Abstract
We report on the presence of a functional hydroxyectoine biosynthesis gene cluster, ectABCD-ask, in Pseudomonas stutzeri DSM5190T and evaluate the suitability of P. stutzeri DSM5190T for hydroxyectoine production. Furthermore, we present information on heterologous de novo production of the compatible solute hydroxyectoine in Escherichia coli. In this host, the P. stutzeri gene cluster remained under the control of its salt-induced native promoters. We also noted the absence of trehalose when hydroxyectoine genes were expressed, as well as a remarkable inhibitory effect of externally applied betaine on hydroxyectoine synthesis. The specific heterologous production rate in E. coli under the conditions employed exceeded that of the natural producer Pseudomonas stutzeri and, for the first time, enabled effective hydroxyectoine production at low salinity (2%), with the added advantage of simple product processing due to the absence of other cosolutes.
As reviewed extensively elsewhere, halotolerant and halophilic microorganisms can adapt to extreme salinity by accumulation or biosynthesis of a range of osmolytes (8, 14, 21, 32, 36). Extreme halophiles like the archaeon Halobacterium salinarum usually recruit inorganic ions, while others, in particular, highly adaptive organisms, make use of a more flexible strategy and employ compatible solutes (also called extremolytes) to obtain osmotic equilibrium. Unlike inorganic ions and even at molar concentrations, these organic low-molecular-weight osmolytes do not interfere negatively with normal cell metabolism, even at molar concentrations. In addition to their function as osmoprotectants for whole living cells, compatible solutes also protect biological macromolecules against physical stress in vitro. Therefore, they have found their way into a range of biochemical applications (25, 33) and even skin care products (5).
The compatible solute ectoine (12) is often detected as the main solute in chemoheterotrophic eubacterial halophiles of broad salt tolerance. Its biosynthesis (see Fig. S01 in the supplemental material) proceeds as an off-branch from the aspartate amino acid family. Enzymes for the three final biosynthetic steps are usually encoded in an ectABC-type cluster (29), although there are notable exceptions which have an incomplete cluster or even only a solitary ectC (22). The ectoine derivative hydroxyectoine is usually formed by a subsequent biosynthetic step (6), provided that the required enzyme (ectoine hydroxylase, often EctD) is encoded in the genome. According to current knowledge, hydroxylation of ectoine seems to be linked to conditions where survival strategies become important, for example, during imminent desiccation at extreme salinity and elevated temperature in the Halomonadaceae family (15). Therefore, the industrial production strain for ectoine, Halomonas elongata, can be manipulated to increase its hydroxyectoine content (to a maximum of approximately 50%) but as yet not to such an extent that hydroxyectoine becomes the sole compatible solute. Fermentation under extreme conditions and subsequent chromatographic separation procedures increase production costs. As hydroxyectoine is in high demand due to its superior protective effect on whole cells (30) and biological macromolecules (4, 17, 27), the search is on for natural and heterologous production strains.
Since the work of Smith and Smith (41), we have known that the primary compatible solute of Pseudomonas species and Rhizobium is N-γ-acetylglutaminyl glutamine 1-amide (NAGGN). So far this compound has been found only in combination with other cosolutes, e.g., trehalose, glucosyl glycerol, and mannitol (10, 18, 34). Although Pseudomonas stutzeri is a well-investigated organism (23), hydroxyectoine production was reported only recently as a surprise discovery in strain DSM5190T by our group (20). This is the first time ever that NAGGN was encountered in combination with hydroxyectoine as a cosolute in a Pseudomonas species. Subsequently, an ectABC-type gene cluster was detected in the genome sequence of P. stutzeri A1505 (44). Downstream of ectABC, two open reading frames were located and annotated as a putative proline hydroxylase (PST_0178) and aspartokinase (PST_0177). Both the similarity of PST_0178 to other ectoine hydroxylases and the proximity of PST_0177 to the ectABC gene cluster indicated a complete hydroxyectoine biosynthesis gene cluster, including an aspartokinase. Until now, such an arrangement was known only from Marinomonas sp. MWYL1 (GenBank accession no. CP000749) and a few alphaproteobacteria, e.g., Hyphomonas neptunium (GenBank accession no. CP000158). As the availability of aspartic acid semialdehyde was reported to be critical for heterologous ectoine production (2), a complete biosynthetic cluster including aspartokinase appears to be an ideal prerequisite for genetic engineering. However, experimental evidence of the function of this constellation in the gammaproteobacterium Pseudomonas stutzeri and its potential use for heterologous expression was still lacking.
Thus, the aim of this study was not only to demonstrate that the putative ectABCD-ask gene cluster has a metabolic function but also to evaluate hydroxyectoine production for industrial use in both the natural producer P. stutzeri and the heterologous host Escherichia coli.
