Abstract
In two previous studies dealing with lactic acid bacteria (LAB) from modified-atmosphere-packaged (MAP) broiler products and a broiler processing plant, several isolates remained unidentified. According to 16S rRNA gene sequence analysis, 36 isolates were assigned to the genus Enterococcus. Numerical analysis of combined HindIII and EcoRI ribopatterns of these isolates resulted in species-specific clusters that were congruent with the clusters obtained by both DNA-directed RNA polymerase subunit A (rpoA) and phenylalanyl-tRNA synthetase α chain (pheS) housekeeping gene analyses. In the analyses, a group of five isolates distinct from any known enterococcal species clustered together. The five isolates were positioned in the Enterococcus avium group, with E. devriesei being the closest phylogenetic neighbor. The DNA-DNA hybridization levels with E. devriesei ranged from 28.8 to 54.3% and indicated that these strains represented a novel species. The name Enterococcus viikkiensis sp. nov. is proposed, with strain DSM 24043T (LMG 26075T) being the type strain. Our study demonstrated that the identification of enterococci within the E. avium phylogenetic group demands polyphasic taxonomic approaches. The rpoA and pheS gene similarities (99.0 to 99.2% and 94.3 to 95.4%, respectively) between E. viikkiensis and its closest phylogenetic neighbor, E. devriesei, were higher than those previously reported within the enterococci. In addition, the phenotypic profiles of the species in the E. avium group were also highly similar, and some traits were found to be misleading for enterococci, such as E. viikkiensis does not grow at 45°C. The numerical analysis of combined HindIII and EcoRI ribopatterns was of considerable assistance in distinguishing enterococcal species within the E. avium group.
Enterococci are Gram-positive, catalase-negative, facultatively anaerobic cocci typically associated with the gastrointestinal tract of humans and animals. Enterococcus strains are also present in many food products, in fermented food, on plants, and in water and soil (1, 14, 15, 17, 22). Enterococcus faecium and Enterococcus faecalis are among the leading causes of nosocomial infections, and the recent increase in antibiotic-resistant strains has caused serious concern (51). The genus was described in 1984 (39), and on the basis of 16S rRNA gene sequence analysis, several phylogenetic groups have been distinguished (E. faecium, E. faecalis, Enterococcus avium, Enterococcus casseliflavus, Enterococcus dispar, Enterococcus saccharolyticus, and Enterococcus cecorum species groups) (16, 22, 52). Several novel species have recently been described (24, 30, 41, 43, 44, 45), and 40 Enterococcus species are currently recognized (13) (last full update, 8 September 2010).
The identification of enterococcal species is often challenging. Conventional phenotypic characterization of enterococci is laborious and may present problems in the interpretation of the results or even cause errors, because many enterococcal species vary by only one phenotypic trait (23, 27, 38). Several molecular methods have been used to differentiate Enterococcus species. Among these, numerical taxonomic methods (3, 12, 31) or sequence-based methods (28, 29, 35) have been the most commonly used. Numerical analysis of ribopatterns is one of the taxonomic approaches that has been successfully applied in the identification of enterococci (18, 19, 24, 25, 36, 42). However, previous studies have included only a limited number of enterococcal species, and to our knowledge, the method has not yet been used to establish species identification libraries. Recently, Naser et al. (29) applied multilocus sequence analysis to differentiate Enterococcus species. DNA-directed RNA polymerase subunit A (rpoA) and phenylalanyl-tRNA synthetase α chain (pheS) gene sequences were both able to discriminate even closely related Enterococcus species showing very high 16S rRNA sequence similarity.
In previous studies by our group, several isolates of lactic acid bacteria (LAB) from modified-atmosphere-packaged (MAP) broiler products (5) and a broiler processing plant (49) remained unidentified by use of a database constructed from the HindIII ribotypes of 260 LAB type and reference strains, including 33 strains of Enterococcus spp. (4, 6, 26). This study was designed to identify these strains to the species level and provide data on the LAB species associated with broiler production. Sequencing of the 16S rRNA gene, a restriction fragment polymorphism (RFLP) database combining EcoRI with HindIII ribopatterns, and sequence analysis of housekeeping genes pheS and rpoA were applied. In these analyses, five strains did not cluster together with any known Enterococcus type strain, and their taxonomic status was investigated using a polyphasic taxonomic approach.
MATERIALS AND METHODS
Isolation of strains and growth conditions.
Strains isolated from the air of a broiler processing facility (Table 1) originated from a study by Vihavainen et al. (49). They had been plated using Reuter centrifugal air (RCS) samplers (Biotest AG, Dreieich, Germany) on a strip of MRS agar (Oxoid, Basingstoke, United Kingdom). Samples from broiler carcasses had been psychrotrophically enriched by incubation in MRS broth at 6°C for 38 days. LAB from MAP broiler products (5, 49) had been isolated using MRS medium and anaerobic conditions at 25°C for 5 to 6 days.
TABLE 1.
