Abstract
Lactococcus lactis is a lactic acid bacterium of proven safety for use in human oral applications. For this purpose, surface display of recombinant proteins is important, and new approaches for it are being sought. Analysis of the bacterial surface proteome is essential in identifying new candidate carrier proteins for surface display. We have made two different predictions of surface-associated proteins of L. lactis MG1363 by using Augur and LocateP software, which yielded 666 and 648 proteins, respectively. Surface proteins of L. lactis NZ9000, a derivative of MG1363, were identified by using a proteomics approach. The surface proteins were cleaved from intact bacteria, and the resulting peptides were identified by mass spectrometry. The latter approach yielded 80 proteins, 34 of which were not predicted by either software. Of the 80 proteins, 7 were selected for further study. These were cloned in frame with a C-terminal hexahistidine tag and overexpressed in L. lactis NZ9000 using nisin-controlled expression. Proteins of correct molecular weight carrying a hexahistidine tag were detected. Their surface localization was confirmed with flow cytometry. Basic membrane protein A (BmpA) was exposed at the highest level. To test BmpA as a candidate carrier protein, the hexahistidine tag was replaced by the B domain of staphylococcal protein A in the genetic construct. The B domain was displayed on the surface with BmpA as a carrier. The advantage of covalent BmpA binding was demonstrated. BmpA was thus shown to be a suitable candidate for a carrier protein in lactococcal surface display.
Lactic acid bacteria (LAB) are among the most intensively exploited microorganisms in the dairy industry. They are used in the fermentation of foodstuffs and as probiotics on account of their health benefits (11). Lactococcus lactis is a model LAB with industrially important applications. The genomes of several L. lactis strains have been sequenced, but strains MG1363 (33) and IL1403 (4) are most commonly used in laboratories.
Various biotechnological applications of surface display of recombinant proteins in bacteria have been suggested, with vaccine delivery being the most popular in LAB (20, 34). Other uses include the display of enzymes (35) as whole-cell biocatalysts, display of binding molecules such as antibodies or affibodies (35) (in diagnostics as biosensors, in therapy for pathogen or toxin removal, and in bioremediation for heavy metal binding [36]), and display of proteins for protein engineering by using combinatorial libraries and in vitro selection (8).
The majority of surface display techniques have been developed for Gram-negative bacteria, with autodisplay being probably the most efficacious technique (16). However, several other approaches for surface display in Gram-positive bacteria have also been described. Transmembrane proteins, lipoproteins, LPXTG-like proteins, and cell wall-binding proteins have been displayed on the surfaces of Gram-positive bacteria (10). Two of these display types have been commonly exploited in L. lactis. Lactococcal proteinase PrtP (24), proteinase PrtB from Lactobacillus delbrueckii subsp. bulgaricus (17), M6 protein from Streptococcus pyogenes (23), and Staphylococcus aureus protein A (29) are all LPXTG-like proteins. Their C-terminal parts are recognized by sortases and covalently attached to the cell surface. The C-terminal domains of the cell wall-binding proteins lactococcal autolysin AcmA (5, 22) and the recently discovered bacteriophage endolysin Lyb5 (15) contain LysM repeats which can bind noncovalently to peptidoglycan.
Two-dimensional gel electrophoresis has been widely applied in proteomic studies for protein separation on the basis of differences in isoelectric point and molecular weight. Poor solubility of the transmembrane proteins has been the main obstacle in analyzing surface proteome by two-dimensional gel electrophoresis. In order to overcome the limitations of the gel-based approach, several proteomic strategies have been devised to reveal the bacterial surface proteome (7). A novel strategy uses protease treatment of whole bacterial cells or “bacterial shaving” (26). Bacterial cells are removed, and the cleaved peptides are separated and identified by mass spectrometry. This technique is particularly suitable for Gram-positive bacteria owing to the thicker cell wall, which prevents cell lysis and the leakage of cytoplasmic proteins. This approach can yield reliable information on proteins or protein regions that are actually surface exposed. This is of special importance in the discovery of virulence factors of pathogenic bacteria and in understanding the mechanisms of interaction of bacteria with their environment. The technique has been applied in searching for candidate antigens for vaccines against pathogenic bacteria (12, 26, 27). It has also led to identification of the surface proteome of the model Gram-positive bacterium Bacillus subtilis (30). A recent improvement of surface shaving involves the introduction of a false-positive strategy, in which control cells are treated without protease. Proteins identified in this manner are considered false-positive hits (28).
We have applied bacterial shaving to identify candidate carrier proteins, which could serve as a novel tool for surface display in the LAB L. lactis NZ9000.
MATERIALS AND METHODS
Digestion of surface proteins of L. lactis NZ9000.
Surface proteins were digested in a manner similar to that reported by Rodriguez-Ortega et al. (26). L. lactis NZ9000 (9, 21) was grown in 200 ml of M17 medium supplemented with 0.5% glucose (GM17) to an optical density at 600 nm (OD600) of 0.40. The bacteria were centrifuged at 3,500 × g for 10 min at 4°C, and the pellet was washed with phosphate-buffered saline (PBS) four times. The cells were resuspended in 800 μl of PBS containing 40% sucrose and 5 mM dithiothreitol (DTT), and digested with 10 μg of trypsin (Promega) for 30 min at 37°C. The cells were pelleted as before, and the supernatant was filtered (0.22-μm-pore-size filter). The protease reaction was stopped by adding 0.1% formic acid, and the mixture was desalted by using Vivapure C18 microspin columns (Sartorius) according to the manufacturer's instructions.
High-pressure liquid chromatography-chip mass spectrometry (HPLC-Chip/MS) analysis.
