Abstract
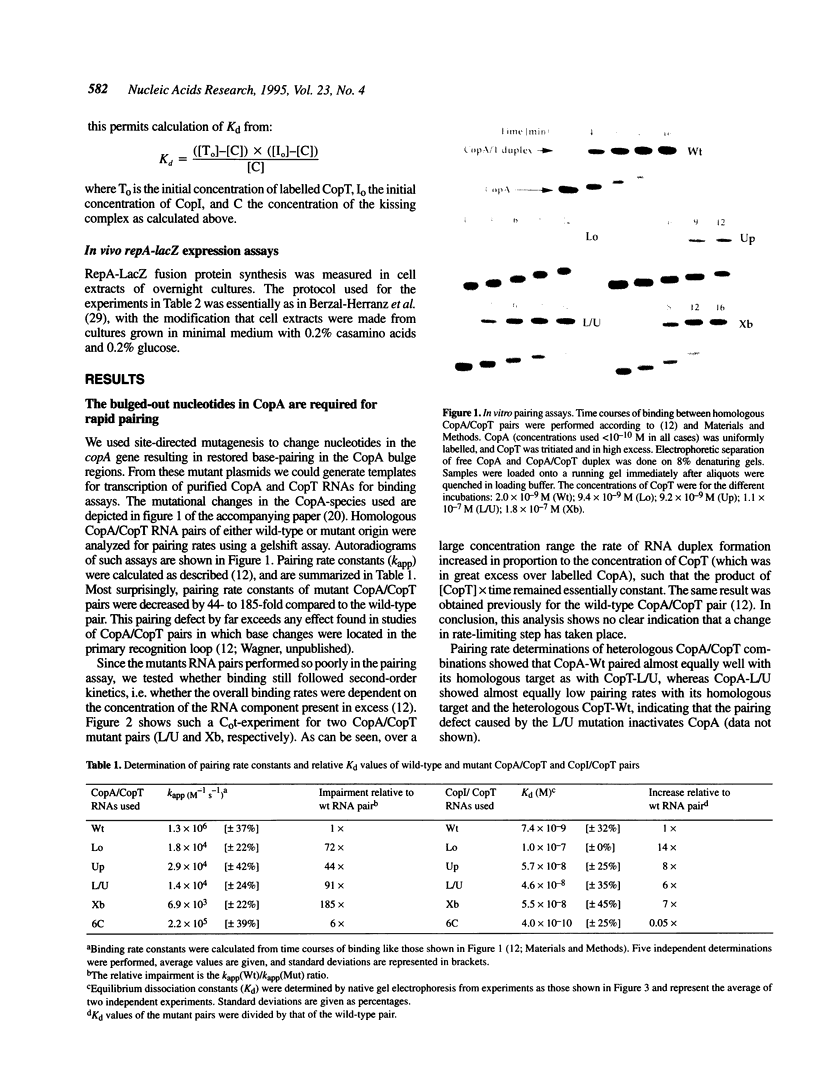
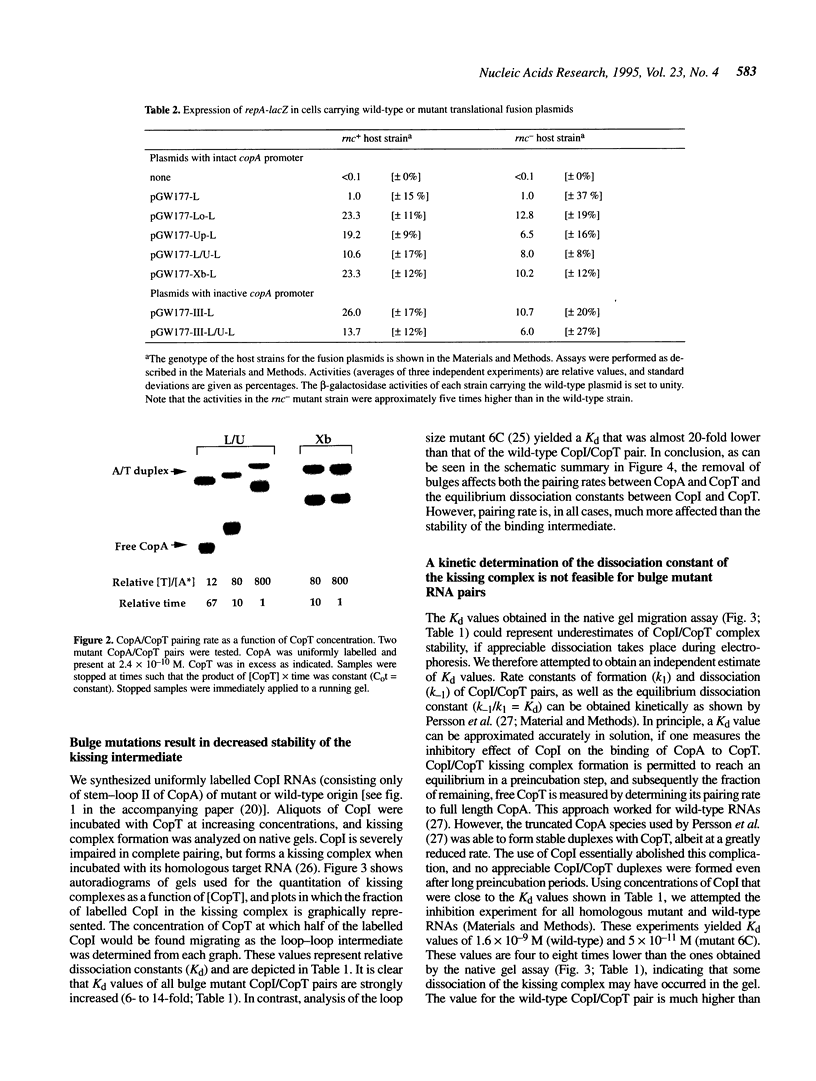
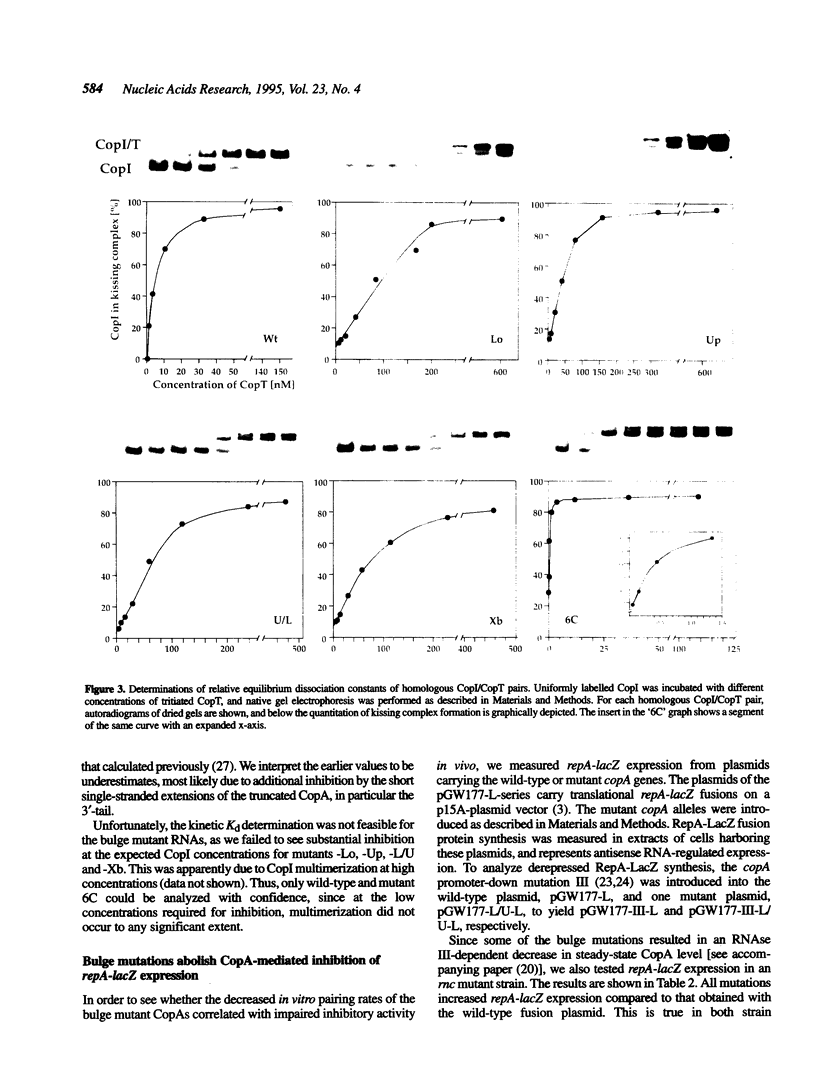
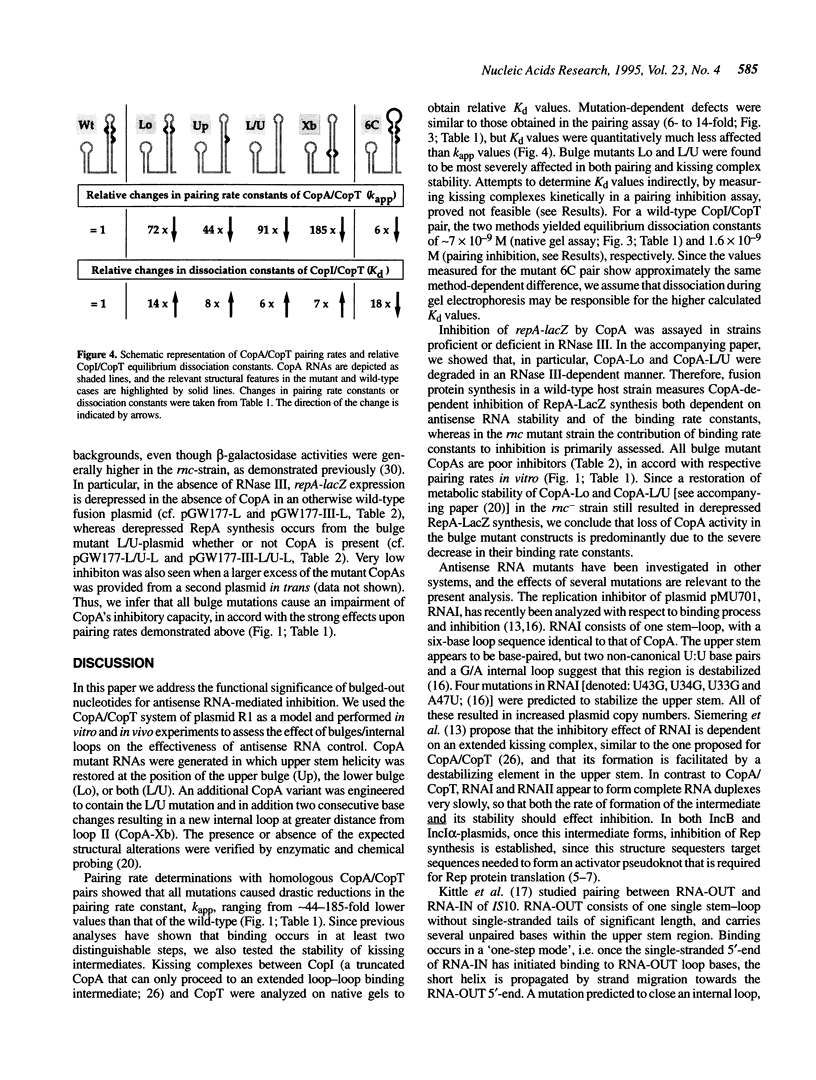
Naturally occurring antisense RNAs in prokaryotes are generally short, highly structured and untranslated. Stem-loops are always present, and loop regions serve as primary recognition structures in most cases. Single-stranded tails or internal unstructured regions are required for initiation of stable pairing between antisense and target RNA. Most antisense RNAs contain bulged-out nucleotides or small internal loops in upper stem regions. Here we investigated the role of the bulged-out nucleotides of CopA (the copy number regulator of plasmid R1) in determining the binding properties of this antisense RNA to its target in vitro and the efficiency of a translational inhibition in vivo. The introduction of perfect helicity in the region of the two bulges in CopA decreased pairing rate constants by up to 180-fold, increased equilibrium dissociation constants of the 'kissing intermediate' up to 14-fold, and severely impaired inhibition of repA expression. A previously described loop size mutant of CopA showed decreased pairing rates, but, in contrast to the bulge-less mutant CopAs, shows a decreased dissociation constant of the 'kissing complex'. We conclude that removal of the specific bulges/internal loops within the stem-loop II of CopA impairs the inhibitor, and that creation of an internal loop at a different position does not restore activity, emphasizing the optimal folding of wild-type CopA. The accompanying paper shows that an additional function of bulges can be protection from RNase III cleavage.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano K., Kato A., Moriwaki H., Hama C., Shiba K., Mizobuchi K. Positive and negative regulations of plasmid CoLIb-P9 repZ gene expression at the translational level. J Biol Chem. 1991 Feb 25;266(6):3774–3781. [PubMed] [Google Scholar]