Enterococcus species isolated from MAP broiler products and a broiler processing plant
| Species | Strain(s) | Source |
|---|---|---|
| E. avium | IEI.4, IE11.4 | Broiler processing plant aira |
| E. casseliflavus | IE2.5, IE7.3 | Broiler processing plant aira |
| NJ4a | Broiler carcass, oropharynxa | |
| MSRL42, MSRL45 | MAP broiler producta | |
| 361, 368 | MAP broiler productb | |
| E. durans | IE12.1, IE14.1, IE18.5 | Broiler processing plant aira |
| HJ6, HJ2 | Broiler carcass, feather shafta | |
| E. faecium | IE18.4 | Broiler processing plant aira |
| E. gallinarum | IE6.1, RSIB1 | Broiler processing plant aira |
| MSRL33 | MAP broiler producta | |
| 381, 393, 391, 383 | MAP broiler productb | |
| E. gilvus | IEII.3B, IE5.5, IE23.4, IE35.1, IE35.5, IE44.6, RSID4 | Broiler processing plant aira |
| 340 | MAP broiler productib | |
| E. malodoratus | IE9.2 | Broiler processing plant aira |
| E. viikkiensis sp. nov. | IEII.2B, IE3.2, IE35.3, IE36.1, IE42.2 | Broiler processing plant aira |
All isolates had been maintained in MRS broth at −70°C. In the present study, the isolates were grown in MRS broth and agar at 25°C. The plates were incubated in anaerobic jars in a CO2-enriched atmosphere (9 to 13% CO2, according to the manufacturer; Anaerogen [Oxoid]). The enterococcal reference strains used in this study are presented in Table S1 in the supplemental material.
Phenotypic tests.
Growth tests at different temperatures and NaCl concentrations were performed and carbohydrate fermentation profiles, Lancefield antigen D, hemolysis, the production of ammonia from arginine, and the formation of typical colonies for enterococci were determined as described by Koort et al. (24).
Isolation of DNA.
Cells harvested from 1 to 2 ml of MRS broth culture were used for DNA analysis. DNA for all analyses was isolated as described by Björkroth and Korkeala (4). The guanidium thiocyanate method of Pitcher et al. (33) was modified by using lysozyme (25 mg/ml) and mutanolysin (200 U/ml) in the cell lysis solution.
Ribotyping.
Ribotyping was performed as described previously (4). EcoRI and HindII restriction enzymes were used to digest 8 μg of DNA, as specified by the manufacturer (New England BioLabs, Beverly, MA). DNA fragments were separated by agarose gel electrophoresis, and Southern blotting was performed using a Vacugene blotting system (Pharmacia, Uppsala, Sweden). The fragments containing the 16S or 23S rRNA gene were detected with a digoxigenin-labeled oligonucleotide probe mixture, OligoMix5 (37). The membranes were hybridized at 58°C, and the labeled fragments were detected as recommended by Roche Molecular Biochemicals. Scanned (Scan Jet 4c/T; Hewlett Packard, Palo Alto, CA) ribopatterns were analyzed using Bionumerics (version 5.10) software (Applied Maths, Sint-Martens-Latem, Belgium). The Dice coefficient correlation and unweighted-pair group method using average linkages (UPGMA) were used for construction of the dendrograms, with a pattern optimization of 0.6% and a band position tolerance of 1.5% being used.
16S rRNA gene sequence analysis.
16S rRNA gene sequence analysis was performed as described by Vihavainen et al. (49). The nearly complete 16S rRNA gene was amplified using a universal primer pair comprising primer F8-27 (5′-AGAGTTTGATCCTGGCTGAG-3′) and primer R1541-1522 (5′-AAGGAGGTGATCCAGCCGCA-3′). The PCR product was purified (QIAquick PCR purification kit; Qiagen) and sequenced by Sanger's dideoxynucleotide chain termination method using two long reactions with primers F1-38 (5′-CTGGCTCAGGAYGAACGCTG-3′) and R1541-1522 and two shorter reactions with primer F926 (5′-AACTCAAAGGAATTGACGG-3′). Samples were run in a Global IR2 sequencing device with e-Seq (version 2.0) software (LiCor, Lincoln, NE), according to the manufacturer's instructions. The consensus sequences were created with AlignIR software (LiCor).
pheS and rpoA gene sequence analysis.
The sequence analysis of housekeeping genes pheS and rpoA was performed as described by Naser et al. (29). Primer pairs pheS-21-F/pheS-22-R (5′-CAYCCNGCHCGYGAYATGC-3′/5′-CCWARVCCRAARGCAAARCC-3′), pheS-21-F/pheS-R008 (5′-CCAGCHCCHAGHACTTCAATCCA-3′), pheS-F004/pheS-R011 (5′-ATGAATCTDCCWAAAGATCAYCC-3′/5′-TAAGAAACGTAARTCATTTTGATARAA-3′), rpoA-21-F/rpoA-23-R (5′-ATGATYGARTTTGAAAAACC-3′/5′-ACHGTRTTRATDCCDGCRCG-3′), and rpoA-21-F/rpoA-R009 (5′-TCWARYTCTTCRATNGTCAT-3′) were used for amplification and sequencing of the genes. PCR was performed using a PTC-200 apparatus (version 3.8; MJ Research, MA). PCR products were checked by 1% agarose gel electrophoresis (Seakem LE agarose, Rockland, ME). Sequencing was performed with a BigDye Terminator cycle sequencing kit (Applied Biosystems, Foster City, CA) and an ABI 3700 capillary DNA sequencer (GMI, Ramsey, MN). Sequences were assembled using the Staden package (Medical Research Council Laboratory of Molecular Biology, Cambridge, United Kingdom).
