, et al. Ann Neurol. 1990;27:428–437. doi: 10.1002/ana.410270412. [DOI] [PubMed] [Google Scholar]
- 3.Evans D A, Hebert L E, Beckett L A, Scherr P A, Albert M S, Chown M J, Pilgrim D M, Taylor J O. Arch Neurol (Chicago) 1997;54:1399–1405. doi: 10.1001/archneur.1997.00550230066019. [DOI] [PubMed] [Google Scholar]
- 4.Callahan C M, Hall K S, Hui S L, Musick B S, Unverzagt F W, Hendrie H C. Arch Neurol (Chicago) 1996;53:134–140. doi: 10.1001/archneur.1996.00550020038013. [DOI] [PubMed] [Google Scholar]
- 5.Unverzagt F W, Hall K S, Torke A M. Clin Neuropsychol. 1996;10:180–190. [Google Scholar]
- 6.Unverzagt F W, Hui S L, Farlow M R, Hall K S, Hendrie H C. Neurology. 1998;50:181–185. doi: 10.1212/wnl.50.1.181. [DOI] [PubMed] [Google Scholar]
- 7.Gilleard C J. Aging and Mental Health. 1997;1:33–46. [Google Scholar]
- 8.Stern Y, Gurland B, Tatemichi T K, Tang M X, Wilder D, Mayeux R. J Am Med Assoc. 1994;271:1004–1010. [PubMed] [Google Scholar]
- 9.Mortimer J, Graves A B. Neurology. 1993;43:S39–S44. [Google Scholar]
- 10.Friedland R P. Neurology. 1993;43:246–249. doi: 10.1212/wnl.43.2.246. [DOI] [PubMed] [Google Scholar]
- 11.Friedland R P, Smyth K, Esteban-Santillan C, Koss E, Cole R, Lerner A J, Strauss M, Whitehouse P J, Petot G, Rowland D Y, et al. In: Proceedings of the Fifth International Conference on Alzheimer's Disease and Related Disease. Iqbal K, Winblad B, Nishimura T, Takeda M, Wisniewski H, editors. New York: Wiley; 1997. pp. 33–37. [Google Scholar]
- 12.Bauer J. Behav Brain Res. 1996;78:1–2. doi: 10.1016/0166-4328(95)00212-x. [DOI] [PubMed] [Google Scholar]
- 13.Wilson R S, Bennett D A, Gilley D W, Beckett L A, Barnes L L, Evans D A. Arch Neurol (Chicago) 2000;57:1718–1723. doi: 10.1001/archneur.57.12.1718. [DOI] [PubMed] [Google Scholar]
- 14.Bull C N. In: Social Roles and Social Participation. Research Instruments in Social Gerontology. Mangen D, Peterson WA, editors. Minneapolis: Univ. of Minnesota Press; 1982. pp. 477–533. [Google Scholar]
- 15.Selkoe D J. Nature (London) 1999;399:A23–A31. doi: 10.1038/399a023. [DOI] [PubMed] [Google Scholar]
- 16.Hultsch D F, Hertzog C, Small B J, Dixon R A. Psychol Aging. 1999;14:245–263. doi: 10.1037//0882-7974.14.2.245. [DOI] [PubMed] [Google Scholar]
- 17.Schooler C. Intelligence. 1984;8:259–281. [Google Scholar]
- 18.Snowdon D A, Kemper S J, Mortimer J A, Greiner L H, Wekstein D R, Markesbery W R. J Am Med Assoc. 1996;275:528–532. [PubMed] [Google Scholar]
- 19.Holmberg I, Persson G. Acta Psychiatr Scand. 1986;74:168–177. doi: 10.1111/j.1600-0447.1986.tb10601.x. [DOI] [PubMed] [Google Scholar]
- 20.Fabrigoule C, Letenneur L, Dartigues J F, Zarrouk M, Commenges D, Barberger-Gateau P. J Am Geriatr Soc. 1995;43:485–490. doi: 10.1111/j.1532-5415.1995.tb06093.x. [DOI] [PubMed] [Google Scholar]
- 21.McKhann G, Drachman D, Folstein M F, Katzman R, Price D, Stadlan E M. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 22.Friedland R P, Farrer L A, Cupples L A, Debanne S M, Lerner A J the MIRAGE Study Group. Lancet. 1998;352:819. doi: 10.1016/s0140-6736(05)60714-3. [DOI] [PubMed] [Google Scholar]