- Asano K., Moriwaki H., Mizobuchi K. An induced mRNA secondary structure enhances repZ translation in plasmid ColIb-P9. J Biol Chem. 1991 Dec 25;266(36):24549–24556. [PubMed] [Google Scholar]
- Berzal-Herranz A., Wagner E. G., Díaz-Orejas R. Control of replication of plasmid R1: the intergenic region between copA and repA modulates the level of expression of repA. Mol Microbiol. 1991 Jan;5(1):97–108. [PubMed] [Google Scholar]
- Blomberg P., Engdahl H. M., Malmgren C., Romby P., Wagner E. G. Replication control of plasmid R1: disruption of an inhibitory RNA structure that sequesters the repA ribosome-binding site permits tap-independent RepA synthesis. Mol Microbiol. 1994 Apr;12(1):49–60. doi: 10.1111/j.1365-2958.1994.tb00994.x. [DOI] [PubMed] [Google Scholar]
- Blomberg P., Nordström K., Wagner E. G. Replication control of plasmid R1: RepA synthesis is regulated by CopA RNA through inhibition of leader peptide translation. EMBO J. 1992 Jul;11(7):2675–2683. doi: 10.1002/j.1460-2075.1992.tb05333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg P., Wagner E. G., Nordström K. Control of replication of plasmid R1: the duplex between the antisense RNA, CopA, and its target, CopT, is processed specifically in vivo and in vitro by RNase III. EMBO J. 1990 Jul;9(7):2331–2340. doi: 10.1002/j.1460-2075.1990.tb07405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantl S., Birch-Hirschfeld E., Behnke D. RepR protein expression on plasmid pIP501 is controlled by an antisense RNA-mediated transcription attenuation mechanism. J Bacteriol. 1993 Jul;175(13):4052–4061. doi: 10.1128/jb.175.13.4052-4061.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Eguchi Y., Itoh T., Tomizawa J. Antisense RNA. Annu Rev Biochem. 1991;60:631–652. doi: 10.1146/annurev.bi.60.070191.003215. [DOI] [PubMed] [Google Scholar]
- Eguchi Y., Tomizawa J. Complex formed by complementary RNA stem-loops and its stabilization by a protein: function of CoIE1 Rom protein. Cell. 1990 Jan 26;60(2):199–209. doi: 10.1016/0092-8674(90)90736-x. [DOI] [PubMed] [Google Scholar]
