Abstract
In this study, the inactivating properties of liquid hydrogen peroxide (L-H2O2), vaporized hydrogen peroxide (V-H2O2), UV light, and a combination of V-H2O2 and UV light were tested on murine norovirus 1 (MNV-1) and bacteriophages (φX174 and B40-8) as models for human noroviruses. Disinfection of surfaces was examined on stainless steel discs based on European Standard EN 13697 (2001). For fresh-produce decontamination, a mixture of the viruses was inoculated onto shredded iceberg lettuce and treated after overnight incubation at 2°C. According to our results, L-H2O2 (2.1%) was able to inactivate MNV-1 and φX174 on stainless steel discs by approximately 4 log10 units within 10 min of exposure, whereas for B40-8, 15% of L-H2O2 was needed to obtain a similar reduction in 10 min. Only a marginal reduction (≤1 log10 unit after 5 min of exposure) by V-H2O2 (2.52%) was achieved for the tested model viruses, although in combination with UV light, a 4-log10-unit decrease within 5 min of treatment was observed on stainless steel discs. Similar trends were observed for the decontamination of shredded iceberg lettuce, but the viral decline was reduced. These results demonstrated that both L-H2O2 and a combination of V-H2O2 and UV light can be used for norovirus inactivation on surfaces; V-H2O2 (2.52%) in combination with UV light is promising for decontamination of fresh produce with much less consumption of water and disinfectant.
In recent years, noroviruses (NoVs) have been increasingly recognized as the most important agents causing outbreaks as well as sporadic cases of acute gastroenteritis worldwide (19, 24, 28, 37). Transmitted mainly through the fecal-oral route, large-scale NoV outbreaks are often the result of a combination of several transmission routes. As reviewed by Patel et al. (31), people from different age groups and with different histo-blood group antigens (HBGA) show various host susceptibilities. Often a sensitive population is exposed to the virus through contaminated food or water, followed by transmission via direct person-to-person contact or a contaminated environment (14). Within these transmission routes, contamination of environmental surfaces plays a key role (22, 41). Fresh produce is also a major indicated source of NoV outbreaks (13, 21, 26). Consequently, studies simulating practical conditions for effective disinfection of surfaces and fresh produce are crucial for the prevention of virus transmission.
Since NoVs were first detected by immunoelectron microscopy during a 1968 outbreak of gastroenteritis in Norwalk, OH, the lack of suitable animal models and the inability to propagate the viruses in cell cultures have hampered their further study (11). Straub et al. (34) have described a highly differentiated three-dimensional cell culture model that supports the natural growth of human NoVs. However, this system is rather complex and not yet confirmed by other groups; therefore, surrogate viruses that share common pathological and/or molecular features with human NoVs are used instead. Feline calicivirus (FCV) and coliphage MS2 were almost exclusively used as a surrogate virus in previous studies (1, 10, 16, 36). In 2003, a NoV that infects mice, namely, murine norovirus 1 (MNV-1), was identified (23). A study investigating the applicability of MNV-1 and FCV as surrogates for the human NoVs with regard to the stability and inactivation of NoVs demonstrated that MNV-1 was more acid tolerant than FCV, thus probably making it a more suitable surrogate for the human NoVs (7). Both coliphage φX174 and Bacillus fragilis phage B40-8 have been proposed as indicator systems for enteric viral pathogens and as tools for studying viral disinfection (3, 4, 6, 17, 32). Bacteriophages are preferred to MNV-1, as they are not pathogenic, are easy to detect and cultivate, and can be even used outside the laboratory environment (3).
Hydrogen peroxide is a commercial sterilizing agent which has been widely used in the food industry and has been approved by the Food and Drug Administration (9). Recently, hydrogen peroxide vapor has been increasingly used for decontamination, and its effect on other microorganisms has already been proven by others (29, 30). In this study, the reduction in infectivity of MNV-1, φX174, and B40-8 on surfaces of stainless steel discs treated with liquid hydrogen peroxide (L-H2O2), vaporized hydrogen peroxide (V-H2O2), UV light, and a combination of V-H2O2 and UV light was studied. The efficacy of these treatments in reducing the viral load on fresh-cut iceberg lettuce was also investigated.
MATERIALS AND METHODS
Preparation of MNV-1 lysate and plaque assay.
Cells of the murine macrophage cell line RAW 264.7 (ATCC TIB-71; kindly provided by H. W. Virgin, Washington University School of Medicine, St. Louis, MO) were grown in complete Dulbecco's modified Eagle's medium (DMEM) at 37°C under a 5% CO2 atmosphere. Complete DMEM consisted of DMEM (Lonza, Walkersville, MD) containing 10% low-endotoxin fetal bovine serum (HyClone, Logan, UT), 100 U/ml penicillin, 100 μg/ml streptomycin (Lonza), 10 mM HEPES (Lonza), and 2 mM l-glutamine (Lonza).