MATERIALS AND METHODS
Strains and culture conditions.
Pseudomonas stutzeri DSM5190T (37) and E. coli DH5α (16) were obtained from the DSMZ (Braunschweig, Germany). We mainly used minimal medium MM63 (24) for growth and production experiments. Luria-Bertani (LB) medium (1) was employed for cloning purposes and to study uptake of solutes from complex medium. Antibiotics were added and NaCl content was modified where necessary. P. stutzeri and E. coli were grown at 37°C in liquid cultures with shaking at 180 rpm. Growth data were collected from cultures in shaking flasks with appendages for optical density measurements.
Molecular biology methods.
We amplified the complete P. stutzeri hydroxyectoine gene cluster, including the aspartokinase, by PCR and cloned it into pUC18 (31), making use of primers clus_for (5′-ATC AGA TCG CGG AGC TCG GG-3′) and clus_rev (5′-CCG GAC TCG ATC ACA TAT GTC TTA-3′), located upstream and downstream of the ectABCD-ask cluster, respectively (numbered PST_00181 to PST_00177, respectively), and modified for NdeI and SacI restriction sites (underlined). We included a sequence 400 bp upstream of ectA to ensure that expression of the whole gene cluster remained under the control of the native promoters. In addition, to exclude expression under the control of the pUC18 lac promoter, the gene cluster was oriented in the opposite direction with respect to the direction of this promoter. The construct was named pSB01. In a second construct, named pSB02, the aspartokinase was excised from pSB01 with ApaI and BstXI. Both plasmids were transformed into E. coli DH5α. We named the resulting organisms E. coli DH5α/pSB01 and E. coli DH5α/pSB02, respectively (see Fig. S02 in the supplemental material for plasmid maps).
Analysis of solute content.
If not noted otherwise, cell material was harvested from an exponentially growing shaking culture and freeze-dried. Extraction followed a modified protocol of Bligh and Dyer (3), as described previously (13). Solute content was analyzed by isocratic high-pressure liquid chromatography (HPLC) on a Nucleosil 100-3 NH2 reversed-phase column (Macherey-Nagel, Düren, Germany), and 80%/20% (vol/vol) acetonitrile-water was used as the mobile phase at a flow rate of 1 ml min−1. Additionally, 1H and 13C nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Avance 300 DPX spectrometer. Spectra were calibrated to the internal standard trimethylsilanepropionic acid (TMSP).
Sequencing.
Sequencing reactions were carried out by Sequiserve (Vaterstetten, Germany).
Nucleotide sequence accession number.
Complete sequence data for P. stutzeri DSM5190T ectABCD-ask are available via the EMBL gene bank under accession number FN868642.
RESULTS
Sequence of P. stutzeri DSM5190T ectABCD-ask.
Complete sequence data for P. stutzeri DSM5190T ectABCD-ask were compared with those for the strain A1501 genome (GenBank accession no. CP000304), and the sequences showed high degrees of homology, as expected: ectA, ectB, ectC, ectD, and ask displayed 5, 27, 1, 6, and 14 differences in the DNA sequence, respectively. Most differences in the nucleotide sequence are silent at the amino acid level. In the type strain, glycine 160 is replaced by serine and glutamine 403 is replaced by lysine in EctB. In addition, glutamate 90 is replaced by aspartate in EctC.
Solute spectrum of P. stutzeri DSM5190T.
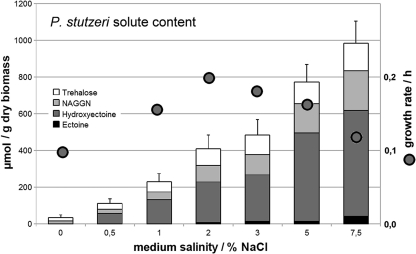
In response to increasing salinity, the organism synthesized hydroxyectoine, NAGGN, and trehalose de novo as main osmolytes. At all salinities tested, hydroxyectoine was the main compatible solute (>50%). In addition, very small amounts of ectoine were detectable at 2% NaCl and higher (Fig. 1). When it was grown on complex (LB) medium, the organism also accumulated large amounts of betaine (comparable to those of hydroxyectoine) and histidine (4% [wt/wt] of total solutes) either directly from the medium or from suitable precursors (see Fig. S03 in the supplemental material for a 13C NMR spectrum of an extract from a culture grown in LB medium). In the presence of sufficient amounts of betaine, the level of biosynthesis of other solutes was reduced by approximately 10% (data not shown).
FIG. 1.