- 23.Hultsch D F, Hammer M, Small B J. J Geront Psych Sci. 1993;48:1–11. doi: 10.1093/geronj/48.1.p1. [DOI] [PubMed] [Google Scholar]
- 24.Nagelkerke N J D. Biometrika. 1991;78:691–692. [Google Scholar]
- 25.Rauramaa R, Salonen J T, Seppanen K, Salonen R, Venalainen J M, Ihanainen M, Rissanen V. Circulation. 1986;74:939–944. doi: 10.1161/01.cir.74.5.939. [DOI] [PubMed] [Google Scholar]
- 26.Small G W, Mazziotta J C, Collins M T, Baxter L R, Phelps M E, Mandelkern M A, Kaplan A, La Rue A, Adamson C F, Chang L, et al. J Am Med Assoc. 1995;273:942–947. [PubMed] [Google Scholar]
- 27.Reiman E M, Caselli R J, Yun L S, Chen K, Bandy D, Minoshima S, Thibodeau S N, Osborne D. N Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 28.Caselli R J, Graff-Radford N R, Reiman E M, Weaver A, Osborne D, Lucas J, Uecker A, Thibodeau S N. Neurology. 1999;53:201–207. doi: 10.1212/wnl.53.1.201. [DOI] [PubMed] [Google Scholar]
- 29.Linn R T, Wolf P A, Bachman D L, Knoefel J E, Cobb J L, Belanger A J, Kaplan E F, D'Agostino RB. Arch Neurol (Chicago) 1995;52:485–490. doi: 10.1001/archneur.1995.00540290075020. [DOI] [PubMed] [Google Scholar]
- 30.Lemere C A, Blusztajn J K, Yamaguchi H, Wisniewski T, Saido T C, Selkoe D J. Neurobiol Dis. 1996;3:16–31. doi: 10.1006/nbdi.1996.0003. [DOI] [PubMed] [Google Scholar]
- 31.Graham P, Jackson R. Am J Epidemiol. 1993;138:443–452. doi: 10.1093/oxfordjournals.aje.a116876. [DOI] [PubMed] [Google Scholar]
- 32.Kondo K, Niino M, Shido K. Dementia. 1994;5:314–326. doi: 10.1159/000106741. [DOI] [PubMed] [Google Scholar]
- 33.Young D R, Masaki K H, Curb J D. J Am Geriatr Soc. 1995;43:845–854. doi: 10.1111/j.1532-5415.1995.tb05525.x. [DOI] [PubMed] [Google Scholar]
- 34.Schooler, C. & Mulatu, M. S. Psychology and Aging (in press).
- 35.Eaton S B, Konner M, Shostak M. Am J Med. 1988;84:739–749. doi: 10.1016/0002-9343(88)90113-1. [DOI] [PubMed] [Google Scholar]
- 36.Wilson R S, Bennett D A, Beckett L A, Morris M C, Gilley D W, Bienias J L, Scherr P A, Evans DA. J Gerontol Psych Sci. 1999;54:P155–P160. doi: 10.1093/geronb/54b.3.p155. [DOI] [PubMed] [Google Scholar]
- 37.Swaab D F. Neurobiol Aging. 1991;12:317–324. doi: 10.1016/0197-4580(91)90008-8. [DOI] [PubMed] [Google Scholar]
- 38.Chandra V, Ganguli M, Pandav R, Johnston J, Belle S, DeKosky S T. Neurology. 1998;51:1000–1008. doi: 10.1212/wnl.51.4.1000. [DOI] [PubMed] [Google Scholar]
- 39.Hendrie H C, Osuntokun B O, Hall K S, Ogunniyi A O, Hui S L, Unverzagt F W, Gureje O, Rodenberg C A, Baiyewu O, Musick B S. Am J Psychiatry. 1995;152:1485–1492. doi: 10.1176/ajp.152.10.1485. [DOI] [PubMed] [Google Scholar]
- 40.Office of Surveillance and Analysis, Centers for Disease Control. Morbid Mortal Wkly Rep. 1994;48:894–899. [Google Scholar]
- 41.Clark L M, Bosworth H B, Welsh-Bohmer K A, Dawson D V, Siegler I C. Neuropsych Neuropsychol Behav Neurol. 2000;13:39–47. [PubMed] [Google Scholar]
- 42.Demissie, S., Green, R. C., Mucci, L., Tziavas, S., Martelli, K., Bang, K., Coons, L., Bourque, S., Buchillon, D., Johnson, K., et al. (2001) Neuroepidemiology, in press. [DOI] [PubMed]