RAW 264.7 cells were infected with MNV-1.CW1, passage 6, at a multiplicity of infection (MOI) of 0.05 (MNV-1 to cells) for 2 days. After two freeze-thaw cycles, low-speed centrifugation was used to remove cellular debris from the virus lysate, as described by Wobus et al. (40). The lysate was stored in aliquots at −75°C.
The titer of MNV-1 (PFU/ml) was determined by plaque assay, as described by Wobus et al. (40). Briefly, RAW 264.7 cells were seeded into six-well plates at a density of 2 × 106 viable cells per well. On the following day, 10-fold dilutions of the samples of unknown viral titer were prepared in complete DMEM, and 1 ml per dilution of the sample was plated onto two wells at 0.5 ml per well. Plates were incubated for 1 h at room temperature and manually rocked every 15 min before the inoculum was aspirated and the cells were overlaid with 1.5% SeaPlaque agarose (Cambrex, Rockland, ME) in minimum essential medium Eagle (MEME) (Lonza) supplemented with 10% low-endotoxin fetal bovine serum, 1% HEPES, 1% penicillin-streptomycin, and 2% glutamine (complete MEME) per well. Plates were incubated at 37°C and 5% CO2 for 2 days. To visualize plaques, cells were stained with 1.5% SeaKem agarose in complete MEME containing 1% neutral red (Sigma Aldrich, St. Louis, MO) per well for 6 h.
Preparation of B40-8 lysate and phage assay.
Bacteriophage B40-8 and the host strain B. fragilis HSP40 were kindly provided by M. Muniesa and J. Jofre (University of Barcelona, Barcelona, Spain). B. fragilis HSP40 was grown in bacteroides phage recovery medium (BPRM) as described by Tartera et al. (35). B40-8 lysate was prepared by the infection of B. fragilis HSP40 grown in BPRM broth anaerobically at 37°C until it reached 2 × 108 CFU/ml at a MOI of 0.1. After 16 to 20 h, chloroform (0.3 volume; VWR, Fontenay-sous-Bois, France) was added to the culture at room temperature. The next day, the top layer was taken from the chloroform-culture mixture and centrifuged (10 min, 4,000 × g, 4°C). The supernatant (approximately 10.6 log PFU/ml) was stored at 4°C.
The number of B40-8 PFU/ml was determined by the double-agar-layer plaque assay as described by Araujo et al. (2). Briefly, 10-fold dilutions of the sample with unknown viral titer were prepared in peptone physiological salt solution (PPS) (1 g of peptone per liter [Oxoid, Basingstoke, United Kingdom] and 8.5 g of NaCl per liter [Sigma Aldrich, Steinheim, Germany]). From each dilution of the sample, 1 ml was added to tubes containing 2.5 ml of melted semisolid BPRM (ssBPRM) agar. Subsequently, 1 ml of B. fragilis strain HSP40 grown until the exponential phase was added to the sample-ssBRPM suspension. After gentle mixing, the suspension was poured onto BPRM agar in a 90-mm petri dish. The ssBPRM agar was allowed to solidify, and plates were incubated in an anaerobic jar at 37°C for 24 h.
Preparation of φX174 lysate and phage assay.
Coliphage φX174 and the host strain Escherichia coli CB390 were kindly provided by M. Muniesa and J. Jofre (University of Barcelona, Barcelona, Spain). E. coli CB390 was grown in Trypticase-yeast extract-glucose (TYG) broth containing 10 g/liter Trypticase peptone (Becton Dickinson Company, Franklin Lakes, NJ), 1 g/liter yeast extract (Oxoid), and 8 g/liter NaCl (Sigma Aldrich) supplemented with 50 mg/liter ampicillin (Merck, Darmstadt, Germany). The titer of φX174 was determined by a double-agar-layer plaque assay. Briefly, 10-fold dilutions of the sample with unknown viral titer were prepared in PPS. From each dilution of the sample, 1 ml was added to tubes containing 2.5 ml of melted ssTYG-agar [TYG broth with 7.5 g/liter agar (Oxoid), 4 mM CaCl2 (Sigma Aldrich), and 6 mM d-(+)-glucose (Sigma Aldrich)]. Subsequently, 1 ml of E. coli CB390 grown until the exponential phase was added to the sample-ssTYG suspension. After gentle mixing, the suspension was poured onto TYG agar (TYG broth with 15 g/liter agar [Oxoid]) in a 90-mm petri dish. Plates were incubated at 37°C for 18 h.
Since it is hard to propagate φX174 in liquid medium, φX174 lysate was prepared by performing the φX174 plaque assay with a sample containing φX174 to induce confluent lysis on the plate. After 18 h of incubation at 37°C, the soft agar was scraped from 10 agar plates and mixed with 10 ml PPS. Chloroform (2 ml) was added and incubated at room temperature for 30 min. The supernatant, i.e., φX174 lysate, was taken after centrifugation at 4,000 × g for 10 min at 20°C and stored at 4°C.
Surface disinfection test using stainless steel discs.