Peptide samples were loaded on a 1200 Series HPLC System coupled to an LC/MSD Trap XCT Ultra mass spectrometer (both from Agilent Technologies). Peptides were separated on a protein identification chip (Agilent Technologies) incorporating a 40-nl enrichment column and a 75-μm-by-43-mm analytical column, both packed with Zorbax 300 SB C18. The mobile phase was 0.1% formic acid and acetonitrile. Peptides were eluted by using a 42 min gradient from 3 to 90% acetonitrile. The data were analyzed by using Spectrum Mill software (Agilent Technologies), and identified peptides were searched against the NCBInr database.
Surface-associated proteins of the entire proteome of L. lactis MG1363 (parent strain of NZ9000 strain) were predicted with Augur (3) (http://bioinfo.mikrobio.med.uni-giessen.de/augur/) and LocateP (37) (http://www.cmbi.ru.nl/locatep-db/cgi-bin/locatepdb.py). Transmembrane helices were predicted with TMHMM Server v.2.0 (http://www.cbs.dtu.dk/services/TMHMM/) and lipoprotein processing with LipPred (http://www.darrenflower.info/LipPred/). Proteins with transmembrane domains, lipoproteins, and LPXTG proteins were visualized schematically with TOPO2 (http://www.sacs.ucsf.edu/TOPO-run/wtopo.pl).
Cloning of candidate genes.
Genomic DNA of L. Lactis NZ9000 was isolated with Wizard Genomic DNA purification kit (Promega), applying the protocol for Gram-positive bacteria and using mutanolysin (Sigma-Aldrich) instead of lysostaphin. Genomic DNA was used as a template in PCRs, using combinations of primers listed in Table S1 in the supplemental material. PCR was performed with Dream Taq DNA polymerase (Fermentas) under the following conditions: 5 min at 94°C; followed by 30 cycles of 30 s at 94°C, 1 min at 46°C, and 1 min at 72°C; with a final 5 min at 72°C. Seven PCR products were purified and ligated to pGEM-T Easy (Promega), yielding pGEM::llmg_0551, pGEM::llmg_2020, pGEM::llmg_2232, pGEM::llmg_2330, pGEM::llmg_0168, pGEM::llmg_1907, and pGEM::llmg_1064. PCR, with pNZ::HAV as a template and Hav-F-NcoI and Hav-R-6His primers, yielded a hav gene with downstream hexahistidine coding sequence, surrounded by NcoI and XbaI restriction sites and containing a BamHI site between the hav gene and the hexahistidine sequence. The product was ligated to pGEM-T Easy and cloned to pNZ8148 via NcoI/XbaI restriction sites, yielding pNZ::HAV-his6. This plasmid was used as a vector for seven candidate lactococcal genes, which were inserted via NcoI/BamHI sites, whereby the antigen gene was removed from the plasmid. This yielded pNZ::llmg_0551-his6, pNZ::llmg_2020-his6, pNZ::llmg_2232-his6, pNZ::llmg_2330-his6, pNZ::llmg_0168-his6, pNZ::llmg_1907-his6, and pNZ::llmg_1064-his6. The B-domain gene was PCR amplified with Bdom-F-BamHI and Bdom-R-XbaI primers using pSDLBA3b as a template. It was ligated to pGEM-T Easy and further cloned to pNZ::llmg_1064-his6 via BamHI and XbaI sites, thereby replacing the hexahistidine tag sequence and yielding pNZ::llmg_1064-B.
Plasmid DNA was isolated by using Wizard Plus Minipreps DNA purification system (Promega, Madison, WI). An additional lysozyme treatment step was used before alkaline lysis when isolating DNA from lactococcal cells. Lactococci were transformed with electroporation according to the method of Holo and Nes (14), using a Gene Pulser II apparatus (Bio-Rad). Restriction enzymes were from New England BioLabs. All plasmids were sequenced at Eurofins MWG Operon (Germany).
Expression of candidate genes in L. lactis NZ9000.
L. lactis NZ9000 was transformed with plasmids containing candidate carrier genes fused to hexahistidine coding sequence (pNZ::llmg_0551-his6, pNZ::llmg_2020-his6, pNZ::llmg_2232- his6, pNZ::llmg_2330-his6, pNZ::llmg_0168-his6, pNZ::llmg_1907-his6, and pNZ::llmg_1064-his6). Overnight cultures were diluted (1:100) in 10 ml of fresh GM-17 medium and grown to A600 of 0.8. Expression was induced with 25 ng of nisin (Fluka)/ml. At 3 h after induction, the culture was split in two. Then, 5 ml was centrifuged at 5,000 × g for 10 min. The cell pellet was resuspended in 0.1 M potassium phosphate buffer (pH 7.00) and frozen for SDS-PAGE and Western blot analysis. The remaining 5 ml of cell culture was stored at 4°C for flow cytometry.
SDS-PAGE and Western blotting.
SDS-PAGE was performed according to the method of Laemmli (18), using a Mini-Protean II apparatus (Bio-Rad). Page Ruler Plus Prestained standard (Fermentas) was used for molecular weight comparison. Samples were thawed on ice, briefly sonicated, and denatured by heating at 100°C in the presence of DTT before loading. Proteins were stained with Coomassie brilliant blue or transferred to polyvinylidene fluoride (PVDF) membrane (Immobilon-P; Millipore) by Western blotting (Mini-Protean II blotting module; Bio-Rad). The membrane was blocked in 1% Western blocking solution (Roche) and incubated overnight at 4°C with fluorescein isothiocyanate (FITC)-conjugated rabbit polyclonal antibody to His6 tag (Abcam catalog no. ab1206; 1:2,000 dilution in 0.5% Western blocking solution). The membrane was washed with 0.05% TBST (50 mM Tris-HCl, 150 mM NaCl, 0.05% Tween 20 [pH 7.5]), and fluorescence was detected with a Typhoon 9410 (GE Healthcare) imager with blue excitation (488 nm).