Construction of phylogenetic trees.
The 16S rRNA, pheS, and rpoA gene sequences were subjected to the BLAST search program (2) and aligned with sequences of representative strains from the same phylogenetic group retrieved from GenBank using Clustal X software (46). The construction of phylogenetic trees was performed with Bionumerics software (version 5.10; Applied Maths) using the neighbor-joining method (see Fig. 2 and 3 and Fig. S2 to S4 in the supplemental material) and maximum parsimony method. The reliability of the groups was evaluated by bootstrap analysis with 500 resamplings. Tetragenococcus solitarius LMG 12890 was used as an outgroup.
DNA-DNA reassociation and GC content.
DNA-DNA reassociation was performed by DSMZ, Braunschweig, Germany. Briefly, DNA was isolated using a French pressure cell (Thermo Spectronic) and was purified by chromatography on hydroxyapatite as described by Cashion et al. (7). DNA-DNA hybridization was carried out as described by De Ley et al. (9) under consideration of the modifications described by Huss et al. (21) using a model Cary 100 Bio UV/visible spectrophotometer equipped with a Peltier-thermostatted 6-by-6 multicell changer and a temperature controller with an in situ temperature probe (Varian).
The DNA GC content of strains IE3.2 and IE35.3 was determined as described by Xu et al. (53). The melting point curves were determined in 1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) with a LightCycler instrument (Roche Diagnostics) using SYBR green I dye (Roche Diagnostics). E. devriesei LMG 14595T and LMG 13603 were used as reference organisms, and E. hermanniensis LMG 12317 was used as the control.
Nucleotide sequence accession numbers.
The gene sequences were submitted to the GenBank database and were given the following nucleotide sequence accession numbers: HQ378509 to HQ378524 for 16S rRNA genes, HQ378525 to HQ378560 for rpoA, and HQ378561 to HQ378593 for pheS.
RESULTS
Numerical analyses of ribopatterns.
The ribopatterns and a dendrogram based on the combined data from the numerical analysis of the HindIII and EcoRI ribopatterns of the isolates and selected Enterococcus type and reference strains are shown in Fig. 1. Table 1 presents the HindIII and EcoRI analysis-deduced clusters considered species specific on the basis of the locations of the type and reference strains. The species detected in MAP broiler products were considered to be Enterococcus gallinarum, E. casseliflavus, and Enterococcus gilvus. Strains that were isolated from the air of the broiler processing plant were assigned to the species E. gilvus, E. gallinarum, E. casseliflavus, Enterococcus durans, Enterococcus malodoratus, E. avium, and E. faecium and a novel species with the proposed name E. viikkiensis sp. nov. E. durans and E. casseliflavus were detected in broiler carcasses. The clusters considered species specific had intraspecific pattern similarities of 68% or more. Isolates belonging to the E. avium phylogenetic group clustered together at a similarity level of 62%. Other phylogenetic species groups (E. faecium, E. casseliflavus, E. dispar, E. saccharolyticus, E. cecorum, and E. faecalis species groups) were spread out in the dendrogram. The fact that numerical analyses of ribotypes or other macromolecular patterns did not necessarily produce a phylogenetic grouping has also been noted previously (3, 31). Compared to the dendrogram based on HindIII ribopatterns alone (see Fig. S1 in the supplemental material), the isolates clustered together with the type and reference strains more clearly when HindIII and EcoRI data were combined. Strains belonging to the species E. devriesei, E. avium, and E. durans, as well as a novel species with the proposed name E. viikkiensis sp. nov., were not identified to the species level with HindIII alone.
FIG. 1.
UPGMA dendrogram based on combined HindIII and EcoRI ribopatterns of 31 Enterococcus type and reference strains and 36 isolates from MAP broiler products and a broiler processing plant. The HindIII and EcoRI banding patterns are also shown. The molecular masses of the bands range from 1 kb (right side) to 23 kb (left side).
In the combined HindIII and EcoRI ribopattern analysis, five isolates (IEII.2B, IE3.2, IE42.2, IE36.1, and IE35.3) formed a cluster with a pattern similarity level of 84% or more that was clearly distinct from any type strain. The ribopatterns of these five strains were most similar to the patterns of strains belonging to the E. avium species group, with E. gilvus and E. devriesei possessing the highest pattern similarities.
rpoA, pheS, and 16S rRNA gene sequence analysis.