The surface disinfection test was based on EN 13697, 2001 (12). Stainless steel discs (20-mm diameter) were incubated in a 5% (vol/vol) Decon 90 solution (Decon Laboratories Ltd., Hove, England) for 1 h. Afterwards the discs were rinsed twice with distilled water for 10 s and were then placed in 70% isopropanol (Sigma Aldrich, Steinheim, Germany) for 15 min. Finally, the discs were dried by evaporation in sterile petri dishes.
For B40-8 and φX174, virus inocula were prepared by 10 times dilution of virus lysate in order to eliminate the interfering effect of the medium and mixing with bovine serum albumin (BSA) as an interfering substance (0.3 g/liter as the final concentration for clean conditions and 3 g/liter for dirty conditions). Since the initial titer of MNV-1 lysate was comparatively lower, the MNV-1 lysate was directly used as the virus inoculum. The protein concentration of MNV-1 lysate was determined to be 3.8 g/liter by a Bradford protein assay, which is similar to the interfering protein concentration defined as the dirty condition.
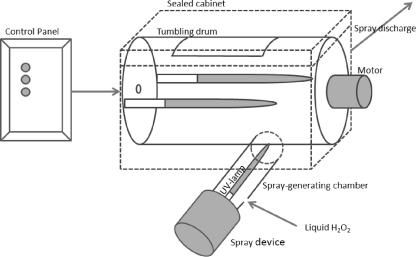
The virus inoculum of MNV-1, φX174, or B40-8 (50 μl) was pipetted separately in the middle of a clean and disinfected stainless steel disc and dried at 37°C for 30 min. Different concentrations of hydrogen peroxide were freshly prepared from EcoClearProx, a stabilized hydrogen peroxide solution containing 42.1% H2O2 (Advanced Biological Technologies [ABT] Belgium, Belgium), in hard water (12) and used within 2 h. For L-H2O2 treatment, 100 μl of the test solution was pipetted on the inoculated discs and incubated for 5 or 10 min. As a positive control, the inoculated disc received 100 μl of hard water instead of a hydrogen peroxide solution. For V-H2O2 and UV treatment, inoculated discs were transferred in the middle of the drum of a prototype apparatus (ABT Belgium in cooperation with DDR systems, Voorde-Ninove, Belgium) with principle similar to that described by Vandekinderen et al. (38) and received treatment of 5 min without tumbling. The H2O2 (2.52%) was vaporized by a spray device (Nocospray, 230V; Airel, France), while UV irradiation was done with three low-pressure mercury lamps (TUVN4 4K P 165 lamp, 4 W each; UV-Technik Speziallampen GmbH, Wolfsberg OT Wümbach, Germany) producing mainly 254-nm UV light. One of these three lamps was fixed in the spray-generating chamber, while the other two were fixed in the middle of the drum (diameter, 33 cm by 48 cm) inside the sealed cabinet (50 cm by 35 cm by 35 cm) (Fig. 1).
FIG. 1.
Scheme of the prototype apparatus (ABT Belgium in cooperation with DDR systems).
The discs representing the positive-control and treated samples were transferred into 25-ml sterile containers, which were previously filled with 10 ml of neutralizer (5 g/liter sodium thiosulfate; Sigma Aldrich, Steinheim, Germany) to stop the inactivating activity and 5 g of glass beads (0.25- to 0.50-mm diameter) to increase virus recovery by mechanical abrasion. After a neutralization of 5 min (including a shaking of 1 min), the neutralized treated virus solution (positive-control and treated samples) was stored at −75°C until the PFU of MNV-1, φX174, and B40-8 were determined according to the assays described above.
Decontamination test on fresh-cut iceberg lettuce.
Iceberg lettuce was purchased from a local store and freshly cut. A viral suspension (50 ml) of approximately 6 log PFU/ml each of MNV-1, φX174, and B40-8 was spotted on 500 g fresh-cut iceberg lettuce in a sterile stomacher bag. The procedure of spotting included several steps: (i) adding 20 ml of viral suspension in drops on lettuce pieces present on one side of the stomacher bag, (ii) turning the stomacher bag, (iii) again adding 20 ml of viral suspension in drops, (iv) shaking the sample manually and gently, (v) adding 10 ml of viral suspension, and finally (vi) again shaking manually and gently to distribute the viral inoculum uniformly throughout the lettuce pieces. Subsequently, the bags were sealed and stored overnight at 2°C.
For L-H2O2 treatment, 50 g of inoculated fresh-cut iceberg lettuce was treated in 500 ml H2O2 solution (1.26% or 2.52% H2O2) for 5 min. Treated fresh-cut iceberg lettuce was rinsed in tap water (5 min) afterwards. Washing of fresh-cut iceberg lettuce with water was performed similarly to the L-H2O2 treatment except that 500 ml tap water was used instead of an H2O2 solution.
For V-H2O2 and UV treatment, 500 g of fresh-cut iceberg lettuce was transferred to the drum of the prototype apparatus. The drum tumbled at a speed of 77 rpm during treatment. The consumption of liquid H2O2 (2.52%) was 80 ml per treatment.