Washing and staining of cells and flow cytometry.
Portions (10 μl) of cell cultures (2 × 109 cells/ml) were added to 500 μl of TBS (50 mM Tris-HCl, 150 mM NaCl [pH 7.5]) and centrifuged for 3 min at 5,000 × g at 4°C. Pellets were resuspended in 300 μl of TBS and 3 μg of specific, FITC-conjugated rabbit polyclonal antibody to His6 tag (Abcam) (or Alexa Fluor 488-conjugated rabbit anti-mouse antibody for B domain) was added to the suspensions, followed by incubation for 2 h at room temperature with constant shaking at 100 rpm. The cells were then washed with 200 μl of 0.1% TBST and resuspended in 300 μl of TBS. The stained cells were analyzed by flow cytometry (FACSCalibur; Becton Dickinson, Inc.) using 488-nm excitation and 530-nm emission in the FL1 channel. Cells were gated using forward scatter (FSC) versus side scatter (SSC) to isolate the bacterial cells. The FSC and SSC voltage detectors were set (log mode) to E1 and 310, respectively. The mean fluorescence intensity (MFI) of at least 100,000 bacterial cells was measured. The average of three independent experiments, each with two repeated measurements, was considered.
Additional washing was used before the cell staining step to compare the surface binding intensity of B domain fused to AcmA protein (encoded by plasmid pSDLBA3b) and B domain fused to BmpA protein (encoded by plasmid pNZ::llmg_1064-B). The same amounts of cells as for staining were resuspended in 500 μl of TBS+ (50 mM Tris-HCl, 1 M NaCl, 1% SDS [pH 7.5]) and centrifuged for 3 min at 5,000 × g at 4°C. Washing with TBS+ was repeated twice and followed by a TBS wash. The cells were stained as described above using Alexa Fluor 488-conjugated rabbit anti-mouse antibody (Invitrogen).
RESULTS
Prediction and analysis of the surface proteome of L. lactis NZ9000.
Two different web servers, Augur and LocateP, were used to predict the secreted and surface-bound proteins of L. lactis MG1363 (the parent strain of L. lactis NZ9000). The servers differ in their abilities to predict distinct types of surface display and secretion. Both predict lipoproteins, LPXTG-like proteins, and transmembrane proteins. Augur can also predict noncovalent binding motifs such as glycine-tryptophan (GW) anchor, leucine-rich repeats, peptidoglycan binding LysM repeats, and the NLPC/P60 domain. Augur can also recognize proteins with a signal peptide for trafficking to the cell membrane. However, in addition to secreted proteins this category often includes lipoproteins, LPXTG-like proteins, and proteins with a single transmembrane domain, all of which contain a similar signal peptide. Augur can therefore classify a single protein into different classes of surface display and secretion. The sum of classified proteins is therefore larger than the number of distinct proteins classified as surface displayed or secreted. LocateP, on the other hand, classifies a single protein into a single class. It predicts secreted proteins separately. It also differentiates between multiple transmembrane domain proteins and proteins with a single N- or C-terminal transmembrane domain used as anchor. The complete Augur and LocateP predictions are given in Table S2 in the supplemental material.
The quantitative results of the prediction are summarized in Table 1 . The predictions of Augur and LocateP match well and overlap to a large extent. Of a total of 2,434 predicted lactococcal proteins in the genome of L. lactis MG1363, 666 different proteins were predicted for secretion and/or surface binding by Augur and 648 by LocateP. Of these, 628 proteins were predicted by both servers. The most abundant are proteins with transmembrane domains (582 with Augur and 556 altogether with LocateP). Both servers identified similar numbers of lipoproteins and of proteins with a sortase signal. LocateP predicted 48 secreted proteins, which is less than a quarter of those containing a signal peptide predicted by Augur. LocateP also predicted 123 proteins with a single transmembrane domain or an N-terminal anchor (none with a C-terminal anchor), which represents 19% of all surface or secreted proteins. This could not be deduced from the Augur prediction.
TABLE 1.
Quantitative summary of proteins predicted to be on the bacterial surface by Augur and LocateP software and identified by bacterial shavinga
| Type of surface displayb | No. of proteins predicted by: |
|||
|---|---|---|---|---|
| Augur | Augur and identified | LocateP | LocateP and identified | |
| All types | 666 | 80 | 648 | 80 |
| Lipoprotein | 40 | 11 | 35 | 8 |
| GW | 4 | 0 | NA | NA |
| LPXTG | 11 | 1 | 9 | 1 |
| LRR | 18 | 0 | NA | NA |
| LysM | 5 | 0 | NA | NA |
| NLPC/P60 | 2 | 0 | NA | NA |
| SP | 214 | 28 | 48 | 7 |
| TMH | 582 | 31 | 556 | 30 |
| N-anchor | NA | NA | 123 | 20 |
| C-anchor | NA | NA | 0 | 0 |
| Multi-TMH | NA | NA | 433 | 10 |
| Not on the surface | 1,768 | 35 | 1,786 | 34 |
In Augur predictions, a single protein can be attributed to several types of surface display; in LocateP predictions a single protein is attributed to a single type of surface display. However, in the total number of Augur surface-predicted proteins, only distinct proteins were considered. The number of proteins containing transmembrane helices in LocateP predictions was calculated as the sum of proteins with a N-terminal anchor, a C-terminal anchor, and multiple transmembrane helices. NA, not applicable.