P. stutzeri DSM5190T salinity-dependent growth rate and solute biosynthesis. The main solutes synthesized de novo in MM63 by P. stutzeri DSM5190T are trehalose, NAGGN, and hydroxyectoine. Ectoine is present only in minor amounts. Relative to dry biomass, the amount of all solutes increases with salinity, as does, consequently, the total amount (given in μmol/g dry biomass). Growth rates are indicated by circles and are given in h−1. The organism's growth optimum in minimal medium is at about 2% salinity, but growth rates do not vary much at between 1% and 5% NaCl. Due to poor growth of the strain, data could not be reproduced sufficiently at salinities above 7.5%.
In mineral salt medium MM63, the relative proportion of solutes remained independent of the NaCl concentration (Fig. 1), and the actual amounts were largely growth phase independent (see Fig. S04 in the supplemental material). Within experimental error, total solute content increased in a nearly linear fashion from 1% to 5% NaCl, being 760 μmol/(g dry biomass [DBM]) at 5% NaCl. At NaCl concentrations above 7.5%, the organism did not grow well and measurements did not yield reproducible results. In medium MM63, P. stutzeri DSM5190T displayed a maximum growth rate of 0.2 h−1 at 2% NaCl.
Heterologous production of hydroxyectoine in E. coli DH5α.
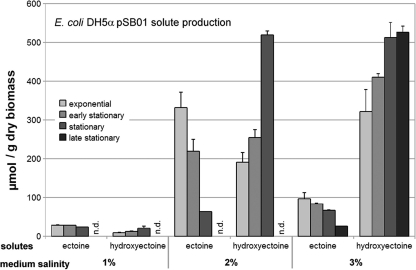
Expression of the complete ectABCD-ask gene cluster under the control of the native P. stutzeri promoters (pSB01) successfully led to salt-induced production of hydroxyectoine (Fig. 2). Contrary to the situation in the donor, however, a remarkable growth phase dependence was observed: hydroxyectoine production was clearly delayed and increased at the expense of ectoine production when the organism entered stationary phase. In late stationary phase, the final contribution of ectoine to the solute pool was below 5% of the total (at 3% NaCl) or was undetectable. The total hydroxyectoine content displayed a more than 20-fold increase from 25 to over 500 μmol/g dry biomass when salinity was raised from 1% to 2% NaCl. Beyond that salinity, no further increase was observed (Fig. 2). Only negligible traces of trehalose were detected (data not shown). Against expectations, a comparison of the results obtained with pSB01 and pSB02 (the gene cluster without aspartokinase) revealed that hydroxyectoine production in E. coli was not dependent on coexpression of aspartokinase (data not shown). Neither plasmid conferred enhanced osmotolerance to E. coli DH5α.
FIG. 2.
E. coli DH5α/pSB01 salinity-dependent solute production. When the ectABCD-ask gene cluster is expressed in E. coli DH5α, the strain produces ectoine and hydroxyectoine (indicated on the x axis), depending on the medium salinity (given in percent [wt/wt] NaCl) and growth phase (bars in different shades of gray). Ectoine levels decrease and hydroxyectoine levels increase over time.
Growth in supplemented medium.
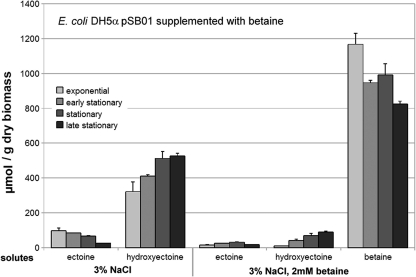
E. coli cells harboring pSB01 (Fig. 3) or pSB02 (data not shown) accumulated betaine in very large amounts when they were supplemented with this solute. In this situation, total biosynthesis of ectoines was reduced to approximately 1/10 of that for cells grown in minimal medium.
FIG. 3.
Heterologous solute production is reduced by betaine uptake. E. coli DH5α/pSB01 production of hydroxyectoine and ectoine (indicated on the x axis) in minimal medium at 3% salinity is shown. When cells are supplemented with 2 mM betaine, betaine is taken up and production of ectoines is reduced to approximately 1/10 of the original value.
Specific hydroxyectoine production rate.
As the product hydroxyectoine is directly related to the biomass grown at a certain salinity, the specific production rate [μmol/(g DBM·h)] is a simple function of the hydroxyectoine content and growth rate. As shown in Table 1, the natural producer P. stutzeri DSM5190T and the donor of the ectABCD-ask gene cluster have their highest specific hydroxyectoine production rates at 5% NaCl [76.8 μmol/(g DBM·h)]. The heterologous production system in E. coli DH5α/pSB01 reaches a specific production rate 2.2 times higher [175 μmol/(g DBM·h)] at a much lower salinity of only 2% NaCl. The higher productivity of the genetically engineered strain is caused by two factors: (i) the apparent repression of other endogenous compatible solutes such as trehalose, enabling a higher hydroxyectoine content, and (ii) its faster growth rate in medium MM63 (0.35 h−1, compared to 0.2 h−1 at 2% NaCl).