After treatment, the model viruses (MNV-1, φX174, and B40-8) were extracted according to the procedure described by Baert et al. (4). Briefly, 10 g of treated iceberg lettuce was washed with 30 ml of elution buffer (0.1 M Tris-HCl [Sigma], 3% beef extract powder [Sigma], and 0.05 M glycine [Sigma], pH 9.5) in a stomacher bag with a filter compartment (full filter blender bag, 190 by 300 mm, FBAG-04; Novolab, Geraardsbergen, Belgium) on a shaking platform (rotating at 120 rpm) for 20 min. The filtrate was centrifuged at 10,000 × g for 15 min at 4°C, and the pH of the supernatant was adjusted to 7.2 to 7.4. Polyethylene glycol (PEG) 6000 (Sigma) and NaCl were added to obtain a final concentration of 10% PEG and 0.3 M NaCl. The samples were placed on a shaking platform (rotating at 120 rpm) overnight at 4°C. The next day, the samples were centrifuged at 10,000 × g for 30 min at 4°C. The supernatant was discarded, and the pellet was dissolved in 1 ml of phosphate-buffered saline (PBS) (Lonza). Virus extracts were stored at −75°C until the PFU of MNV-1, φX174, and B40-8 were determined by the assays described above.
Data analysis.
Data from the surface disinfection using stainless steel discs were obtained by comparison with positive controls, which were processed in a manner similar to that for the inactivated groups except that they did not undergo the H2O2 or UV treatment but were treated with hard water. Samples were tested in duplicate for the surface disinfection using stainless steel discs and in triplicate for the decontamination of shredded iceberg lettuce. Each error bar represents the data range. Statistical analyses of the reductions obtained by the decontamination of fresh-cut iceberg lettuce were performed by one-way analysis of variance (ANOVA) (the Tukey HSD test was used as a post hoc test) with SPSS 17.0 for Windows (SPSS Inc.). Differences were considered significant when the P value was <0.05.
RESULTS
Initially, the experimental setup of the surface disinfection test was evaluated. It was shown that the difference between the titers of virus lysate and the positive control was no greater than 2 log10 units (data not shown), which means that the treatments other than H2O2 or UV had no observable inactivating effects. Less than a 0.3-log10-unit difference in viral titer was observed between the viral contents of an inoculated disc immersed in neutralizer supplemented with 100 μl hard water and those of an inoculated disc immersed in neutralizer supplemented with 100 μl 20% H2O2 solution (data not shown), suggesting complete neutralization.
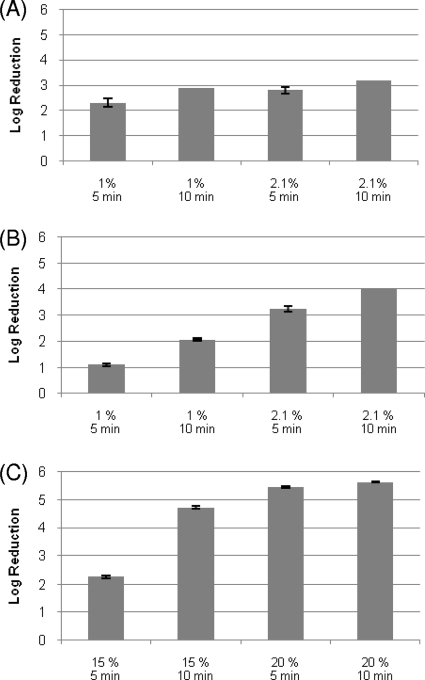
The treatment with L-H2O2 at a concentration of 2.1% was able to inactivate MNV-1 and φX174 on stainless steel discs by approximately 4 log10 units with a contact time of 10 min (Fig. 2 A and B), whereas for B40-8, a similar reduction was not obtained within the same contact time until the concentration of L-H2O2 was increased to 15% (Fig. 2C). The influence of the protein load on the efficacy of H2O2 was tested on φX174 (Table 1) and B40-8 (data not shown) by the addition of 3 g/liter BSA (dirty conditions) and 0.3 g/liter BSA (clean conditions). Less than a 0.5-log10-unit difference was observed for both viruses; therefore, only results obtained under dirty (worse) condition are shown here.
FIG. 2.
(A) Reductions of infectious titer of MNV-1 on stainless steel discs by liquid H2O2 treatment. The concentration of H2O2 and exposure time are shown on the horizontal axis. Each data point is an average of duplicates, and each error bar represents the data range. The reduction of MNV-1 treated with H2O2 (2.1%) for 10 min reached the detection limit. (B) Reductions of infectious titer of φX174 on stainless steel discs by liquid H2O2 treatment. The concentration of H2O2 and exposure time are shown on the horizontal axis. Each data point is an average of duplicates, and each error bar represents the data range. (C) Reductions of infectious titer of B40-8 on stainless steel discs by liquid H2O2 treatment. The concentrations of H2O2 and exposure times are shown on the horizontal axis. Each data point is an average of duplicates, and each error bar represents the data range. The reduction of B40-8 treated with H2O2 (20%) for 10 min reached the detection limit. All experiments were performed at room temperature.
TABLE 1.