GW, Gly-Trp anchor; LPXTG, sortase signal; LRR, leucine-rich repeat; LysM, peptidoglycan binding domain; NLPC/P60, NLPC/P60 domain at the C terminus; SP, secretory signal; TMH, transmembrane helices; N-anchor, single transmembrane helix on N terminus; C-anchor, single transmembrane helix on C terminus; Multi-TMH, multiple transmembrane helices.
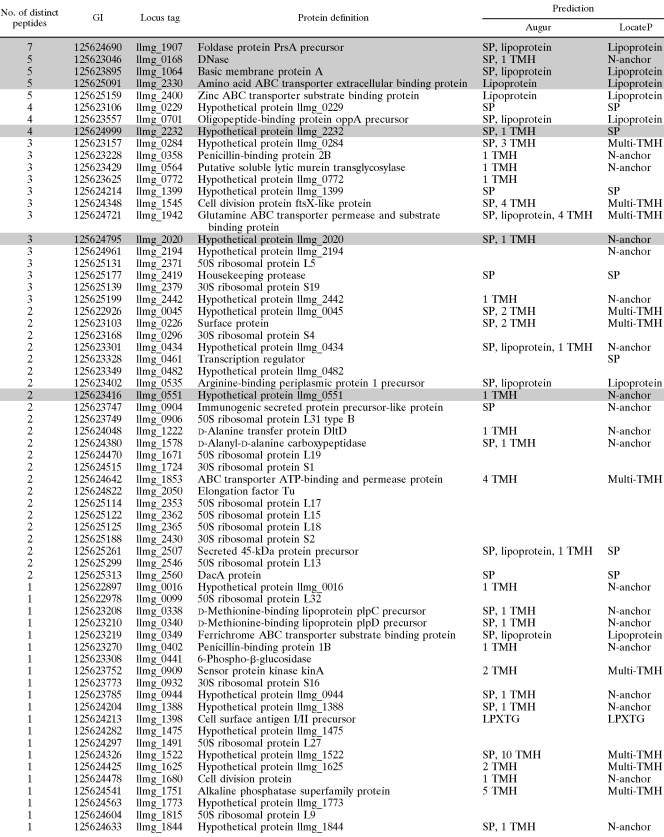
Approximately 13% of the predicted proteins were identified by using mass spectrometry of trypsin shaved peptides. A total of 80 different proteins were identified, 44 unambiguously with at least two peptides and 36 with only one peptide. The identified proteins are listed in Table 2 according to the number of distinct peptides and locus tag number. The majority of the identified proteins with known or predicted function are expected at the bacterial surface, being transmembrane proteins, proteins with signal peptide, and lipoproteins. Only one LPXTG-like protein was identified (cell surface antigen I/II precursor). No proteins with GW anchor, leucine-rich repeats, peptidoglycan binding LysM repeats, or NLPC/P60 domains were identified. The largest class of identified proteins comprises 18 proteins with an N-terminal single transmembrane domain anchor, as predicted by LocateP. A total of 35 identified proteins were not predicted by Augur, and 34 were not predicted by LocateP. Of these, 26 are ribosomal proteins (Table 2).
TABLE 2.
Proteins identified on the surface of L. lactis NZ9000 by bacterial shaving and mass spectrometrya
Proteins are arranged according to the number of different peptides identified for each protein and the locus tag number. The Augur and LocateP predictions are indicated. Abbreviations are as defined for Table 1. The number of Augur-predicted transmembrane helices is also given. Proteins highlighted in gray were selected for further optimization.
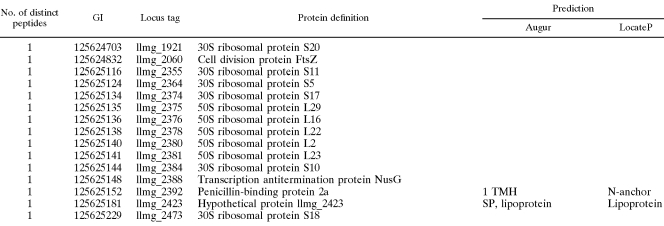
Proteins with transmembrane helices, lipoproteins, and LPXTG-like proteins were modeled and visualized (Fig. 1). Peptides identified by mass spectrometry are shown on the proteins. With a few exceptions (e.g., llmg_0909, llmg_1625, and llmg_1680), they are located on the predicted extracellular parts of the proteins.
FIG. 1.
Schematic representation of identified surface proteins that were predicted to be lipoproteins or to contain either transmembrane regions or the LPXTG sortase motif. The two horizontal lines represent the membrane. Proteins are listed as in Table 2. The upper part of each protein is extracellular and the lower part cytoplasmic. Identified peptides are highlighted in orange. Proteins that were selected for cloning are encircled.
Selection and cloning of candidate carrier proteins for surface display.
Seven proteins were selected from those identified on the surface of L. lactis cells and are highlighted in Table 2. The selection criteria were as follows: (i) the C-terminal part of the protein predicted to be outside the cell, (ii) the protein identified with several peptides, (iii) the C-terminal peptide identified, and (iv) size of the protein encoding gene not exceeding 1,000 bp because of cloning feasibility. Selected proteins were either lipoproteins or single transmembrane helix proteins, in which the transmembrane helix may represent a secretion signal. Protein-encoding genes were amplified from the genome and cloned in frame with a downstream hexahistidine tag encoding sequence. Genes encoding proteins with a C-terminal hexahistidine tag label were under the control of the inducible nisin promoter of pNZ8148 plasmid (9, 21). The constructed plasmids are described in Table S1 in the supplemental material.
Expression of candidate proteins.