TABLE 1.
Specific hydroxyectoine production rates of donor and acceptor strains
| Strain vector | Salinity (%) | Growth rate (h−1) | Yield(s) (μmol/g DBM)a | Specific production rate [μmol/(g DBM·h)] |
|---|---|---|---|---|
| P. stutzeri DSM5190T | 1.0 | 0.15 | 140 HOE, 55 T, 45 N | 21.0 |
| 2.0 | 0.20 | 220 HOE, 90 T, 90 N | 44.0 | |
| 3.0 | 0.18 | 250 HOE, 110 T, 110 N | 45.0 | |
| 5.0 | 0.16 | 480 HOE, 120 T, 160 N | 76.8 | |
| 7.5 | 0.12 | 580 HOE, 150 T, 215 N | 69.6 | |
| E. coli DH5α/pSB01 | 1.0 | 0.32 | 25 HOE | 8.0 |
| 2.0 | 0.35 | 500 HOE | 175 | |
| 3.0 | 0.2 | 500 HOE | 100 |
Solute contents are shown for hydroxyectoine (HOE), trehalose (T), and NAGGN (N). Ectoine is neglected here due to small amounts (<5% of total solutes). See Fig. S05 in the supplemental material for a comparative graphic representation of the data for 1% to 3% NaCl.
DISCUSSION
P. stutzeri DSM5190T-compatible solutes.
P. stutzeri DSM5190T is the first Pseudomonas sp. where hydroxyectoine production was reported (20) and the complete ectABCD-ask gene cluster was identified. Compared to other halotolerant microorganisms, the salinity-dependent total solute content is in the expected range, but production of hydroxyectoine along with trehalose and NAGGN as cosolutes is unusual. Trehalose is commonly employed as an osmolyte only at lower salinities (8, 14, 36), a fact that can be attributed to its relatively high energy cost, approximately double that of ectoine (32). In addition, hydroxylation of ectoine has so far always been linked to extreme conditions triggering survival strategies (15). In particular, the seemingly unnecessary hydroxylation step under standard growth conditions appears to lack economic reason. With respect to these considerations and in unison with the pronounced natural competence of several P. stutzeri subspecies (23), we were intrigued by the idea that genes for hydroxyectoine biosynthesis might have been acquired by lateral gene transfer. Unfortunately, we did not find significant variations in codon usage or in GC content compared to those for the rest of the P. stutzeri genome to support this theory, at least not in the strain A1501 genome. We also could not find unusual trehalose biosynthesis pathways which might help to explain the use of trehalose as a third solute together with NAGGN and hydroxyectoine. Therefore, the presence of the ectoine/hydroxyectoine biosynthetic pathway may be specific for the genomovar P. stutzeri, which is characterized by its true marine lifestyle. This group of organisms is very versatile, uses cheap substrates (for example, ethylene glycol), and is easy to cultivate, even under high-cell-density conditions. P. stutzeri strains therefore qualify as potential industrial production strains. As use of complex media leads to accumulation of external compatible solutes (in particular, glycine betaine, which represses hydroxyectoine biosynthesis), production processes are confined to the use of mineral salt media devoid of complex components, such as yeast extract. Unfortunately, the growth rate of P. stutzeri DSM5190T in medium MM63 at 2% NaCl is considerably lower than that of E. coli (Table 1). Although medium improvements are certainly an option, the intrinsic disadvantage of P. stutzeri for hydroxyectoine production remains, namely, that hydroxyectoine is always produced in combination with other solutes. This not only reduces content and hydroxyectoine productivity but also imposes the need for subsequent downstream separation procedures to obtain a pure product.
While uptake of betaine in complex medium was to be expected because it is energetically favorable to de novo biosynthesis of solutes, accumulation of histidine came as a surprise. Its function remains unclear, especially as this compound has so far not been reported to play a role in osmoadaptation. Thus, it is very well possible that scavenging histidine in connection with osmotic adaptation is only a side effect of other transport processes. Genes encoding histidine uptake systems like hisJQMP (7) or the histidine utilization pathway (hut) (45) underlie complex but not yet fully understood regulation. According to current knowledge, there is no osmolarity-dependent regulation. In the P. stutzeri A1501 genome (GenBank accession no. CP000304), no transport system is annotated and similarities to verified His and Hut proteins are insufficient to propose homologues.
Heterologous expression in E. coli DH5α.
