Abstract
Transcriptional profiles of uropathogenic Escherichia coli CFT073 exposed to cranberry-derived proanthocyanidins (PACs) were determined. Our results indicate that bacteria grown on media supplemented with PACs were iron deprived. To our knowledge, this is the first time that PACs have been shown to induce a state of iron limitation in this bacterium.
Urinary tract infection (UTI) refers to clinical signs and symptoms arising from the presence of microorganisms in the genitourinary tract. Furthermore, a wide range of UTI-related nosocomial pathologies may be caused by microbial contamination of invasive medical devices (15). The principal treatment intervention in patients with acute UTIs is oral therapy with antibiotics. The standard treatment for managing recurrent, uncomplicated UTIs in women is continuous or postcoital prophylaxis with low-dose antimicrobials or intermittent self-treatment with antimicrobials (2, 13, 23). However, the development of bacterial resistance to antibiotics has resulted in rising rates of clinical failure (2, 3, 17), emphasizing the need to develop alternate options for infection prevention and treatment.
For over 100 years, North American cranberries (Vaccinium macrocarpon Ait., family Ericaceae) have been linked to UTI prevention (4). It was initially believed that the acidity of cranberries reduced bacterial growth levels and hence reduced the incidence of UTIs (4). However, subsequent studies revealed that the pH of urine is not significantly altered by the consumption of cranberries (1, 16). Also, cranberry products have been proven to be nonbacteriostatic (27). Recent research suggests that cranberries prevent UTIs by hindering bacterial attachment to surfaces via phytochemicals known as proanthocyanidins (PACs) (5, 10-12, 19, 25). However, a mechanistic understanding of the way in which cranberry extracts alter bacterial adhesion is still lacking. Investigation of the bacterial response to cranberry PACs at the genome level may facilitate the unveiling of these mechanisms and identify the factors that may be involved. The purpose of the work presented here was to perform a genome-wide transcriptional analysis using microarray technology to determine how PACs affect gene expression of the uropathogen Escherichia coli CFT073 (28).
For this study, dry PACs (Marucci Center for Blueberry and Cranberry Research, Rutgers University) were solubilized in distilled, deionized water and sterilized by filtration. The average molecular mass of the PACs used was ∼15 kDa. For the transcriptome analysis, the bacteria were grown in LB broth alone (controls) or in LB broth supplemented with PACs at a final concentration of 100 μg/ml. After 5 h of incubation at 37°C and 200 rpm, RNA was extracted by following the standard protocol with an RNeasy minikit (Qiagen). Figure 1 shows optical density versus time for the bacteria grown in the presence or absence of PACs and confirms that PACs, under these conditions, are not inhibitory of growth. Additionally, no inhibition of growth was observed for bacteria that were grown in the presence of any of the higher concentrations of PACs we tested, including a concentration as high as 1,600 μg/ml (data not included).
FIG. 1.
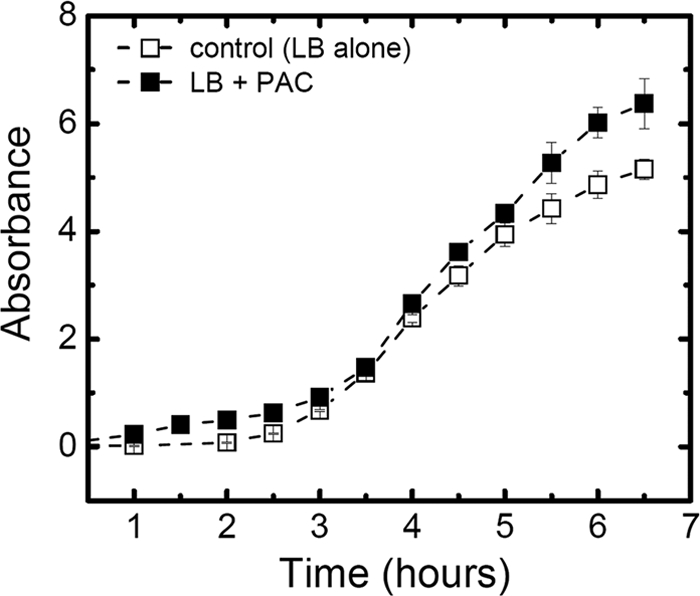
Growth curves of cultures used for transcriptional profiling. E. coli CFT073 was grown in 500-ml flasks containing 150 ml of LB broth at 37°C in the presence (▪) or absence (□) of 100 μg/ml cranberry-derived proanthocyanidins. The culture was harvested for RNA extraction 5 h after seeding.