- Eguchi Y., Tomizawa J. Complexes formed by complementary RNA stem-loops. Their formations, structures and interaction with ColE1 Rom protein. J Mol Biol. 1991 Aug 20;220(4):831–842. doi: 10.1016/0022-2836(91)90356-b. [DOI] [PubMed] [Google Scholar]
- Gerhart E., Wagner H., Nordström K. Structural analysis of an RNA molecule involved in replication control of plasmid R1. Nucleic Acids Res. 1986 Mar 25;14(6):2523–2538. doi: 10.1093/nar/14.6.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjalt T. A., Wagner E. G. Bulged-out nucleotides protect an antisense RNA from RNase III cleavage. Nucleic Acids Res. 1995 Feb 25;23(4):571–579. doi: 10.1093/nar/23.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjalt T., Wagner E. G. The effect of loop size in antisense and target RNAs on the efficiency of antisense RNA control. Nucleic Acids Res. 1992 Dec 25;20(24):6723–6732. doi: 10.1093/nar/20.24.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittle J. D., Simons R. W., Lee J., Kleckner N. Insertion sequence IS10 anti-sense pairing initiates by an interaction between the 5' end of the target RNA and a loop in the anti-sense RNA. J Mol Biol. 1989 Dec 5;210(3):561–572. doi: 10.1016/0022-2836(89)90132-0. [DOI] [PubMed] [Google Scholar]
- Nordström K., Wagner E. G. Kinetic aspects of control of plasmid replication by antisense RNA. Trends Biochem Sci. 1994 Jul;19(7):294–300. doi: 10.1016/0968-0004(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Iordanescu S., Projan S. J., Kornblum J., Edelman I. pT181 plasmid replication is regulated by a countertranscript-driven transcriptional attenuator. Cell. 1989 Oct 20;59(2):395–404. doi: 10.1016/0092-8674(89)90300-0. [DOI] [PubMed] [Google Scholar]
- Ohman M., Wagner E. G. Regulation of replication of plasmid R1: an analysis of the intergenic region between copA and repA. Mol Gen Genet. 1991 Nov;230(1-2):321–328. doi: 10.1007/BF00290683. [DOI] [PubMed] [Google Scholar]