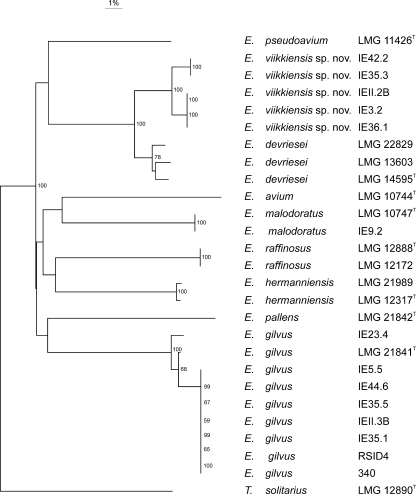
Sequence analysis of housekeeping genes pheS and rpoA from 36 isolates was performed to evaluate the use of the numerical analysis of ribopatterns in the species identification of enterococci and to investigate the taxonomic positions of the 5 unknown isolates. The clusters obtained with numerical analysis of the HindIII and EcoRI ribopatterns were congruent with both the rpoA and pheS gene sequence trees (see Fig. S2 and S3 in the supplemental material). The five isolates (IEII.2B, IE3.2, IE42.2, IE36.1, and IE35.3) formed a distinct branch that was closest to E. devriesei in both rpoA and pheS gene sequence analysis (Fig. 2 and 3). In the analysis of the partial rpoA gene sequences (585 bp), the sequences of these strains were most similar to those of E. devriesei strains LMG 14595T, LMG 22829, and LMG 13603 (99.0 to 99.1% sequence similarity), as well as those of E. avium (95.7%), E. malodoratus (95.8%), E. raffinosus (94.5%), E. gilvus (93.5%), and E. pseudoavium (93.2%). The partial pheS gene sequences (369 bp) of these five isolates were most similar to those of E. devriesei strains LMG 14595T, LMG 22829, and LMG 13603 (94.9 to 95.7%), as well as those of E. malodoratus (89.7%), E. pseudoavium (84.6%), E. avium (82.9%), E. hermanniensis (82.6%), and E. gilvus (82.5%).
FIG. 2.
Neighbor-joining dendrogram based on the rpoA sequences of 12 Enterococcus type and reference strains and 17 isolates from MAP broiler products and a broiler processing plant positioned in the E. avium phylogenetic group. Bootstrap percentages (≥50%) after 500 replicates are shown. Tetragenococcus solitarius is included as an outgroup.
FIG. 3.
Neighbor-joining dendrogram based on the pheS sequences of 12 Enterococcus type and reference strains and 14 isolates from MAP broiler products and a broiler processing plant positioned in the E. avium phylogenetic group. Bootstrap percentages (≥50%) after 500 replicates are shown. Tetragenococcus solitarius is included as an outgroup.
The almost complete 16S rRNA gene sequences of 16 representative isolates, including the 5 unknown isolates, were determined. The 16S rRNA gene sequences of isolates IEII.2B, IE3.2, IE42.2, IE36.1, and IE35.3 were identical and most similar to those of strains from the E. avium species group. In the analysis, the 16S rRNA gene sequences of these isolates possessed the highest sequence similarities with E. devriesei strains LMG 14595T, LMG 22829, LMG 13603, and LMG 22830 (99.9 to 100%), as well as E. pseudoavium (99.9%), E. avium (99.5%), E. raffinosus (99.5%), E. gilvus (99.5%), and E. malodoratus (99.4%). In the phylogenetic tree based on 16S rRNA sequences (see Fig. S4 in the supplemental material), the five isolates were positioned in the E. avium group, with E. devriesei and E. pseudoavium being the closest neighbors.
DNA-DNA reassociation experiment and mean base composition of DNA.
DNA-DNA hybridization was performed with strains IE3.2 and IE35.3 and the phylogenetically closest neighbors E. devriesei strains LMG 14595T and LMG 13603. E. hermanniensis LMG 12317T was tested with E. devriesei LMG 14595T as the negative control. The DNA-DNA hybridization value between strains IE3.2 and IE35.3 was 103.6%, which clearly indicated that these strains represent the same species. The relatedness between strains IE3.2 and IE35.3 and E. devriesei strains LMG 14595T and LMG 13603 ranged from 28.8 to 54.3%. Thus, strains IE3.2 and IE35.3 do not belong to the species E. devriesei, when the recommendation of a threshold value of 70% DNA-DNA similarity is considered (50). The hybridization value between E. devriesei LMG 114595T and E. hermanniensis LMG 12317T was 10.8%. The GC contents of strains IE3.2 and IE35.3 were 39.8 and 39.9%, respectively.
Phenotypic properties.
Details of the phenotypic properties are given in the species description and Table 2. Isolates IE3.2, IEII.2B, IE36.1, IE42.2, and IE35.3 did not grow at 4°C.
TABLE 2.
Characteristics of Enterococcus viikkiensis and the closest phylogenetic neighbors in the E. avium group
| Characteristic | Finding for the following speciesa: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Growth at: | |||||||||
| 10°C | + | + | + | D | + | D | + | + | + |
| 45°C | − | − | − | + | + | + | D | + | + |
| Group D antigen | + | ND | − | D | − | D | + | + | + |
| Acid production from: | |||||||||
| l-Arabinose | − | D | − | + | − | + | − | − | + |
| l-Arabitol | − | D | − | + | − | + | + | ND | ND |
| Dulcitol | − | − | − | D | − | − | D | ND | ND |
| Glycerol | + | D | − | + | − | + | D | + | + |
| Inositol | − | − | − | − | − | + | − | ND | ND |
| Lactose | + | + | − | + | + | + | + | + | + |
| Melezitose | − | D | − | + | − | + | − | ND | ND |
| Melibiose | − | − | − | − | − | + | + | + | + |
| Raffinose | − | D | − | − | − | + | + | + | + |
| Rhamnose | − | D | D | + | − | + | + | ND | ND |
| Sorbitol | − | D | − | + | + | + | + | + | + |
| l-Sorbose | − | D | − | + | + | + | + | + | + |
| d-Tagatose | − | D | − | + | − | + | + | ND | ND |
Species: 1, E. viikkiensis sp. nov.; 2, E. devriesei; 3, E. hermanniensis; 4, E. avium; 5, E. pseudoavium; 6, E. raffinosus; 7, E. malodoratus; 8, E. gilvus; 9, E. pallens. Characteristics are scored as follows: +, positive; −, negative; D, either positive or negative; ND, not determined. Data for taxa other than E. viikkiensis were obtained from Koort et al. (24), Svec et al. (44), Tyrrell et al. (48), Devriese et al. (11), and Collins et al. (8).