Comparison of the infectious titers of φX174 in the presence of a low protein concentration (0.3 g/liter BSA) and a high protein concentration (3 g/liter BSA) on stainless steel discs with L-H2O2 treatment
| L-H2O2 treatment | Mean titer (log PFU/ml) ± SD |
|
|---|---|---|
| Low protein concn | High protein concn | |
| Positive control | 7.12 ± 0.01 | 6.65 ± 0.05 |
| 1% | ||
| 5 min | 5.71 ± 0.01 | 5.52 ± 0.06 |
| 10 min | 5.13 ± 0.05 | 4.59 ± 0.06 |
| 2.1% | ||
| 5 min | 4.18 ± 0.03 | 3.42 ± 0.03 |
| 10 min | 3.01 ± 0.12 | 2.61 ± 0.09 |
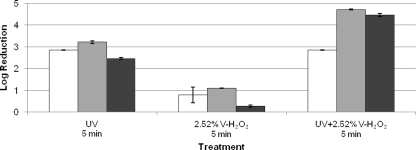
V-H2O2 (2.52%) induced only a marginal reduction (≤1 log10 unit after 5 min of exposure) for the tested model viruses, although UV light induced a considerable higher decrease (3 log10 units) within 5 min of treatment. When V-H2O2 (2.52%) was combined with UV light, a 3-log10-unit reduction (detection limit reached) for MNV-1 and 4-log10-unit reductions for φX174 and B40-8 were obtained (Fig. 3).
FIG. 3.
Reductions of infectious titers of MNV-1 (white bars), φX174 (gray bars), and B40-8 (black bars) on stainless steel discs. The treatments are shown on the horizontal axis. All experiments were performed at room temperature. Each data point is an average of duplicates, and each error bar represents the data range. Reductions of MNV-1 treated with UV and the combination of UV and 2.52% H2O2 and of φX174 treated with the combination of UV and 2.52% H2O2 reached the detection limits.
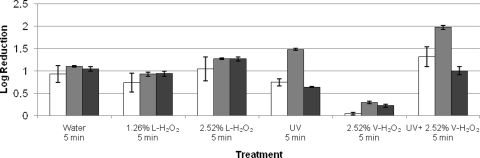
Similar trends were obtained for H2O2 treatments with or without UV on fresh-cut iceberg lettuce as on stainless steel discs (Fig. 4). L-H2O2 (2.52%, 5 min) was more effective than V-H2O2 (2.52%, 5 min) (P < 0.05) for all three model viruses. The combination of UV and V-H2O2 (2.52%) could induce a significantly higher reduction of φX174 than the traditional method of washing with water (P < 0.05). B40-8, which was quite persistent on stainless steel discs, showed a reduction similar to that for MNV-1 and φX174 in the case of washing of iceberg lettuce with water or treating with L-H2O2 (P > 0.05). φX174 was the most sensitive of the three model viruses to UV treatment as well as the combination of UV and V-H2O2 (2.52%). No significant change of appearance or sensorial quality for fresh-cut lettuce was observed after H2O2 treatments with or without UV (data not shown).
FIG. 4.
Reductions of infectious titers of MNV-1 (white bars), φX174 (gray bars), and B40-8 (black bars) on fresh-cut iceberg lettuce. The treatments are shown on the horizontal axis. All experiments were performed at room temperature. Each data point is an average of triplicates, and each error bar represents the data range.
DISCUSSION
Multiple studies evaluating NoV inactivation on surfaces and fresh produce have been recently performed, since they may play an important role in the spread of human NoVs (1, 4, 8, 18, 27). Magulski et al. (27) tested the inactivating properties of several chemical biocides using MNV-1 as a surrogate for human NoVs and suggested products based upon peroxyacetic acid (0.1%), glutaraldehyde (0.25%), ethanol (50%, vol/vol), and isopropanol (30%, vol/vol) which could inactivate MNV-1 on stainless steel discs by ≥ 4 log10 units within 5 min. In 2010, Girard et al. (18) also evaluated the effectiveness of different household disinfectants and found that sodium hypochlorite (3%) could produce a ≥3-log10-unit reduction of genomic copies of both MNV-1 and human NoVs after 10 min. According to our results, liquid H2O2 at a concentration of 2.1% enabled more than 4-log10-unit reductions of MNV-1 and φX174 on stainless steel discs in 10 min.
As reviewed by Beuchat (5), disinfection using chemical disinfectants and sanitizers has been identified as an important procedure for fresh produce to lower the risk of enteric pathogens. However, reductions of viral infectivity on fresh produce are always lower than reductions on surfaces. Allwood et al. (1) conducted a series of experiments to compare the survival of Escherichia coli, feline calicivirus, and F-specific coliphage MS2 on cabbage and lettuce with and without disinfection, suggesting that viruses were resistant to sodium bicarbonate (10%, 5 min), sodium hypochlorite (0.02%, 2 min), peroxyacetic acid (0.008%, 2 min), and hydrogen peroxide (3%, 2 min) (<3-log10-unit PFU reductions). In 2005, Dawson et al. (8) tested the efficacy of chlorine wash (0.01% free chlorine) to reduce MS2 on fresh fruit and vegetables, obtaining only a 0.89-log10-unit reduction as the mean value across all produce types. In our study, a reduction of only 1 log10 unit was obtained in the test of decontamination of fresh-cut iceberg lettuce by liquid H2O2 at a concentration of 2.52% for MNV-1, φX174, and B40-8.