- Persson C., Wagner E. G., Nordström K. Control of replication of plasmid R1: formation of an initial transient complex is rate-limiting for antisense RNA--target RNA pairing. EMBO J. 1990 Nov;9(11):3777–3785. doi: 10.1002/j.1460-2075.1990.tb07591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson C., Wagner E. G., Nordström K. Control of replication of plasmid R1: kinetics of in vitro interaction between the antisense RNA, CopA, and its target, CopT. EMBO J. 1988 Oct;7(10):3279–3288. doi: 10.1002/j.1460-2075.1988.tb03195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson C., Wagner E. G., Nordström K. Control of replication of plasmid R1: structures and sequences of the antisense RNA, CopA, required for its binding to the target RNA, CopT. EMBO J. 1990 Nov;9(11):3767–3775. doi: 10.1002/j.1460-2075.1990.tb07590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumbridge J. A., Dondon J., Nakamura Y., Grunberg-Manago M. Effect of NusA protein on expression of the nusA,infB operon in E. coli. Nucleic Acids Res. 1985 May 10;13(9):3371–3388. doi: 10.1093/nar/13.9.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyle A. M., McSwiggen J. A., Cech T. R. Direct measurement of oligonucleotide substrate binding to wild-type and mutant ribozymes from Tetrahymena. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8187–8191. doi: 10.1073/pnas.87.21.8187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemering K. R., Praszkier J., Pittard A. J. Interaction between the antisense and target RNAs involved in the regulation of IncB plasmid replication. J Bacteriol. 1993 May;175(10):2895–2906. doi: 10.1128/jb.175.10.2895-2906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemering K. R., Praszkier J., Pittard A. J. Mechanism of binding of the antisense and target RNAs involved in the regulation of IncB plasmid replication. J Bacteriol. 1994 May;176(9):2677–2688. doi: 10.1128/jb.176.9.2677-2688.