Affymetrix GeneChip E. coli genome 2.0 arrays were used to run the microarray analysis. Targets were prepared by following the prokaryotic target preparation protocol from Affymetrix (Affymetrix GeneChip expression analysis technical manual). Hybridization and chip analysis were performed by using the Functional Genomics Platform at McGill University and Génome Québec Innovation Centre. Microarray data analysis was done using the FlexArray 1.4.1 software program (http://genomequebec.mcgill.ca/FlexArray/). The data were normalized using the robust multiarray average algorithm (RMA) (14). Comparison between the two data sets was done using the randomized variance model (RVM) with the cutoff being a 2-fold increase or decrease and with a P value of 0.05 (30). The expression level of each of 5,379 open reading frames (ORFs) was determined. Overall, 616 genes were found to be upregulated and 419 were found to be downregulated.
The microarray results revealed that the most upregulated gene was ryhB (13.8-fold increase) (Table 1). This gene encodes a small regulatory RNA that acts as a quick off-switch for a suite of dispensable iron storage and iron-using proteins when the metal becomes limiting, and therefore leaves more iron available for essential proteins (6). RyhB downregulates the expression of the sdhCDAB operon that encodes a succinate dehydrogenase, two genes that encode proteins in the tricarboxylic acid cycle, the acnA and fumA genes, two ferritin genes, the ftnA and bfr genes, and a gene for superoxide dismutase, the sodB gene (21, 26). Consistent with the upregulation of the ryhB gene, we observed the downregulation of the acnA, ftnA, and sodB genes in the culture exposed to PACs (Table 1). Additionally, 33 genes that encode proteins involved in iron uptake and assimilation were upregulated in the presence of PACs (Table 1). These include the ent genes, encoding enterobactin, the iuc genes, encoding aerobactin, and the iutA gene, the aerobactin receptor. Also, the fhu genes, for ferrichrome and rhodotorulic acid transport, the feo genes, for ferric iron transport, the chu genes, required for hemin utilization, and the sit genes, which encode an iron transport system, were upregulated. Furthermore, the tonb and exbD genes that encode proteins that form the TonB-ExbB-ExbD complex, which provides energy for ferrisiderophore transport across the outer membrane, were upregulated. The observed changes in gene expression signify that E. coli adapts to the presence of PACs by reducing iron storage and upregulating iron acquisition systems. Taken together, our results indicate that cranberry PACs limit iron availability.
TABLE 1.
E. coli CFT073 iron metabolism genes up- or downregulated in the presence of cranberry proanthocyanidinsa
| Gene | Gene product and/or function | Fold change | P value (<0.05) |
|---|---|---|---|
| Upregulated genesb | |||
| ryhB | RyhB, a small regulatory RNA involved in downregulation of Fe storage proteins and nonessential Fe proteins when iron is limiting | 13.83 | 1.6 × 10−10 |
| iucA | Aerobactin protein IucA | 2.52 | 1.2 × 10−4 |
| iucB | Aerobactin protein IucB | 2.54 | 3.4 × 10−5 |
| iucC | Aerobactin protein IucC | 3.11 | 8.3 × 10−7 |
| iucD | Aerobactin protein IucD | 5.1 | 3.2 × 10−8 |
| iutA | Outer membrane aerobactin receptor | 3.95 | 3.2 × 10−7 |
| entA | Enterobactin system dehydrogenase | 3.33 | 1.0 × 10−5 |
| entB | EntB, a subunit of apo-EntB | 4.69 | 1.6 × 10−7 |
| entC | Isochorismate synthase EntC, enterobactin system | 3.47 | 4.9 × 10−6 |