DISCUSSION
The discriminatory power of 16S and 23S rRNA gene RFLPs in the identification of enterococci was enhanced by using two restriction enzymes, HindIII and EcoRI, and by combining the data from the numerical analysis of ribopatterns. With a combination of HindIII and EcoRI we were able to obtain more distinct species-specific clusters than we could with HindIII alone. The clustering was confirmed to be species specific by sequence analysis of housekeeping genes rpoA and pheS, which have been reported by Naser et al. (29) to be reliable in the species identification of enterococci.
It is known that enterococci have a high level of 16S rRNA gene sequence similarity between phylogenetically closely related species (32, 52). 16S and 23S rRNA gene RFLPs can discriminate closely related species better than 16S rRNA sequence analysis, because in ribopatterns the labeled fragments consist of ribosomal operon and flanking sequences of up to 23 kbp. Enterococci possess five or six rrn operons (40), which explains the good number of bands obtained. On average, EcoRI produced more bands (9 to 14 bands) and was more discriminatory than HindIII (4 to 14 bands). This has also been reported by Pryce et al. (36) and Svec et al. (42), who used Escherichia coli and E. faecalis 16S and 23S rRNA gene probes. We used a chemically labeled set of five oligonucleotides targeting the 16S and 23S rRNA genes (OligoMix5) (37) and obtained strong banding patterns for all 31 Enterococcus species and 36 isolates studied.
The results of this study demonstrated that the five LAB strains isolated from the air of a broiler processing plant represent a novel species for which we propose the name Enterococcus viikkiensis sp. nov. The 16S rRNA gene sequence analysis clearly assigned these strains to the genus Enterococcus and to its E. avium phylogenetic group. Numerical analysis of HindIII and EcoRI ribopatterns, as well as sequence analysis of housekeeping genes pheS and rpoA, distinguished these strains as being separate from the other members of the E. avium group. Sequence analysis of housekeeping genes indicated that the E. viikkiensis strains formed a group that is closely related to E. devriesei. The rpoA and pheS gene sequences of the E. viikkiensis strains had higher similarities with E. devriesei (99.0 to 99.2% and 94.3 to 95.4%, respectively) than the interspecies heterogeneity reported by Naser et al. (29). DNA-DNA reassociation studies differentiated the two species E. viikkiensis and E. devriesei. E. viikkiensis strains had 16S rRNA gene sequences identical to those of E. devriesei and E. pseudoavium type strains. However, the 16S rRNA gene sequence similarity of previously described enterococcus species in the E. avium group was also high (97.2 to 99.9%) (32, 44, 52). Moreover, identification of these species on the basis of biochemical characteristics can be misleading (44). Even genus-level identification is problematic for the recently described species in the E. avium group that do not grow at 45°C or react with Lancefield group D antisera (24, 42).
All our 36 Enterococcus strains were associated with broilers and originated from broiler carcasses, processing plant air, and MAP broiler products (5, 49). The five E. viikkiensis strains were isolated from processing plant air (49). Enterococci are ubiquitous in the environment and are commonly found in meat, the intestinal tract of chickens, and the poultry production environment (10, 20, 34, 47). Strains of the recently described species E. devriesei and E. hermanniensis, being closely related to E. viikkiensis, also originated from poultry processing plant air and MAP, marinated broiler legs (24, 44). E. viikkiensis, like E. avium and E. hermanniensis, does not grow at 4°C and is not very likely to be a spoilage organism if food is refrigerated. It has been shown that enterococci are poor competitors in cold-stored MAP broiler products and are easily overgrown by psychrotrophic LAB during storage (5).
In this study we demonstrated that the five unknown LAB strains isolated from poultry processing plant air represent a novel species, E. viikkiensis sp. nov. This study also indicated that 16S and 23S rRNA gene RFLPs with HindIII and EcoRI have a greater resolution power than 16S rRNA gene sequence analysis or phenotypic tests in the identification of enterococci, especially within the species in the E. avium group.
Description of Enterococcus viikkiensis sp. nov.