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T., Itoh T. Control of ColE2 DNA replication: in vitro binding of the antisense RNA to the Rep mRNA. Nucleic Acids Res. 1993 Dec 25;21(25):5972–5977. doi: 10.1093/nar/21.25.5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thisted T., Sørensen N. S., Wagner E. G., Gerdes K. Mechanism of post-segregational killing: Sok antisense RNA interacts with Hok mRNA via its 5'-end single-stranded leader and competes with the 3'-end of Hok mRNA for binding to the mok translational initiation region. EMBO J. 1994 Apr 15;13(8):1960–1968. doi: 10.1002/j.1460-2075.1994.tb06465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa J. Control of ColE1 plasmid replication: the process of binding of RNA I to the primer transcript. Cell. 1984 Oct;38(3):861–870. doi: 10.1016/0092-8674(84)90281-2. [DOI] [PubMed] [Google Scholar]
- Tomizawa J., Itoh T. Plasmid ColE1 incompatibility determined by interaction of RNA I with primer transcript. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6096–6100. doi: 10.1073/pnas.78.10.6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa J., Som T. Control of ColE1 plasmid replication: enhancement of binding of RNA I to the primer transcript by the Rom protein. Cell. 1984 Oct;38(3):871–878. doi: 10.1016/0092-8674(84)90282-4. [DOI] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Wagner E. G., Simons R. W. Antisense RNA control in bacteria, phages, and plasmids. Annu Rev Microbiol. 1994;48:713–742. doi: 10.1146/annurev.mi.48.100194.003433. [DOI] [PubMed] [Google Scholar]
- Wilson I. W., Praszkier J., Pittard A. J. Mutations affecting pseudoknot control of the replication of B group plasmids. J Bacteriol. 1993 Oct;175(20):6476–6483. doi: 10.1128/jb.175.20.6476-6483.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R., Wang X., Womble D. D., Rownd R. H. Expression of the repA1 gene of IncFII plasmid NR1 is translationally coupled to expression of an overlapping leader peptide. J Bacteriol. 1992 Dec;174(23):7620–7628. doi: 10.1128/jb.174.23.7620-7628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]