Although they have effective inactivating properties, liquid disinfectants still have a series of limitations in practical usage. As for surfaces, it is difficult to ensure enough contact time between the contaminated surface and the aqueous sanitizer. In food disinfection, aqueous sanitizers often fail to reach pathogens located in inaccessible sites, and they require large amount of potable water and disinfectant. With a high penetrating activity and broad spectrum of applicable sanitizers, aerosolization may be an alternative sanitizer delivery system for the food industry. The antiviral activity of hydrogen peroxide spray in combination with UV light has already been reported. Xie et al. (42) evaluated the efficacy of inactivation of MS2 inoculated onto 5-cm2 iceberg lettuce sections and found a reduction of 0.5 to 1.0 log10 PFU by UV light for 20 to 60 s and a 3-log10-unit reduction with 2% H2O2 spray at 50°C. An even higher reduction (4.12 log10 units) was obtained with H2O2 spray followed by UV illumination. In our experiments, only a marginal reduction by V-H2O2 was achieved for the tested model viruses, although in combination with UV light, a 4-log10-unit decrease within 5 min of treatment could be established for φX174 and B40-8 in the surface disinfection test. For the treatment of iceberg lettuce, the combination of UV and V-H2O2 (2.52%) induced a 2-log10-unit reduction of φX174, which was significantly higher than that obtained by the traditional method of washing with water (P < 0.05), representing a good method to enhance the microbiological safety of fresh produce. The additive effect of this combination can be explained by the fact that radical formation from hydrogen peroxide is greatly enhanced in the presence of UV light (33), while the further mechanism still should be explored in the future.
A number of previous studies have shown that a higher organic load could result in a decreased effectiveness of viral inactivation (15, 25, 39). In the surface disinfection test, φX174 and B40-8 suspensions were mixed with bovine serum albumin as an interfering substance to simulate clean (0.3% BSA) and dirty (3% BSA) conditions (12). Interestingly, there were no significant differences in virus reduction on surfaces under clean or dirty conditions. Nevertheless, for viruses inoculated on lettuce, the viral decline was indeed lower. This result, being consistent with some previous studies (16, 27), indicates that due to the sheltering or inaccessibility of the food structures, the interfering effect of real food might be too complicated to be simulated by one simple protein.
Up to now, MNV-1 has been recognized as the most representative surrogate for human NoVs. It is a member of the genus Norovirus and infects via the gastrointestinal tract. However, Girard et al. (18) compared the inactivation effect of household disinfectants on both MNV-1 and human NoVs by real-time reverse transcription-PCR (RT-PCR), revealing that MNV-1 was more sensitive than human NoVs to chemical disinfectants. Bacteriophages have also been proposed as indicator systems for enteric viral pathogens and as tools for studying viral disinfection. As they are genetically less closely related to human NoVs, the correlation between bacteriophages and enteroviruses has been and is still under exploration (3, 8, 20). In this study, we performed treatments on MNV-1, coliphage φX174, and B. fragilis phage B40-8. Although B40-8 was more persistent with L-H2O2 than MNV-1 and φX174 in the surface disinfection test, they tended to have similar reductions under L-H2O2 treatment on iceberg lettuce. φX174 was more sensitive than the other two model viruses to UV treatment as well as the combination of UV and V-H2O2 (2.52%) in the decontamination test on fresh-cut iceberg lettuce.
In summary, both L-H2O2 and a combination of V-H2O2 and UV light could be used for NoV inactivation on surfaces. V-H2O2 (2.52%) in combination with UV light might be promising for the decontamination of fresh produce with much less consumption of water and disinfectant, suggesting an economical processing method to enhance the microbiological safety of fresh produce for the industry.
Acknowledgments
This work was finished at Ghent University and the Institute for Agricultural and Fisheries Research. The research leading to these results has received funding from the European Community's Seventh Framework Programme (FP7) under grant agreement no. 244994 (project VEG-i-TRADE). This work was also supported by Ghent University, FWO, and Advanced Biological Technologies Belgium.
Footnotes
Published ahead of print on 23 December 2010.