| entE | Enterobactin synthase subunit E | 2.39 | 4.1 × 10−4 |
| entF | Enterobactin synthase subunit F, aryl carrier protein | 5.41 | 6.1 × 10−9 |
| entH | Proofreading thioesterase in enterobactin biosynthesis | 4.48 | 4.3 × 10−8 |
| fes | Ferric enterobactin esterase | 3.64 | 7.9 × 10−8 |
| fepA | Outer membrane ferrienterobactin receptor precursor | 2.41 | 1.0 × 10−6 |
| fepB | Iron enterobactin transporter periplasmic binding protein | 2.15 | 1.2 × 10−5 |
| ybdZ | Part of the fes-ybdZ-entF-fepE transcriptional unit, an enterobactin synthesis and transport operon | 5.98 | 7.7 × 10−8 |
| fhuA | Ferrichrome outer membrane transporter | 2.25 | 4.6 × 10−6 |
| fhuE | Ferric rhodotorulic acid outer membrane transporter | 2.65 | 3.9 × 10−6 |
| fhuF | Ferric iron reductase involved in ferric hydroximate transport | 4.29 | 1.6 × 10−7 |
| tonB | Cytoplasmic membrane protein that provides energy for the import of iron-siderophore complexes; part of the TonB-ExbB-ExbD complex | 2.1 | 1.5 × 10−4 |
| exbD | Part of the TonB-ExbB-ExbD complex | 3.2 | 7.4 × 10−8 |
| chuX | ChuX, a ShuX-like protein, required for hemin utilization | 4.68 | 1.9 × 10−7 |
| chuT | ChuT, a putative periplasmic binding protein; may be required for hemin utilization | 3.06 | 5.5 × 10−6 |
| chuW | ChuW, a putative coproporphyrinogen III oxidase; may be required for hemin utilization | 3.01 | 4.2 × 10−6 |
| chuY | Hypothetical protein | 2.84 | 7.0 × 10−6 |
| chuU | Putative hemin permease | 2.52 | 7.5 × 10−7 |
| chuV | Hypothetical protein highly similar to ATPases of iron ABC transporters | 2.01 | 7.7 × 10−6 |
| chuA | ChuA, an outer membrane heme/hemoglobin receptor | 3.88 | 4.5 × 10−6 |
| chuS | ChuS, a putative heme/hemoglobin transport protein; may be required for hemin utilization | 3.76 | 4.0 × 10−6 |
| sitA | Iron transport system SitA protein | 5.23 | 2.4 × 10−6 |
| sitB | SitB, an ATP-binding component of the iron transport system | 3.07 | 1.6 × 10−4 |
| sitC | Inner membrane component of the iron transport system | 2.10 | 9.1 × 10−4 |
| feoA | Ferrous iron transport protein A | 2.98 | 1.8 × 10−7 |
| feoB | Ferrous iron transporter | 2.32 | 2.9 × 10−6 |
| Downregulated genesc | |||
| acnA | Aconitate hydratase 1, contains a [4Fe-4S]2+ cluster | 0.26 | 3.6 × 10−7 |
| ftnA | Ferritin iron storage protein FtnA | 0.24 | 4.2 × 10−7 |
| sodB | Superoxide dismutase | 0.27 | 1.5 × 10−8 |
Shown are E. coli CFT073 iron metabolism genes up- or downregulated in the presence of cranberry proanthocyanidins (100 μg/ml) as revealed by Affymetrix GeneChip E. coli genome 2.0 microarrays. Fold change refers to growth in LB broth supplemented with PACs relative to growth in LB broth alone.
Upregulated genes, genes which are upregulated in the presence of PACs.
Downregulated genes, genes whose expression is regulated by RhyB and which are downregulated in the presence of PACs.
Additional genes of interest that were differentially regulated in the presence of PACs include the fliC gene, encoding the flagellar structural protein, which was downregulated 2.4-fold. On the other hand, the flgL gene, which encodes one of the two flagellar hook-filament junction proteins, FlgL, was upregulated 2.7-fold. The fimA gene, encoding the major subunit of the E. coli type 1 fimbriae, and the genes encoding proteins involved in biofilm formation, the ymgA, ymgB (ariR), and ymgC genes, were downregulated. Curli, chemotaxis, and other fimbrial or flagellar genes were not differentially regulated in the presence of PACs.