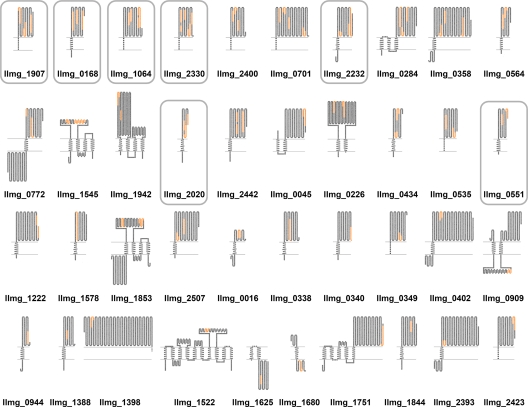
Candidate proteins were overexpressed using nisin-controlled expression under the conditions determined previously, as appropriate for recombinant expression (25). Proteins were overexpressed, as seen from SDS-PAGE gels of cell lysates (Fig. 2A). Proteins llmg_0551, llmg_2330, llmg_1907, and llmg_1064 were expressed at higher yields than llmg_2020, llmg_2232, and llmg_0168. The molecular masses of overexpressed hexahistidine-labeled proteins correspond to those calculated: 23.3 kDa for llmg_0551, 17.3 kDa for llmg_2020, 36.9 kDa for llmg_2232, 32.1 kDa for llmg_2330, 29.6 kDa for llmg_0168, 34.7 kDa for llmg_1907, and 37.9 kDa for llmg_1064. Overexpression of candidate proteins and the presence of hexahistidine tag were confirmed by Western blotting with a specific anti-hexahistidine tag antibody (Fig. 2B). No overexpression of hexahistidine-labeled protein was detected with treated bacteria containing empty plasmid pNZ8148 (control; Fig. 2).
FIG. 2.
SDS-PAGE (A) and Western blot (B) analyses of L. lactis NZ9000 cells expressing candidate carrier proteins. llmg_0551, llmg_2020, and llmg_2232 are putative proteins; llmg_2330 is amino acid ABC transporter extracellular binding protein; llmg_0168 is DNase; llmg_1907 is foldase protein PrsA precursor; and llmg_1064 is BmpA. Arrows denote the positions of overexpressed proteins.
Quantification of the candidate proteins on the bacterial surface.
Candidate proteins labeled with hexahistidine tag were overexpressed in L. lactis NZ9000. Equally treated bacteria containing empty plasmid pNZ8148 were again used as a control. The MFIs of all candidate proteins were significantly higher than that of the control (Fig. 3). llmg_1064 (basic membrane protein A [BmpA]) had the highest fluorescence of all of the candidate proteins (almost 2-fold higher than the others and almost 3-fold higher than the control, when average values are compared). BmpA was therefore chosen for further optimization.
FIG. 3.
Flow cytometric analyses of L. lactis NZ9000 cells expressing candidate carrier proteins, which were detected with FITC-conjugated antibody against hexahistidine tag. llmg_0551, llmg_2020, and llmg_2232 are putative proteins; llmg_2330 is an amino acid ABC transporter extracellular binding protein; llmg_0168 is DNase; llmg_1907 is foldase protein PrsA precursor; and llmg_1064 is BmpA. Cells expressing candidate proteins have a significantly higher MFI than the control (pNZ8148), with P < 0.05 (*) or P < 0.005 (**). Vertical lines represent the standard deviations.
Application of BmpA for surface display.
To test the feasibility of BmpA (llmg_1064) as a carrier protein, we replaced the hexahistidine tag with B domain of staphylococcal protein A as a C-terminal fusion. The B domain was detected on the bacterial surface with fluorescently labeled rabbit antibody, which was bound via its Fc region. BmpA-B domain fusion (plasmid pNZ::llmg_1064-B) was compared to our system for surface display (25) consisting of Usp45-B domain-AcmA fusion (plasmid pSDLBA3b). The MFI of lactococcal cells expressing BmpA-B domain fusion was considerably higher than that of the control which contained empty plasmid. On the other hand, it was lower than MFI of cells expressing the Usp45-B domain-AcmA fusion (Fig. 4).
FIG. 4.
Flow cytometric analyses of L. lactis NZ9000 cells expressing the fusion of B domain of staphylococcal protein A with AcmA peptidoglycan-binding domain (pSDLBA3b), the fusion of B domain with BmpA (pNZ::llmg_1064-B), and control cells (pNZ8148). The MFI is shown. Cells were stained with Alexa Fluor 488-conjugated rabbit anti-mouse antibody which bound to the B domain. Before staining with antibody, cells were washed with TBS (dark gray) or TBS+ (TBS containing 1 M NaCl and 1% SDS; light gray) as described in Materials and Methods. Vertical lines represent the standard deviations.
BmpA is a putative lipoprotein that binds to the cell wall covalently, in contrast to Usp45-B domain-AcmA fusion where binding is noncovalent, via LysM repeats. To compare the strength of the binding of the B domain to the surfaces of lactococci with the two systems, we applied stringent washing (buffer with high ionic strength and containing detergent). A decrease in MFI was observed when a Usp45-B domain-AcmA fusion was used, whereas the MFI of the BmpA-B domain fusion increased (Fig. 4).
DISCUSSION
L. lactis is a biotechnologically important organism due to its safety and feasibility for human oral application. Its surface proteome can be analyzed by the “bacterial shaving” approach since it is a Gram-positive bacterium. L. lactis MG1363 is one of the predominant laboratory strains, and its genome has been sequenced. We have used its derivative L. lactis NZ9000, which has nisRK genes inserted into the pepN gene and therefore differs only by the presence of three genes.