REFERENCES
- 1.Allwood, P. B., Y. S. Malik, C. W. Hedberg, and S. M. Goyal. 2004. Effect of temperature and sanitizers on the survival of feline calicivirus, Escherichia coli, and F-specific coliphage MS2 on leafy salad vegetables. J. Food Prot. 67:1451-1456. [DOI] [PubMed] [Google Scholar]
- 2.Araujo, R., et al. 2001. Optimisation and standardisation of a method for detecting and enumerating bacteriophages infecting Bacteroides fragilis. J. Virol. Methods 93:127-136. [DOI] [PubMed] [Google Scholar]
- 3.Baert, L., M. Uyttendael, E. Van Coillie, and J. Debevere. 2008. The reduction of murine norovirus 1, B. fragilis HSP40 infecting phage B40-8 and E. coli after a mild thermal pasteurization process of raspberry puree. Food Microbiol. 25:871-874. [DOI] [PubMed] [Google Scholar]
- 4.Baert, L., et al. 2009. The efficacy of sodium hypochlorite and peroxyacetic acid to reduce murine norovirus 1, B40-8, L. monocytogenes and E. coli O157:H7 on shredded iceberg lettuce and in residual wash water. J. Food Prot. 72:1047-1054. [DOI] [PubMed] [Google Scholar]
- 5.Beuchat, L. R. 1998. Surface decontamination of fruits and vegetables eaten raw: a review. WHO/FSF/FOS/98.2. World Health Organization, Geneva, Switzerland.
- 6.Busta, F. F., et al. 2003. The use of indicators and surrogate microorganisms for the evaluation of pathogens in fresh and fresh-cut produce. Crit. Rev. Food Sci. 2(Suppl. 1):179-185. [Google Scholar]
- 7.Cannon, J. L., et al. 2006. Surrogates for the study of norovirus stability and inactivation in the environment: a comparison of murine norovirus and feline calicivirus. J. Food Prot. 69:2761-2765. [DOI] [PubMed] [Google Scholar]
- 8.Dawson, D. J., A. Paish, L. M. Staffell, I. J. Seymour, and H. Appleton. 2005. Survival of viruses on fresh produce, using MS2 as a surrogate for norovirus. J. Appl. Microbiol. 98:203-209. [DOI] [PubMed] [Google Scholar]
- 9.Demirkol, O. 2009. Effects of hydrogen peroxide treatment on thiol contents in fresh-cut asparagus (Asparagus officinalis) spears. Int. J. Food Sci. Nutr. 60:80-88. [DOI] [PubMed] [Google Scholar]
- 10.Doultree, J. C., J. D. Druce, C. J. Birch, D. S. Bowden, and J. A. Marshall. 1999. Inactivation of feline calicivirus, a Norwalk virus surrogate. J. Hosp. Infect. 41:51-57. [DOI] [PubMed] [Google Scholar]
- 11.Duizer, E., et al. 2004. Laboratory efforts to cultivate noroviruses. J. Gen. Virol. 85:79-87. [DOI] [PubMed] [Google Scholar]
- 12.European Committee for Standardization. 2001. European Standard EN 13697. Chemical disinfectants and antiseptics—quantitative non-porous surface test for the evaluation of bactericidal and/or fungicidal activity of chemical disinfectants used in food, industrial, domestic and institutional areas—test method and requirements (phase 2, step 2), 2001. European Committee for Standardization, Brussels, Belgium.
- 13.Falkenhorst, G., et al. 2005. Imported frozen raspberries cause a series of norovirus outbreaks in Denmark. Euro Surveill. 10:2795. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=2795. [DOI] [PubMed] [Google Scholar]
- 14.FAO/WHO. 2008. Microbiological hazards in fresh leafy vegetables and herbs: meeting report. Microbiological risk assessment series, no. 14. FAO/WHO, Rome, Italy.
- 15.Ferrier, A., D. Garin, and J. M. Crance. 2004. Rapid inactivation of vaccinia virus in suspension and dried on surfaces. J. Hosp. Infect. 57:73-79. [DOI] [PubMed] [Google Scholar]
- 16.Fino, V. R., and K. E. Kniel. 2008. UV light inactivation of hepatitis A virus, Aichi virus, and feline calicivirus on strawberries, green onions, and lettuce. J. Food Prot. 71:908-913. [DOI] [PubMed] [Google Scholar]
- 17.Gantzer, C., A. Maul, J. M. Audic, and L. Schwartzbrod. 1998. Detection of infectious enteroviruses, enterovirus genomes, somatic coliphages, and Bacteroides fragilis phages in treated wastewater. Appl. Environ. Microbiol. 64:4307-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Girard, M., S. Ngazoa, K. Mattison, and J. Jean. 2010. Attachment of noroviruses to stainless steel and their inactivation, using household disinfectants. J. Food Prot. 73:400-404. [DOI] [PubMed] [Google Scholar]
- 19.Hansman, G. S., et al. 2004. Genetic diversity of norovirus and sapovirus in hospitalized infants with sporadic cases of acute gastroenteritis in Chiang Mai, Thailand. J. Clin. Microbiol. 42:1305-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Havelaar, A. H., M. Vanolphen, and Y. C. Drost. 1993. F-specific RNA bacteriophages are adequate model organisms for enteric viruses in fresh-water. Appl. Environ. Microbiol. 59:2956-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hjertqvist, M., et al. 2006. Four outbreaks of norovirus gastroenteritis after consuming raspberries, Sweden, June-August 2006. Euro Surveill. 11:3038. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=3038. [DOI] [PubMed] [Google Scholar]