Further validation of the biological reproducibility of our array data was obtained by conducting reverse transcription-PCR (RT-PCR) to determine the relative quantities of selected transcripts in the presence and absence of PACs. Four genes were selected for this test, two (the chuX and iucD genes) that were observed to be upregulated during the microarray analysis and two (the ftnA and sodB genes) that were downregulated. Reverse transcription was performed with RNA taken from the same aliquots as those used in the microarray study. Three genes, the arcA, dnaG, and grpE genes, were used as a reference, and calculations were performed using the qBase package (9). The RT-PCR confirmed that in the presence of PACs, there was significant stimulation of the chuX and iucD genes, and the ftnA and sodB genes were downregulated (data not shown).
Additional confirmation of the iron-chelating capability of PACs was obtained by monitoring the formation of complexes between PACs and ferrous and ferric ions. Changes in the UV-visible spectrum were tracked as described by Guo et al. (7). Briefly, small aliquots of 1 mM Fe2+ or Fe3+ solutions were added sequentially to a 10 μM PAC solution and homogenized. Spectra were recorded after each iron addition using an 8453 Hewlett-Packard spectrophotometer (Hewlett-Packard Company, Palo Alto, CA); results are shown in Fig. 2 A. Guo et al. (7) observed a peak near 372 nm, which was assigned to the π-π* transition in the B ring of quercitin, one of the phenolic phytochemicals present in cranberries. Binding of quercitin to Fe2+ or Fe3+ resulted in the disappearance of this band, while a new one appeared at 425 nm. Cranberry-derived PACs are a mixture of oligomers, each of which may exhibit several unique electronic transitions in the region near 372 nm. This can result in the disappearance of a distinctive narrow peak corresponding to a single π-π* transition in the B ring but the appearance of a broader peak that is less sensitive to small amounts of iron cations. The addition of Fe2+ to the PAC solution led to the appearance of new bands at 405 nm and 545 nm, consistent with the formation of PAC-Fe2+ complexes.
FIG. 2.
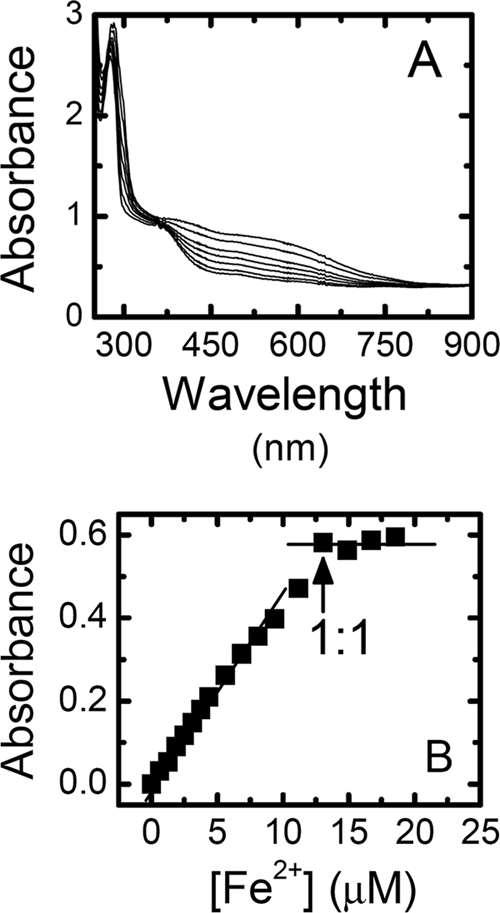
(A) Spectra obtained for the titration of ∼10 μM cranberry-derived proanthocyanidins (PACs) in 0.1 M HEPES buffer at pH 7.2 with (from bottom to top) 0, 2, 5, 7, 9, 11, and 16 μM Fe2+. (B) Titration curve for the formation of the PAC-Fe2+ complexes. The absorbance of the complex at 545 nm minus the absorbance of the Fe2+-free PACs (also at 545 nm) is plotted versus the Fe2+ concentration. 1:1 refers to the 1:1 PAC/Fe2+ complex.
To better understand the stoichiometry of complex formation between PACs and Fe2+, the absorbance obtained for the iron-free PACs was subtracted from the absorbance at 545 nm, and this difference was plotted as a function of the corresponding total [Fe2+] (Fig. 2B). A 1:1 PAC/Fe2+ complex was formed after the addition of 13 μM Fe2+ to ∼10 μM PAC. This small disparity in the concentration equivalence between PACs and Fe2+ is attributable to the heterogeneity of the PAC solution. Identical experiments were carried out with Fe3+ cations, and comparable results were obtained, thus confirming complex formation between PACs and Fe3+ cations (data not shown).