The surface proteome of L. lactis was explored by using two software predictive packages, Augur and LocateP. These are based on finding characteristic sequences that could be involved in surface trafficking or binding, with certain evident limitations. Augur does not unambiguously classify a single protein into a single class of surface display but returns all possible classifications, which may be mutually exclusive. Due to the similarity of signal peptides, Augur can classify a single protein as secreted, lipoprotein, or protein with a single transmembrane domain at the same time, as in the example of Usp45 protein (llmg_2507), which has been shown experimentally to be a major lactococcal secreted protein (31). Augur and LocateP differ in the types of surface display they can predict. Nevertheless, the results of the two predictors match relatively well. They both predict approximately one-quarter of the genome to be surface associated. Within that group, they predict from 550 to 580 transmembrane proteins, 35 to 40 lipoproteins, and ca. 10 LPXTG-like proteins. There is a growing awareness that there are mechanisms of trafficking and binding of proteins to the surface additional to those made use of by Augur and LocateP. These mechanisms apparently do not include characteristic signal sequences, and the proteins involved are termed anchorless surface proteins (6).
We have identified experimentally 80 surface proteins by bacterial shaving and mass spectrometry. This low number can be attributed to technical limitations, such as a protein resistance to trypsin cleavage, hindered access to trypsin, low abundance of proteins, or partial peptide separation. Of the 80 proteins, 36 were identified on the basis of only one peptide and are therefore less reliable. The number of identified proteins is very similar to that reported for group A Streptococcus (26). The majority were also predicted by Augur and LocateP. However, considerable numbers of these proteins were not predicted by Augur or by LocateP (35 and 34, respectively). The majority (26) of these putative (on the basis of software prediction) cytoplasmic proteins are ribosomal proteins, which constitute half of the 53 ribosomal proteins present in L. lactis MG1363 (33). Surface exposure of ribosomal proteins has been reported by other surface-shaving studies (27, 30), but not to such an extent. In the study of Tjalsma et al. (30), 13 ribosomal proteins were classified as surface proteins. The mechanism of surface display and the role of displayed ribosomal proteins remain subjects of speculation. However, there is a growing consensus in the literature that the absence of other cytoplasmic proteins indicates that the identification of ribosomal proteins is not the result of cell lysis. The surface exposure of elongation factor Tu was also reported previously (13) and could imply the translocation of the entire ribosomal machinery to the surface. Some ribosomal proteins and elongation factor Tu were together recognized as anchorless surface proteins (2). Other cytoplasmic proteins thus identified include three predicted proteins (llmg_0482, llmg_1475, and llmg_1773), cell division protein FtsZ, transcription anti-termination protein NusG, and 6-phospho-β-glucosidase, the latter being the only carbohydrate metabolism enzyme. This again suggests the absence of cell lysis during bacterial shaving. When the identified surface lactococcal proteins were compared to those of Streptococcus pyogenes (26, 27) or Bacillus subtilis (30), a few common features were found, as expected. Several ABC transporter proteins, which deal with the transport of metal ions, amino acids, or phosphate, were identified in all organisms. Penicillin-binding proteins, which are involved in peptidoglycan synthesis, were also abundant, and at least one protease was identified at the surface. A methodologically different study of lactococcal insoluble proteome (1) yielded 89 predicted surface and/or secreted proteins. Of these, several were lipoproteins, which function mainly as ABC transporter subunits. Transporter proteins were also the most abundant among the membrane proteins. Cell division proteins, sensor kinase proteins, and penicillin-binding proteins were also identified, as in our results. The study, however, encompasses all of the insoluble proteins, which can also be attached to the interior side of the membrane or the cell wall. Surface or secreted proteins are therefore not identified unambiguously. The observation of numerous ribosomal proteins is nevertheless interesting and in accordance with our observations.
Transmembrane proteins, lipoproteins, and LPXTG-like proteins were modeled and presented (Fig. 1) to show the extra- and intracellular protein regions which could be important in the design of a carrier protein for surface display. The identified peptides are highlighted on the graphical model and are, as expected, mainly located extracellularly. The exceptions are proteins llmg_0909 and llmg_1680, where an error in modeling is likely, and llmg_1625, which is a predicted protein. Sensor protein kinase (llmg_0909) contains a kinase region on the C-terminal which should therefore be located intracellularly and suggests wrong prediction of the protein's N-terminal location. Cell division protein (llmg_1680) is described as FtsL protein in L. lactis SK11 strain, and its E. coli homologue has its N terminus in the cytoplasm and its C terminus in the periplasm, which again suggests wrong prediction of the N-terminal location.
Several secreted proteins were also detected on the protein surface, which seems unusual and would be expected to have been removed during the intensive washing prior to trypsin cleavage. The prominent example is Usp45 protein (llmg_2507), which was confirmed experimentally as the most abundant secreted protein in L. lactis (31). Secreted proteins might bind to the surface in an alternative, as-yet-uncharacterized fashion, with an equilibrium between the bound and unbound states. The other possibility is that the secretion machinery fails to process signal peptides completely, so that the secreted protein remains trapped on the surface.
In surface display, carrier proteins exploit cellular machinery to deliver the protein of interest, termed the passenger protein, to the bacterial surface. We have tested seven of the unambiguously identified proteins for their potential to function as carrier proteins in surface display. We used hexahistidine peptide as a model passenger in surface display, since it is a well-established immunogenic tag. Hexahistidine peptide was detected by a specific antibody, which also binds to the cells in an unspecific manner and accounts for the fluorescence detected with control cells. In addition, the detection of hexahistidine peptide can depend on steric hindrance and does not necessarily reflect the amount of the protein. However, it does correlate with the degree of hexahistidine peptide exposure, which is relevant in surface display. The selection of proteins followed the criteria described in Materials and Methods. The number of different peptides identified by mass spectrometry was an important criterion, since it was reported as a relative measure of protein abundance (30). However, this is probably only partially true, because a greater number of identified peptides could correlate with greater exposure of the protein or its susceptibility to trypsin cleavage. Protein exposure on the bacterial surface depends on the distance from the surface and steric hindrance. Greater protein exposure is beneficial for the surface display, since it enables better contact with the molecules from the environment.