In order to avoid the problem of by-product separation, we aimed at establishing a heterologous production process to obtain almost pure hydroxyectoine. So far, de novo heterologous production has been reported only for ectoine (2, 29, 35, 40) and not for hydroxyectoine. In all these studies, a coherent ectABC gene cluster was used. Bestvater et al. identified the availability of the immediate precursor aspartylphosphate—and, thus, the reaction catalyzed by aspartokinase—to be a metabolic bottleneck in ectoine biosynthesis by way of expression of the ectABC gene cluster of Marinococcus halophilus. Only in the presence of a deregulated aspartokinase (plasmid pAKECT1) did they achieve a maximum specific production rate of 140 μmol/(g DBM·h) at 2% NaCl (2). This heterologous productivity was as high as that of the industrial production strain H. elongata at 3% NaCl (9). In view of these reports, the availability of a complete ectABCD-ask gene cluster for hydroxyectoine biosynthesis in P. stutzeri DSM5190T seemed very encouraging, especially as the presence of the aspartokinase gene promised optimal results without the need for further pathway engineering.
Expression of the complete P. stutzeri ectABC-ask gene cluster in E. coli DH5α proved successful, albeit with delayed conversion of ectoine into hydroxyectoine. As demonstrated in Fig. 2, a minimum of 2% NaCl was required for efficient hydroxyectoine synthesis. Total hydroxyectoine content in stationary phase was the same at 2% and 3% NaCl, approximately 500 μmol/(g DBM), and more than twice as high as that in the donor strain at 2% NaCl. With a maximum productivity of 175 μmol/(g DBM·h) at 2% NaCl, the heterologous expression record for ectoine by Bestvater et al. (2) has now been exceeded without the need for engineering of aspartokinase into the process.
Although the drastic increase from 1% NaCl to 2% NaCl indicates a somewhat deregulated function of the P. stutzeri ectABCD-ask gene cluster in E. coli, it is worthy of note that under given conditions, de novo biosynthesis of trehalose is abolished to almost negligible levels and that the presence of external betaine shuts down hydroxyectoine biosynthesis (Fig. 3). One can therefore conclude that at least part of the regulation associated with the native gene cluster still functions in the host. However, contrary to our expectations and contrary to results by Bestvater et al. (2), production of hydroxyectoine was not changed significantly in construct pSB02, which lacks the ask gene. The three E. coli aspartokinases ThrA, MetL, and LysC can provide the necessary precursor for hydroxyectoine biosynthesis. Nevertheless, the concentrations seem to be insufficient due to feedback regulation of the enzyme, at least with the vector construct used by Bestvater et al. (2). Thus, the association of ask with the ectABCD gene cluster, as well as possible implications of this proximity (28) and likely differences of the enzymes involved, might be an interesting object for further research but is beyond the scope of this project.
Comparison of hydroxyectoine production systems.
Hydroxyectoine is at present produced on an industrial scale with H. elongata using the bacterial milking technique developed by Sauer and Galinski (38) or an improvement of this technique based on the continuous fermentation of this organism (26), followed by chromatographic separation of the two ectoines. In order to increase the hydroxyectoine content, H. elongata has to be grown at increased salinity and temperature (unpublished data). Recent work on related organisms such as Halomonas boliviensis (42) and Chromohalobacter salexigens (43) seems to confirm the situation within the Halomonadaceae family that hydroxyectoine is always produced in combination with ectoine (e.g., 55% of total ectoines in H. boliviensis at 18.5% NaCl) and that its relative proportion increases with temperature.
The other native strains which have so far been employed for hydroxyectoine production are from the Gram-positive bacterial genus Marinococcus. Early work with Marinococcus sp. strain M52 has revealed a growth-phase-dependent hydroxyectoine synthesis enabling a high proportion of hydroxyectoine production in stationary-phase cells. Contrary to Halomonas cells, Marinococcus spp. cannot be milked by simply diluting the medium (11). However, as shown by Schiraldi and coworkers (39), osmotic downshock in combination with heat will trigger permeabilization of cells. Unfortunately, Marinococcus spp. produce growth-inhibiting components like acetate and are therefore not suitable for batch and fed-batch fermentation strategies. The maximum growth rate depicted in Table 2 is achieved only at the beginning of a batch culture and is not representative of the entire growth phase. This drawback can, in principle, be resolved by using a dialysis reactor system (19) or a microfiltration technique (39), at the expense of markedly reduced growth rates. These technically demanding fermentation strategies have so far not been exploited for industrial application.
TABLE 2.
Comparison of hydroxyectoine production systems
| Strain vector | Salinity (% NaCl) | Growth rate (μ) (h−1) | Content (μmol/g DBM) | Specific production rate (μmol/g DBM·h)a | Reference or source |
|---|---|---|---|---|---|
| P. stutzeri DSM5190T | 5.0 | 0.16 | 480 | 76.8 | This study |
| E. coli DH5α/pSB01 | 2.0 | 0.35 | 500 | 175 | This studyb |
| Marinococcus sp. strain M52 | 10.0 | 0.25f | 860 | 215 | 11c |
| Marinococcus sp. strain M52 | 10.0 | 0.20 | 670 | 134 | 39d |
| Marinococcus sp. strain M52 | 10.0 | 0.03 | 603 | 18 | 39d |
| H. boliviensis | 18.5 | Multistep process | 950 | 169 | 42e |
Maximum specific hydroxyectoine production rates were calculated on the basis of growth rates and biomass content.