Strains of uropathogenic E. coli (UPEC) have evolved multiple strategies that facilitate bacterial growth and persistence within the unfavorable setting of the host urinary tract. Relative to K-12 and commensal E. coli isolates, UPEC harbor more genes encoding iron acquisition systems (28). These systems enable UPEC to appropriate host iron stores (29) and have been shown to be highly expressed in vivo during human infection (8). Given that PACs induce a state of iron limitation in E. coli CFT073, it is possible that prevention of UTIs by cranberry PACs is partially the result of the extreme reduction in the amount of available iron to UPEC strains in the urinary tract. It is noteworthy that the absorption and metabolism of cranberry PACs by humans have not been thoroughly studied; however, reports from other laboratories have shown that proanthocyanidins and anthocyanins are mostly absorbed into the human circulatory system and transported into the urine in intact form (20, 22, 24). If further information about metabolites or induced compounds of PACs is published, research concerning the effects of these compounds on bacterial metabolism must be conducted in order to obtain a thorough understanding of their potential as agents for the prevention of bacterial adhesion.
This is the first study to investigate the effect that PACs have on the transcriptome of E. coli CFT073. Based on changes in gene expression, we found that PACs induce a state of iron limitation in this bacterium. Observation of the formation of PAC-Fe complexes confirmed that PACs act as iron chelators. A study by Lin et al. (18) reported that exposure of a nonuropathogenic E. coli strain to cranberry juice also resulted in the altered expression of iron metabolism genes. Future work will be oriented toward determining whether the ability of PACs to chelate iron is a factor that influences the anti-adhesive properties of these compounds and hinders biofilm formation.
Microarray data accession number.
Array data were submitted to the Gene Expression Omnibus website (http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE25745.
Acknowledgments
We acknowledge the financial support of NSERC, the Canada Research Chair program, the Wisconsin Cranberry Board, and the Cranberry Institute.
We thank A. Howell (Rutgers University) for technical assistance and for providing cranberry PACs. We are grateful to J. Lipsitz for assistance with the growth curves and to I. A. Eydelnant for helpful discussions.
Footnotes
Published ahead of print on 17 December 2010.
REFERENCES
- 1.Avorn, J., et al. 1994. Reduction of bacteriuria and pyuria after ingestion of cranberry juice. JAMA 271:751-754. [DOI] [PubMed] [Google Scholar]
- 2.Bishop, M. 2004. Uncomplicated urinary tract infection. EAU-EBU Update Ser. 2:143-150. [Google Scholar]
- 3.Bjerrum, L., R. B. Dessau, and J. Hallas. 2002. Treatment failures after antibiotic therapy of uncomplicated urinary tract infections: a prescription database study. Scand. J. Prim. Health Care 20:97-101. [PubMed] [Google Scholar]
- 4.Blatherwick, N. R. 1914. The specific role of foods in relation to the composition of the urine. Arch. Intern. Med. XIV:409-450. [Google Scholar]
- 5.Eydelnant, I. A., and N. Tufenkji. 2008. Cranberry derived proanthocyanidins reduce bacterial adhesion to selected biomaterials. Langmuir 24:10273-10281. [DOI] [PubMed] [Google Scholar]
- 6.Gottesman, S. 2005. Micros for microbes: non-coding regulatory RNAs in bacteria. Trends Genet. 21:399-404. [DOI] [PubMed] [Google Scholar]
- 7.Guo, M., et al. 2007. Iron-binding properties of plant phenolics and cranberry's bio-effects. Dalton Trans. 4951-4961. [DOI] [PMC free article] [PubMed]
- 8.Hagan, E. C., A. L. Lloyd, D. A. Rasko, G. J. Faerber, and H. L. T. Mobley. 2010. Escherichia coli global gene expression in urine from women with urinary tract infection. PLoS Pathog. 6:e1001187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hellemans, J., G. Mortier, A. De Paepe, F. Speleman, and J. Vandesompele. 2007. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 8:R19-R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howell, A. B. 2007. Bioactive compounds in cranberries and their role in prevention of urinary tract infections. Mol. Nutr. Food Res. 51:732-737. [DOI] [PubMed] [Google Scholar]