- 22.Holmes, J. D., and G. C. Simmons. 2009. Gastrointestinal illness associated with a long-haul flight. Epidemiol. Infect. 137:441-447. [DOI] [PubMed] [Google Scholar]
- 23.Karst, S. M., C. E. Wobus, M. Lay, J. Davidson, and H. W. Virgin IV. 2003. STAT 1-dependent innate immunity to a Norwalk-like virus. Science 299:1575-1578. [DOI] [PubMed] [Google Scholar]
- 24.Kirkwood, C. D., R. Clark, N. Bogdanovic-Sakran, and R. F. A. O. Bishop. 2005. A 5-year study of the prevalence and genetic diversity of human caliciviruses associated with sporadic cases of acute gastroenteritis in young children admitted to hospital in Melbourne, Australia (1998-2002). J. Med. Virol. 77:96-101. [DOI] [PubMed] [Google Scholar]
- 25.Lee, J. E., K. D. Zoh, and G. P. Ko. 2008. Inactivation and UV disinfection of murine norovirus with TiO2 under various environmental conditions. Appl. Environ. Microbiol. 74:2111-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Guyader, F. S., et al. 2004. Detection of noroviruses in raspberries associated with a gastroenteritis outbreak. Int. J. Food Microbiol. 97:179-186. [DOI] [PubMed] [Google Scholar]
- 27.Magulski, T., et al. 2009. Inactivation of murine norovirus by chemical biocides on stainless steel. BMC Infect. Dis. 9:107-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noda, M., S. Fukuda, and O. Nishio. 2008. Statistical analysis of attack rate in norovirus foodborne outbreaks. Int. J. Food Microbiol. 122:216-220. [DOI] [PubMed] [Google Scholar]
- 29.Oh, S. W., G. I. Dancer, and D. H. Kang. 2005. Efficacy of aerosolized peroxyacetic acid as a sanitizer of lettuce leaves. J. Food Prot. 68:1743-1747. [DOI] [PubMed] [Google Scholar]
- 30.Otter, J. A., and G. L. French. 2009. Survival of nosocomial bacterial and spores on surfaces and inactivation by hydrogen peroxide vapor. J. Clin. Microbiol. 47:205-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel, M. M., A. J. Hall, J. Vinjé, and U. D. Parashar. 2009. Noroviruses: a comprehensive review. J. Clin. Virol. 44:1-8. [DOI] [PubMed] [Google Scholar]
- 32.Rapp, M. L., T. Thiel, and R. J. Arrowsmith. 1992. Model system using coliphage φX174 for testing virus removal by air filters. Appl. Environ. Microbiol. 58:900-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenfeldt, E. J., K. G. Linden, S. Canonica, and U. V. Gunten. 2006. Comparison of the efficiency of OH radical formation during ozonation and the advanced oxidation processes O3/H2O2 and UV/H2O2. Water Res. 40:3695-3704. [DOI] [PubMed] [Google Scholar]
- 34.Straub, T. M., et al. 2007. In vitro cell culture infectivity assay for human noroviruses. Emerg. Infect. Dis. 13:396-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tartera, C., R. Araujo, T. Michel, and J. Jofre. 1992. Culture and decontamination methods affecting enumeration of phages infecting Bacteroides fragilis in sewage. Appl. Environ. Microbiol. 58:2670-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thurston-Enriquez, J. A., C. N. Haas, J. Jacangelo, K. Riley, and C. P. Gerba. 2003. Inactivation of feline calicivirus and adenovirus type 40 by UV radiation. Appl. Environ. Microbiol. 69:577-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turcios, R. M., M. A. Widdowson, A. C. Sulka, P. S. Mead, and R. I. Glass. 2006. Reevaluation of epidemiological criteria for identifying outbreaks of acute gastroenteritis due to norovirus: United States, 1998-2000. Clin. Infect. Dis. 42:964-969. [DOI] [PubMed] [Google Scholar]
- 38.Vandekinderen, I., et al. 2008. Effect of decontamination agents on the microbial population, sensorial quality, and nutrient content of grated carrots (Daucus carota L.). J. Agric. Food Chem. 56:5723-5731. [DOI] [PubMed] [Google Scholar]
- 39.Weber, D. J., S. L. Barbee, M. D. Sobsey, and W. A. Rutala. 1999. The effect of blood on the viral activity of sodium hypochlorite, a phenolic, and a quaternary ammonium compound. Infect. Control Hosp. Epidemiol. 20:821-827. [DOI] [PubMed] [Google Scholar]
- 40.Wobus, C. E., et al. 2004. Replication of norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol. 2:2076-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu, H. M., et al. 2005. A norovirus outbreak at a long-term care facility: the role of environmental surface contamination. Infect. Control Hosp. Epidemiol. 26:802-810. [DOI] [PubMed] [Google Scholar]
- 42.Xie, Y., C. Hajdok, G. S. Mittal, and K. Warriner. 2008. Inactivation of MS2 F(+) coliphage on lettuce by a combination of UV light and hydrogen peroxide. J. Food Prot. 71:903-907. [DOI] [PubMed] [Google Scholar]