Trehalose production is suppressed almost completely.
The organism does not grow exponentially, and the maximum growth rate is observed only at the beginning of batch phase; hydroxyectoine is retained within the biomass upon osmotic downshock.
Cells were first grown in batch culture (μ = 0.2) and subsequently subjected to continuous dialysis in order to remove growth-inhibiting compounds (μ = 0.03).
Cells were first grown at optimal salinity for biomass production (step 1) and then transferred to a medium of higher salinity for solute production (step 2). Here the highest specific production rates during the hydroxyectoine production phase are compared, not accounting for the time needed for step 1 (24 h) and an adaptation phase (3 h) at the beginning of the production phase.
Maximum.
In this study we have presented two alternative production systems: wild-type P. stutzeri DSM5190T and E. coli expressing the ectABCD-ask gene cluster from P. stutzeri under the control of the donor's promoter region. Both have the advantage that the precursor ectoine is almost completely transformed into hydroxyectoine at low salinity and normal growth temperature. Whereas the donor produces hydroxyectoine in combination with trehalose and NAGGN, the heterologous production system has hydroxyectoine as its sole compatible solute. A comparison of the specific production rates of the donor and the host (Table 1) demonstrates the superiority of the heterologous production system, in particular at low salinity (2% NaCl). With a maximum production rate of 175 μmol/(g DBM·h) and the additional advantage of relatively low salinity (2% NaCl), heterologous hydroxyectoine production in E. coli is superior to any other strategy published so far (Table 2).
Conclusions.
In conclusion, P. stutzeri DSM5190T lends itself as a new potential candidate for the production of the compatible osmolyte hydroxyectoine. Since the strain shows a high degree of physiological variability (23), the use of a range of approaches to further improve cultivation for industrial-scale applications seems feasible. With respect to growth rate (0.16 h−1) and yield (480 μmol hydroxyectoine per g dry biomass), 5% NaCl appears to be the optimum process condition. As P. stutzeri DSM5190T produces NAGGN and trehalose as side products, the use of deletion strains for trehalose and NAGGN in combination with optimized mineral salt medium may provide a promising perspective.
The heterologous E. coli production system based on the ectABCD-ask gene cluster from P. stutzeri DSM5190T suffers the stigma of genetically modified organisms (GMO) and the costs of maintenance of the plasmid, for example, by the addition of antibiotics. On the other hand, the heterologous system provides a number of advantages, mainly, a specific production rate [175 μmol/(g DBM·h)] higher than that of the donor and any other available system so far, a high hydroxyectoine content at low salinity [500 μmol/(g DBM) at 2% NaCl], and an almost complete absence of other contaminating compatible solutes (>95% purity).
Supplementary Material
Acknowledgments
We thank Ute Habermann (Bioreact, Troisdorf, Germany) and Harald Gross (University of Bonn) for interesting discussions and critically reading the manuscript. Many thanks also go to Marlene Stein (University of Bonn) for help with HPLC and interpretation of NMR results. We gratefully acknowledge the help of Binbin He (University of Bonn) with solute content measurements. Special thanks go to Carolyn Lucas for her valuable language input as a native speaker.