All seven proteins were detected in L. lactis NZ9000 upon induction with nisin. Their estimated molecular weights correspond well to the predicted values. The selected proteins were expressed with different yields under the same expression and induction conditions. The total yield of the protein is important for the amount of protein on the surface. It is, however, not the only measure, since the amount on the surface also depends on the mechanism that traffics the protein to the surface. The growth of lactococci that overexpressed the selected proteins did not appear to be affected.
The amounts of the selected proteins on the surface were estimated by flow cytometry, where surface-displayed proteins were detected by using the hexahistidine tag. All selected proteins exhibited significantly higher fluorescence than the control that lacked the hexahistidine tag. Protein llmg_1064 (BmpA) exhibited the highest ability for surface display and was also expressed at high yield. We therefore propose it as a potential carrier protein in surface display in lactococci. BmpA appears never to have been applied in such a way in any bacterial species. The function of BmpA in L. lactis was recently determined to be nucleoside transport (19). It is an extracellular substrate-binding domain of an ABC transporter, which it forms together with NupABC proteins (19). The BmpA homologue from Borrelia burgdorferi is, however, recognized as a surface antigen and is important for infection (32).
To further evaluate BmpA as a carrier, we replaced the hexahistidine peptide with the B domain of staphylococcal protein A as a passenger protein. B domain binds antibodies in a nonspecific manner via their Fc region, and this property can be used for the detection of B domain. We have previously used B domain as a model protein for surface display, in a fusion with Usp45 secretion signal and C-terminal part of AcmA protein, which contains LysM repeats (25). We have therefore compared the amount of B domain delivered to the surface with either Usp45 secretion signal/AcmA fusion or with BmpA as a carrier protein. B domain delivered to the surface of L. lactis with BmpA as a carrier was functional and capable of binding antibody via the Fc region. The amount of B domain delivered with Usp45 secretion signal/AcmA fusion was 4- to 5-fold higher than with BmpA. This is in accordance with our expectations, since the BmpA-based surface display was not optimized in any way and offers room for further studies.
For some practical applications of surface display, the strength of binding of a displayed protein to the surface is important. Covalent binding, such as with lipoproteins or LPXTG-like proteins, would be advantageous over noncovalent binding, such as with peptidoglycan-binding LysM repeats. To compare the strength of binding of lipoprotein BmpA to that of LysM repeats of AcmA, we used additional washing of lactococcal cells with detergent and high-ionic-strength buffer. This decreased noncovalent, LysM-mediated binding by ca. 50%. Surprisingly, the BmpA-mediated covalent binding did not remain on the same level but appeared to be stronger under these conditions. This result could be explained by the removal of some surface components during rigorous washing, which can otherwise cause steric hindrance of the displayed protein.
To summarize, based on the study of the surface proteome of L. lactis NZ9000, BmpA emerges as a potential carrier protein for surface display in lactococci. Its applicability was demonstrated by the successful display of the B domain of staphylococcal protein A.
Supplementary Material
Acknowledgments
This study was supported by Slovenian Research Agency grant P4-0127.
We are grateful to Marko Fonović and Andrijana Leonardi for performing mass spectrometry analysis and to Roger Pain for critical reading of the manuscript.
Footnotes
Published ahead of print on 23 December 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Beganovic, J., et al. 2010. Characterization of the insoluble proteome of Lactococcus lactis by SDS-PAGE LC-MS/MS leads to the identification of new markers of adaptation of the bacteria to the mouse digestive tract. J. Proteome Res. 9:677-688. [DOI] [PubMed] [Google Scholar]
- 2.Bendtsen, J. D., L. Kiemer, A. Fausboll, and S. Brunak. 2005. Non-classical protein secretion in bacteria. BMC Microbiol. 5:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Billion, A., R. Ghai, T. Chakraborty, and T. Hain. 2006. Augur: a computational pipeline for whole genome microbial surface protein prediction and classification. Bioinformatics 22:2819-2820. [DOI] [PubMed] [Google Scholar]
- 4.Bolotin, A., et al. 2001. The complete genome sequence of the lactic acid bacterium Lactococcus lactis subsp. lactis IL1403. Genome Res. 11:731-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosma, T., et al. 2006. Novel surface display system for proteins on non-genetically modified gram-positive bacteria. Appl. Environ. Microbiol. 72:880-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chhatwal, G. S. 2002. Anchorless adhesins and invasins of Gram-positive bacteria: a new class of virulence factors. Trends Microbiol. 10:205-208. [DOI] [PubMed] [Google Scholar]
- 7.Cordwell, S. J. 2006. Technologies for bacterial surface proteomics. Curr. Opin. Microbiol. 9:320-329. [DOI] [PubMed] [Google Scholar]
- 8.Daugherty, P. S. 2007. Protein engineering with bacterial display. Curr. Opin. Struct. Biol. 17:474-480. [DOI] [PubMed] [Google Scholar]
- 9.de Ruyter, P. G., O. P. Kuipers, and W. M. de Vos. 1996. Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl. Environ. Microbiol. 62:3662-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desvaux, M., E. Dumas, I. Chafsey, and M. Hebraud. 2006. Protein cell surface display in Gram-positive bacteria: from single protein to macromolecular protein structure. FEMS Microbiol. Lett. 256:1-15. [DOI] [PubMed] [Google Scholar]