Enterococcus viikkiensis (viik.ki.en′sis. N.L. masc. adj. viikkiensis pertaining to Viikki, a locality in Helsinki, Finland). The cells are Gram-positive, catalase-negative, facultatively anaerobic coccoid and cocci. Colonies on blood or MRS medium are white to light gray and translucent. On bovine blood agar there is alpha-hemolysis. Strains grow slowly on azide-containing enterococcal selective agar as small light pink to colorless colonies and cause blackening of bile-esculin agar. All strains grow well at 10 and 37°C, but no growth is observed at 4°C or at 42 or 45°C. Strains grow well in the presence of 2, 4, or 6.5% NaCl, and two strains (IEII2.B and IE3.2) grow in the presence of 8% NaCl but not 10% NaCl. All strains react positively in Voger-Proskauer and Lancefield D antigen tests and negatively in tests for hippurate hydrolysis, pyrrolidonyl arylamidase, α-galactosidase, β-galactosidase, β-glucuronidase, alkaline phosphatase, leucine arylamidase, and arginine dihydrolase. Esculin hydrolysis may be weak or delayed. Acid is produced from glycerol, d-ribose, d-galactose, d-glucose, d-fructose, d-mannose, d-mannitol, N-acetylglucosamine, amygdalin, arbutin, salicin, d-cellobiose, d-maltose, d-trehalose, and gentibiose. Acid production from lactose and gluconate may be weak or delayed. No acid is produced from erythritol, d-arabinose, l-arabinose, d-xylose, methyl-β-d-xylopyranoside, l-sorbose, l-rhamnose, dulcitol, inositol, d-sorbitol, methyl-α-d-mannopyranoside, methyl-α-\d-glucopyranoside, d-melibiose, d-melezitose, d-raffinose, starch, glycogen, xylitol, d-turanose, d-lyxose, d-fucose, l-fucose, l-arabitol, or 5-ketogluconate. Acid production from d-adonitol, inulin, d-tagatose, d-arabitol, and 2-ketogluconate is weak or absent. Strains IE42.2 and IE35.3 produce acid from d-saccharose. The GC content of DNA ranged from 39.8 to 39.9%. The type strain is DSM 24043T (LMG 26075T, IE3.2T). Strains IE35.3, IE36.1, IE42.2, and IEII.2B were deposited in the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany) as DSM 24044, DSM 24045, DSM 24046, and DSM 24047, respectively, and in the Belgian Co-ordinated Collections of Microorganisms (BCCM/LMG, Gent, Belgium) as LMG 26079, LMG 26077, LMG 26078, and LMG 26076, respectively. All strains were isolated from the air of a broiler processing plant.
Supplementary Material
Acknowledgments
We thank Henna Niinivirta and Erja Merivirta for excellent technical assistance and Timo Nieminen for reading the manuscript.
The Academy of Finland project 110310 (Food-hygienic risks caused by novel lactic acid bacteria) and the funding for the Centre of Excellence in Microbial Food Safety Research are gratefully acknowledged.
Footnotes
Published ahead of print on 23 December 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Abriouel, H., et al. 2008. Comparative analysis of genetic diversity and incidence of virulence factors and antibiotic resistance among enterococcal populations from raw fruit and vegetable foods, water and soil, and clinical samples. Int. J. Food Microbiol. 123:38-49. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., et al. 1997. Gapped blast and psi-blast: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alves, P. I., et al. 2004. Comparison of phenotypic and genotypic taxonomic methods for the identification of dairy enterococci. Antonie Van Leeuwenhoek 85:237-252. [DOI] [PubMed] [Google Scholar]
- 4.Björkroth, J., and H. Korkeala. 1996. rRNA gene restriction patterns as a characterization tool for Lactobacillus sake strains producing ropy slime. Int. J. Food Microbiol. 30:293-302. [DOI] [PubMed] [Google Scholar]
- 5.Björkroth, J., M. Ristiniemi, P. Vandamme, and H. Korkeala. 2005. Enterococcus species dominating in fresh modified-atmosphere-packaged, marinated broiler legs are overgrown by Carnobacterium and Lactobacillus species during storage at 6°C. Int. J. Food Microbiol. 97:267-276. [DOI] [PubMed] [Google Scholar]
- 6.Björkroth, K. J., and H. J. Korkeala. 1997. Use of rRNA gene restriction patterns to evaluate lactic acid bacterium contamination of vacuum-packaged sliced cooked whole-meat product in a meat processing plant. Appl. Environ. Microbiol. 63:448-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cashion, P., M. A. Holder-Franklin, J. McCully, and M. Franklin. 1977. A rapid method for the base ratio determination of bacterial DNA. Anal. Biochem. 81:461-466. [DOI] [PubMed] [Google Scholar]
- 8.Collins, M. D., J. A. Farrow, B. A. Phillips, and O. Kandler. 1983. Streptococcus garvieae sp. nov. and Streptococcus plantarum sp. nov. J. Gen. Microbiol. 129:3427-3431. [DOI] [PubMed] [Google Scholar]
- 9.De Ley, J., H. Cattoir, and A. Reynaerts. 1970. The quantitative measurement of DNA hybridization from renaturation rates. Eur. J. Biochem. 12:133-142. [DOI] [PubMed] [Google Scholar]