Footnotes
Published ahead of print on 17 December 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Bertani, G. 1951. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 62:293-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bestvater, T., P. Louis, and E. A. Galinski. 2008. Heterologous ectoine production in Escherichia coli: by-passing the metabolic bottle-neck. Saline Systems 4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bligh, E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and urification. Can. J. Biochem. Physiol. 37:911-917. [DOI] [PubMed] [Google Scholar]
- 4.Borges, N., A. Ramos, N. D. Raven, R. J. Sharp, and H. Santos. 2002. Comparative study of the thermostabilizing properties of mannosylglycerate and other compatible solutes on model enzymes. Extremophiles 6:209-216. [DOI] [PubMed] [Google Scholar]
- 5.Buenger, J., and H. Driller. 2004. Ectoin: an effective natural substance to prevent UVA-induced premature photoaging. Skin Pharmacol. Physiol. 17:232-237. [DOI] [PubMed] [Google Scholar]
- 6.Bursy, J., A. J. Pierik, N. Pica, and E. Bremer. 2007. Osmotically induced synthesis of the compatible solute hydroxyectoine is mediated by an evolutionarily conserved ectoine hydroxylase. J. Biol. Chem. 282:31147-31155. [DOI] [PubMed] [Google Scholar]
- 7.Caldara, M., P. N. L. Minh, S. Bostoen, J. Massant, and D. Charlier. 2007. ArgR-dependent regulation of arginine and histidine transport genes in Escherichia coli K-12. J. Mol. Biol. 373:251-267. [DOI] [PubMed] [Google Scholar]
- 8.da Costa, M. S., H. Santos, and E. A. Galinski. 1998. An overview of the role and diversity of compatible solutes in Bacteria and Archaea. Adv. Biochem. Eng. Biotechnol. 61:117-153. [DOI] [PubMed] [Google Scholar]
- 9.Dötsch, A., J. Severin, W. Alt, E. A. Galinski, and J. Kreft. 2008. A mathematical model for growth and osmoregulation in halophilic bacteria. Microbiology 154:2956-2969. [DOI] [PubMed] [Google Scholar]
- 10.D'Souza-Ault, M. R., L. T. Smith, and G. M. Smith. 1993. Roles of N-acetylglutaminylglutamine amide and glycine betaine in adaptation of Pseudomonas aeruginosa to osmotic stress. Appl. Environ. Microbiol. 59:473-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frings, E., T. Sauer, and E. A. Galinski. 1995. Production of hydroxyectoine: high cell-density cultivation and osmotic downshock of Marinococcus strain M52. J. Biotechnol. 43:56-63. [Google Scholar]
- 12.Galinski, E. A., H. P. Pfeiffer, and H. G. Trüper. 1985. 1,4,5,6-Tetrahydro-2-methyl-4-pyrimidinecarboxylic acid. A novel cyclic amino acid from halophilic phototrophic bacteria of the genus Ectothiorhodospira. Eur. J. Biochem. 149:135-139. [DOI] [PubMed] [Google Scholar]
- 13.Galinski, E. A., and A. Oren. 1991. Isolation and structure determination of a novel compatible solute from the moderately halophilic purple sulfur bacterium Ectothiorhodospira marismortui. Eur. J. Biochem. 198:593-598. [DOI] [PubMed] [Google Scholar]
- 14.Galinski, E. A. 1995. Osmoadaptation in bacteria. Adv. Microb. Physiol. 37:272-328. [PubMed] [Google Scholar]
- 15.García-Estepa, R., et al. 2006. The ectD gene, which is involved in the synthesis of the compatible solute hydroxyectoine, is essential for thermoprotection of the halophilic bacterium Chromohalobacter salexigens. J. Bacteriol. 188:3774-3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 17.Harishchandra, R. K., S. Wulff, G. Lentzen, T. Neuhaus, and H.-J. Galla. 2010. The effect of compatible solute ectoines on the structural organization of lipid monolayer and bilayer membranes. Biophys. Chem. 150:37-46. [DOI] [PubMed] [Google Scholar]
- 18.Kets, E. P., E. A. Galinski, M. de Wit, J. A. de Bont, and H. J. Heipieper. 1996. Mannitol, a novel bacterial compatible solute in Pseudomonas putida S12. J. Bacteriol. 178:6665-6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krahe, M., G. Antranikian, and H. Märkl. 1996. Fermentation of extremophilic microorganisms. FEMS Microbiol. Rev. 18:271-285. [Google Scholar]
- 20.Kurz, M., C. Montermann, and E. A. Galinski. 2007. A Pseudomonas stutzeri surprise: production of the compatible osmolyte hydroxyectoine, p. 195, abstr. PT015. Abstr. Ann. Conf. Assoc. Gen. Appl. Microbiol.
- 21.Kurz, M. 2008. Compatible solute influence on nucleic acids: many questions but few answers. Saline Systems 4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurz, M., A. Burch, B. Seip, S. E. Lindow, and H. Gross. 2010. Genomic-driven investigation of compatible solute biosynthesis pathways of Pseudomonas syringae pv. syringae and their contribution to water stress tolerance. Appl. Environ. Microbiol. 76:5452-5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lalucat, J., A. Bennasar, R. Bosch, E. García-Valdés, and N. J. Palleroni. 2006. Biology of Pseudomonas stutzeri. Microbiol. Mol. Biol. Rev. 70:510-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larsen, P. I., L. K. Sydnes, B. Landfald, and A. R. Strom. 1987. Osmoregulation in Escherichia coli by accumulation of organic osmolytes: betaines, glutamic acid, and trehalose. Arch. Microbiol. 147:1-7. [DOI] [PubMed] [Google Scholar]
- 25.Lentzen, G., and T. Schwarz. 2006a. Extremolytes: natural compounds from extremophiles for versatile applications. Appl. Microbiol. Biotechnol. 72:623-634. [DOI] [PubMed] [Google Scholar]
- 26.Lentzen, G., and T. Schwarz. 2006b. Kompatible Solute: Mikrobielle Herstellung und Anwendung, p. 355-371. In G. Antranikian (ed.), Angewandte Mikrobiologie. Springer-Verlag, Berlin, Germany.