- 11.de Vrese, M., and J. Schrezenmeir. 2008. Probiotics, prebiotics, and synbiotics. Adv. Biochem. Eng. Biotechnol. 111:1-66. [DOI] [PubMed] [Google Scholar]
- 12.Doro, F., et al. 2009. Surfome analysis as a fast track to vaccine discovery: identification of a novel protective antigen for group B Streptococcus hypervirulent strain COH1. Mol. Cell Proteomics 8:1728-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granato, D., et al. 2004. Cell surface-associated elongation factor Tu mediates the attachment of Lactobacillus johnsonii NCC533 (La1) to human intestinal cells and mucins. Infect. Immun. 72:2160-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holo, H., and I. F. Nes. 1995. Transformation of Lactococcus by electroporation, p. 195-199. In J. A. Nickoloff (ed.), Electroporation protocols for microorganisms, vol. 47. Humana Press, Totowa, NJ. [DOI] [PubMed] [Google Scholar]
- 15.Hu, S., J. Kong, W. Kong, T. Guo, and M. Ji. 2010. Characterization of a novel LysM domain from Lactobacillus fermentum bacteriophage endolysin and its use as an anchor to display heterologous proteins on the surfaces of lactic acid bacteria. Appl. Environ. Microbiol. 76:2410-2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jose, J., and T. F. Meyer. 2007. The autodisplay story, from discovery to biotechnical and biomedical applications. Microbiol. Mol. Biol. Rev. 71:600-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim, T. W., S. Igimi, A. Kajikawa, and H. Y. Kim. 2008. Display of heterologous proteins on the surface of Lactococcus lactis using the H and W domain of PrtB from Lactobacillus delbrueckii subsp. bulgaricus as an anchoring matrix. J. Appl. Microbiol. 104:1636-1643. [DOI] [PubMed] [Google Scholar]
- 18.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 19.Martinussen, J., C. Sorensen, C. B. Jendresen, and M. Kilstrup. Two nucleoside transporters in Lactococcus lactis with different substrate specificities. Microbiology 156:3148-3157. [DOI] [PubMed]
- 20.Mercenier, A., H. Muller-Alouf, and C. Grangette. 2000. Lactic acid bacteria as live vaccines. Curr. Issues Mol. Biol. 2:17-25. [PubMed] [Google Scholar]
- 21.Mierau, I., and M. Kleerebezem. 2005. 10 years of the nisin-controlled gene expression system (NICE) in Lactococcus lactis. Appl. Microbiol. Biotechnol. 68:705-717. [DOI] [PubMed] [Google Scholar]
- 22.Okano, K., et al. 2008. System using tandem repeats of the cA peptidoglycan-binding domain from Lactococcus lactis for display of both N- and C-terminal fusions on cell surfaces of lactic acid bacteria. Appl. Environ. Microbiol. 74:1117-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piard, J. C., et al. 1997. Cell wall anchoring of the Streptococcus pyogenes M6 protein in various lactic acid bacteria. J. Bacteriol. 179:3068-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramasamy, R., et al. 2006. Immunogenicity of a malaria parasite antigen displayed by Lactococcus lactis in oral immunizations. Vaccine 24:3900-3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ravnikar, M., B. Strukelj, N. Obermajer, M. Lunder, and A. Berlec. 2010. Engineered lactic acid bacterium Lactococcus lactis capable of binding antibodies and tumor necrosis factor alpha. Appl. Environ. Microbiol. 76:6928-6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez-Ortega, M. J., et al. 2006. Characterization and identification of vaccine candidate proteins through analysis of the group A Streptococcus surface proteome. Nat. Biotechnol. 24:191-197. [DOI] [PubMed] [Google Scholar]
- 27.Severin, A., et al. 2007. Proteomic analysis and identification of Streptococcus pyogenes surface-associated proteins. J. Bacteriol. 189:1514-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solis, N., M. R. Larsen, and S. J. Cordwell. 2010. Improved accuracy of cell surface shaving proteomics in Staphylococcus aureus using a false-positive control. Proteomics 10:2037-2049. [DOI] [PubMed] [Google Scholar]
- 29.Steidler, L., J. Viaene, W. Fiers, and E. Remaut. 1998. Functional display of a heterologous protein on the surface of Lactococcus lactis by means of the cell wall anchor of Staphylococcus aureus protein A. Appl. Environ. Microbiol. 64:342-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tjalsma, H., L. Lambooy, P. W. Hermans, and D. W. Swinkels. 2008. Shedding and shaving: disclosure of proteomic expressions on a bacterial face. Proteomics 8:1415-1428. [DOI] [PubMed] [Google Scholar]
- 31.van Asseldonk, M., et al. 1990. Cloning of usp45, a gene encoding a secreted protein from Lactococcus lactis subsp. lactis MG1363. Gene 95:155-160. [DOI] [PubMed] [Google Scholar]
- 32.Verma, A., C. A. Brissette, A. Bowman, and B. Stevenson. 2009. Borrelia burgdorferi BmpA is a laminin-binding protein. Infect. Immun. 77:4940-4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wegmann, U., et al. 2007. Complete genome sequence of the prototype lactic acid bacterium Lactococcus lactis subsp. cremoris MG1363. J. Bacteriol. 189:3256-3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wells, J. M., and A. Mercenier. 2008. Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria. Nat. Rev. Microbiol. 6:349-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wernerus, H., and S. Stahl. 2004. Biotechnological applications for surface-engineered bacteria. Biotechnol. Appl. Biochem. 40:209-228. [DOI] [PubMed] [Google Scholar]
- 36.Wu, C. H., A. Mulchandani, and W. Chen. 2008. Versatile microbial surface-display for environmental remediation and biofuels production. Trends Microbiol. 16:181-188. [DOI] [PubMed] [Google Scholar]
- 37.Zhou, M., J. Boekhorst, C. Francke, and R. J. Siezen. 2008. LocateP: genome-scale subcellular-location predictor for bacterial proteins. BMC Bioinformatics 9:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.