- 10.Devriese, L. A., J. Hommez, R. Wijfels, and F. Haesebrouck. 1991. Composition of the enterococcal and streptococcal intestinal flora of poultry. J. Appl. Bacteriol. 71:46-50. [PubMed] [Google Scholar]
- 11.Devriese, L. A., B. Pot, and M. D. Collins. 1993. Phenotypic identification of the genus Enterococcus and differentiation of phylogenetically distinct enterococcal species and species groups. J. Appl. Bacteriol. 75:399-408. [DOI] [PubMed] [Google Scholar]
- 12.Domig, K. J., H. K. Mayer, and W. Kneifel. 2003. Methods used for the isolation, enumeration, characterisation and identification of Enterococcus spp. 2. Pheno- and genotypic criteria. Int. J. Food Microbiol. 88:165-188. [DOI] [PubMed] [Google Scholar]
- 13.Euzéby, J. P. 1997. List of bacterial names with standing in nomenclature: a folder available on the internet. Int. J. Syst. Bacteriol. 47:590-592. http://www.bacterio.cict.fr/. [DOI] [PubMed] [Google Scholar]
- 14.Fisher, K., and C. Phillips. 2009. The ecology, epidemiology and virulence of Enterococcus. Microbiology 155:1749-1757. [DOI] [PubMed] [Google Scholar]
- 15.Foulquié Moreno, M. R. F., P. Sarantinopoulos, E. Tsakalidou, and L. D. Vuyst. 2006. The role and application of enterococci in food and health. Int. J. Food Microbiol. 106:1-24. [DOI] [PubMed] [Google Scholar]
- 16.Franz, C. M., M. E. Stiles, K. H. Schleifer, and W. H. Holzapfel. 2003. Enterococci in foods—a conundrum for food safety. Int. J. Food Microbiol. 88:105-122. [DOI] [PubMed] [Google Scholar]
- 17.Giraffa, G. 2002. Enterococci from foods. FEMS Microbiol. Rev. 26:163-171. [DOI] [PubMed] [Google Scholar]
- 18.Gordillo, M. E., K. V. Singh, and B. E. Murray. 1993. Comparison of ribotyping and pulsed-field gel electrophoresis for subspecies differentiation of strains of Enterococcus faecalis. J. Clin. Microbiol. 31:1570-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall, L. M., B. Duke, M. Guiney, and R. Williams. 1992. Typing of Enterococcus species by DNA restriction fragment analysis. J. Clin. Microbiol. 30:915-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayes, J. R., L. L. English, L. E. Carr, D. D. Wagner, and S. W. Joseph. 2004. Multiple-antibiotic resistance of Enterococcus spp. isolated from commercial poultry production environments. Appl. Environ. Microbiol. 70:6005-6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huss, V. A. R., H. Festl, and K. H. Schleifer. 1983. Studies on the spectrophotometric determination of DNA hybridization from renaturation rates. Syst. Appl. Microbiol. 4:184-192. [DOI] [PubMed] [Google Scholar]
- 22.Klein, G. 2003. Taxonomy, ecology and antibiotic resistance of enterococci from food and the gastro-intestinal tract. Int. J. Food Microbiol. 88:123-131. [DOI] [PubMed] [Google Scholar]
- 23.Knudtson, L. M., and P. A. Hartman. 1992. Routine procedures for isolation and identification of enterococci and fecal streptococci. Appl. Environ. Microbiol. 58:3027-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koort, J., T. Coenye, P. Vandamme, A. Sukura, and J. Björkroth. 2004. Enterococcus hermanniensis sp. nov., from modified-atmosphere-packaged broiler meat and canine tonsils. Int. J. Syst. Evol. Microbiol. 54:1823-1827. [DOI] [PubMed] [Google Scholar]
- 25.Lang, M. M., S. C. Ingham, and B. H. Ingham. 2001. Differentiation of Enterococcus spp. by cell membrane fatty acid methyl ester profiling, biotyping and ribotyping. Lett. Appl. Microbiol. 33:65-70. [DOI] [PubMed] [Google Scholar]
- 26.Lyhs, U., J. Björkroth, and H. Korkeala. 1999. Characterisation of lactic acid bacteria from spoiled, vacuum-packaged, cold-smoked rainbow trout using ribotyping. Int. J. Food Microbiol. 52:77-84. [DOI] [PubMed] [Google Scholar]
- 27.Moore, D. F., et al. 2006. Comparison of 16S rRNA sequencing with conventional and commercial phenotypic techniques for identification of enterococci from the marine environment. J. Appl. Microbiol. 100:1272-1281. [DOI] [PubMed] [Google Scholar]
- 28.Naser, S., et al. 2005. Phylogeny and identification of enterococci by atpA gene sequence analysis. J. Clin. Microbiol. 43:2224-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naser, S. M., et al. 2005. Application of multilocus sequence analysis (MLSA) for rapid identification of Enterococcus species based on rpoA and pheS genes. Microbiology 151:2141-2150. [DOI] [PubMed] [Google Scholar]
- 30.Naser, S. M., et al. 2005. Enterococcus canintestini sp. nov., from faecal samples of healthy dogs. Int. J. Syst. Evol. Microbiol. 55:2177-2182. [DOI] [PubMed] [Google Scholar]
- 31.Pangallo, D., et al. 2008. Evaluation of different PCR-based approaches for the identification and typing of environmental enterococci. Antonie Van Leeuwenhoek 93:193-203. [DOI] [PubMed] [Google Scholar]