- 11.Howell, A. B., et al. 2005. A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry 66:2281-2291. [DOI] [PubMed] [Google Scholar]
- 12.Howell, A. B., N. Vorsa, A. D. Marderosian, and L. Y. Foo. 1998. Inhibition of the adherence of P-fimbriated Escherichia coli to uroepithelial-cell surfaces by proanthocyanidin extracts from cranberries. N. Engl. J. Med. 339:1085-1086. [DOI] [PubMed] [Google Scholar]
- 13.Hummers-Pradier, E., and M. M. Kochen. 2002. Urinary tract infections in adult general practice patients. Br. J. Gen. Pract. 52:752-761. [PMC free article] [PubMed] [Google Scholar]
- 14.Irizarry, R. A., et al. 2003. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4:249-264. [DOI] [PubMed] [Google Scholar]
- 15.Jacobsen, S. M., D. J. Stickler, H. L. T. Mobley, and M. E. Shirtliff. 2008. Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin. Microbiol. Rev. 21:26-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kontiokari, T., et al. 2001. Randomised trial of cranberry-lingonberry juice and Lactobacillus GG drink for the prevention of urinary tract infections in women. BMJ 322:1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lawrenson, R. A., and J. W. Logie. 2001. Antibiotic failure in the treatment of urinary tract infections in young women. J. Antimicrob. Chemother. 48:895-901. [DOI] [PubMed] [Google Scholar]
- 18.Lin, B., B. J. Johnson, R. A. Rubin, A. P. Malanoski, and F. S. Ligler. 2010. Iron chelation by cranberry juice and its impact on Escherichia coli growth. BioFactors [Epub ahead of print.] doi: 10.1002/biof.110. [DOI] [PubMed]
- 19.Liu, Y., M. A. Black, L. Caron, and T. A. Camesano. 2006. Role of cranberry juice on molecular-scale surface characteristics and adhesion behavior of Escherichia coli. Biotechnol. Bioeng. 93:297-305. [DOI] [PubMed] [Google Scholar]
- 20.Manach, C., G. Williamson, C. Morand, A. Scalbert, and C. Rémésy. 2005. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 81:S230-S242. [DOI] [PubMed] [Google Scholar]
- 21.Massé, E., and S. Gottesman. 2002. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 99:4620-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGhie, T. K., G. D. Ainge, L. E. Barnett, J. M. Cooney, and D. J. Jensen. 2003. Anthocyanin glycosides from berry fruit are absorbed and excreted unmetabolized by both humans and rats. J. Agric. Food Chem. 51:4539-4548. [DOI] [PubMed] [Google Scholar]
- 23.Michael, M., E. Hodson, J. Craig, S. Martin, and V. Moyer. 2002. Short compared with standard duration of antibiotic treatment for urinary tract infection: a systematic review of randomised controlled trials. Arch. Dis. Child. 87:118-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohnishi, R., et al. 2006. Urinary excretion of anthocyanins in humans after cranberry juice ingestion. Biosci. Biotechnol. Biochem. 70:1681-1687. [DOI] [PubMed] [Google Scholar]
- 25.Pinzón-Arango, P. A., Y. Liu, and T. A. Camesano. 2009. Role of cranberry on bacterial adhesion forces and implications for Escherichia coli-uroepithelial cell attachment. J. Med. Food 12:259-270. [DOI] [PubMed] [Google Scholar]
- 26.Semsey, S., et al. 2006. Genetic regulation of fluxes: iron homeostasis of Escherichia coli. Nucleic Acids Res. 34:4960-4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sobota, A. E. 1984. Inhibition of bacterial adherence by cranberry juice: potential use for the treatment of urinary tract infections. J. Urol. 131:1013-1016. [DOI] [PubMed] [Google Scholar]
- 28.Welch, R. A., et al. 2002. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 99:17020-17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiles, T. J., R. R. Kulesus, and M. A. Mulvey. 2008. Origins and virulence mechanisms of uropathogenic Escherichia coli. Exp. Mol. Pathol. 85:11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright, G. W., and R. M. Simon. 2003. A random variance model for detection of differential gene expression in small microarray experiments. Bioinformatics 19:2448-2455. [DOI] [PubMed] [Google Scholar]