- 27.Lippert, K., and E. A. Galinski. 1992. Enzyme stabilization by ectoine-type compatible solutes: protection against heating, freezing and drying. Appl. Microbiol. Biotechnol. 37:61-65. [Google Scholar]
- 28.Lo, C., C. A. Bonner, G. Xie, M. D'Souza, and R. A. Jensen. 2009. Cohesion group approach for evolutionary analysis of aspartokinase, an enzyme that feeds a branched network of many biochemical pathways. Microbiol. Mol. Biol. Rev. 73:594-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Louis, P., and E. A. Galinski. 1997. Characterization of genes for the biosynthesis of the compatible solute ectoine from Marinococcus halophilus and osmoregulated expression in Escherichia coli. Microbiology 143:1141-1149. [DOI] [PubMed] [Google Scholar]
- 30.Manzanera, M., S. Vilchez, and A. Tunnacliffe. 2004. High survival and stability rates of Escherichia coli dried in hydroxyectoine. FEMS Microbiol. Lett. 233:347-352. [DOI] [PubMed] [Google Scholar]
- 31.Norrander, J., T. Kempe, and J. Messing. 1983. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene 26:101-106. [DOI] [PubMed] [Google Scholar]
- 32.Oren, A. 1999. Bioenergetic aspects of halophilism. Microbiol. Mol. Biol. Rev. 63:334-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oren, A. 2002. Diversity of halophilic microorganisms: environments, phylogeny, physiology, and applications. J. Ind. Microbiol. Biotechnol. 28:56-63. [DOI] [PubMed] [Google Scholar]
- 34.Pocard, J. A., L. T. Smith, G. M. Smith, and D. Le Rudulier. 1994. A prominent role for glucosylglycerol in the adaptation of Pseudomonas mendocina SKB70 to osmotic stress. J. Bacteriol. 176:6877-6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reshetnikov, A. S., V. N. Khmelenina, and Y. A. Trotsenko. 2006. Characterization of the ectoine biosynthesis genes of haloalkalotolerant obligate methanotroph “Methylomicrobium alcaliphilum 20Z.” Arch. Microbiol. 184:286-297. [DOI] [PubMed] [Google Scholar]
- 36.Roberts, M. F. 2005. Organic compatible solutes of halotolerant and halophilic microorganisms. Saline Systems 1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosselló, R., E. Garcia-Valdes, J. Lalucat, and J. Ursing. 1991. Genotypic and phenotypic diversity of Pseudomonas stutzeri. Syst. Appl. Microbiol. 14:150-157. [Google Scholar]
- 38.Sauer, T., and E. Galinski. 1998. Bacterial milking: a novel bioprocess for production of compatible solutes. Biotechnol. Bioeng. 59:128. [DOI] [PubMed] [Google Scholar]
- 39.Schiraldi, C., C. Maresca, A. Catapano, E. A. Galinski, and M. De Rosa. 2006. High-yield cultivation of Marinococcus M52 for production and recovery of hydroxyectoine. Res. Microbiol. 157:693-699. [DOI] [PubMed] [Google Scholar]
- 40.Schubert, T., T. Maskow, D. Benndorf, H. Harms, and U. Breuer. 2007. Continuous synthesis and excretion of the compatible solute ectoine by a transgenic, nonhalophilic bacterium. Appl. Environ. Microbiol. 73:3343-3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith, L. T., and G. M. Smith. 1989. An osmoregulated dipeptide in stressed Rhizobium meliloti. J. Bacteriol. 171:4714-4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van-Thuoc, D., H. Guzmán, J. Quillaguamán, and R. Hatti-Kaul. 2010. High productivity of ectoines by Halomonas boliviensis using a combined two-step fed-batch culture and milking process. J. Biotechnol. 147:46-51. [DOI] [PubMed] [Google Scholar]
- 43.Vargas, C., et al. 2008. Unravelling the adaptation responses to osmotic and temperature stress in Chromohalobacter salexigens, a bacterium with broad salinity tolerance. Saline Systems 4:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan, Y., et al. 2008. Nitrogen fixation island and rhizosphere competence traits in the genome of root-associated Pseudomonas stutzeri A1501. Proc. Natl. Acad. Sci. U. S. A. 105:7564-7569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang, X. X., and P. B. Raniey. 2007. Genetic analysis of the histidine utilization (hut) genes in Pseudomonas fluorescens SBW25. Genetics 176:2165-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.