- 32.Patel, R., et al. 1998. Determination of 16S rRNA sequences of enterococci and application to species identification of nonmotile Enterococcus gallinarum isolates. J. Clin. Microbiol. 36:3399-3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pitcher, D. G., N. A. Saunders, and R. J. Owen. 1989. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett. Appl. Microbiol. 8:151-156. [Google Scholar]
- 34.Pleydell, E., L. Rogers, E. Kwan, and N. French. 2010. Evidence for the clustering of antibacterial resistance phenotypes of enterococci within integrated poultry companies. Microb. Ecol. 59:678-688. [DOI] [PubMed] [Google Scholar]
- 35.Poyart, C., G. Quesnes, and P. Trieu-Cuot. 2000. Sequencing the gene encoding manganese-dependent superoxide dismutase for rapid species identification of enterococci. J. Clin. Microbiol. 38:415-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pryce, T. M., R. D. Wilson, and J. K. Kulski. 1999. Identification of enterococci by ribotyping with horseradish-peroxidase-labelled 16S rDNA probes. J. Microbiol. Methods 36:147-155. [DOI] [PubMed] [Google Scholar]
- 37.Regnault, B., F. Grimont, and P. A. Grimont. 1997. Universal ribotyping method using a chemically labelled oligonucleotide probe mixture. Res. Microbiol. 148:649-659. [DOI] [PubMed] [Google Scholar]
- 38.Scheidegger, E. M. D., S. A. P. Fracalanzza, L. M. Teixeira, and P. Cardarelli-Leite. 2009. RFLP analysis of a PCR-amplified fragment of the 16S rRNA gene as a tool to identify Enterococcus strains. Mem. Inst. Oswaldo Cruz 104:1003-1008. [DOI] [PubMed] [Google Scholar]
- 39.Schleifer, K. H., and R. Kilpper-Bälz. 1984. Transfer of Streptococcus faecalis and Streptococcus faecium to the genus Enterococcus nom. rev. as Enterococcus faecalis comb. nov. and Enterococcus faecium comb. nov. Int. J. Syst. Bacteriol. 34:31-34. [Google Scholar]
- 40.Sechi, L. A., F. M. Zuccon, J. E. Mortensen, and L. Daneo-Moore. 1994. Ribosomal RNA gene (rrn) organization in enterococci. FEMS Microbiol. Lett. 120:307-313. [DOI] [PubMed] [Google Scholar]
- 41.Sukontasing, S., S. Tanasupawat, S. Moonmangmee, J.-S. Lee, and K.-I. Suzuki. 2007. Enterococcus camelliae sp. nov., isolated from fermented tea leaves in Thailand. Int. J. Syst. Evol. Microbiol. 57:2151-2154. [DOI] [PubMed] [Google Scholar]
- 42.Svec, P., I. Sedlácek, R. Pantcek, L. A. Devriese, and J. V. Doskar. 2001. Evaluation of ribotyping for characterization and identification of Enterococcus haemoperoxidus and Enterococcus moraviensis strains. FEMS Microbiol. Lett. 203:23-27. [DOI] [PubMed] [Google Scholar]
- 43.Svec, P., et al. 2005. Enterococcus aquimarinus sp. nov., isolated from sea water. Int. J. Syst. Evol. Microbiol. 55:2183-2187. [DOI] [PubMed] [Google Scholar]
- 44.Svec, P., et al. 2005. Enterococcus devriesei sp. nov., associated with animal sources. Int. J. Syst. Evol. Microbiol. 55:2479-2484. [DOI] [PubMed] [Google Scholar]
- 45.Tanasupawat, S., S. Sukontasing, and J.-S. Lee. 2008. Enterococcus thailandicus sp. nov., isolated from fermented sausage (‘mum’) in Thailand. Int. J. Syst. Evol. Microbiol. 58:1630-1634. [DOI] [PubMed] [Google Scholar]
- 46.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turtura, G. C., and P. Lorenzelli. 1994. Gram-positive cocci isolated from slaughtered poultry. Microbiol. Res. 149:203-213. [DOI] [PubMed] [Google Scholar]
- 48.Tyrrell, G. J., L. Turnbull, L. M. Teixeira, J. Lefebvre, M. da G. Carvalho, R. R. Facklam, and M. Lovgren. 2002. Enterococcus gilvus sp. nov. and Enterococcus pallens sp. nov. isolated from human clinical specimens. J. Clin. Microbiol. 40:1140-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vihavainen, E., et al. 2007. Role of broiler carcasses and processing plant air in contamination of modified-atmosphere-packaged broiler products with psychrotrophic lactic acid bacteria. Appl. Environ. Microbiol. 73:1136-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wayne, L. G., et al. 1987. International Committee on Systematic Bacteriology. Report of the Ad Hoc Committee on Reconciliation of Approaches to Bacterial Systematics. Int. J. Syst. Bacteriol. 37:463-464. [Google Scholar]
- 51.Werner, G., et al. 2008. Emergence and spread of vancomycin resistance among enterococci in Europe. Euro Surveill. 13:1-11. [PubMed] [Google Scholar]
- 52.Williams, A. M., U. M. Rodrigues, and M. D. Collins. 1991. Intrageneric relationships of enterococci as determined by reverse transcriptase sequencing of small-subunit rRNA. Res. Microbiol. 142:67-74. [DOI] [PubMed] [Google Scholar]
- 53.Xu, H. X., et al. 2000. A rapid method for determining the G+C content of bacterial chromosomes by monitoring fluorescence intensity during DNA denaturation in a capillary tube. Int. J. Syst. Evol. Microbiol. 50(Pt. 4):1463-1469. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.