Abstract
Legionella spp. are frequently isolated in hospital water systems. Heat shock (30 min at 70°C) is recommended by the World Health Organization to control its multiplication. The aim of the study was to evaluate retrospectively the efficacy of heat treatments by using a flow cytometry assay (FCA) able to identify viable but nonculturable (VBNC) cells. The study included Legionella strains (L. pneumophila [3 clusters] and L. anisa [1 cluster]) isolated from four hot water circuits of different hospital buildings in Saint-Etienne, France, during a 20-year prospective surveillance. The strains recovered from the different circuits were not epidemiologically related, but the strains isolated within a same circuit over time exhibited an identical genotypic profile. After an in vitro treatment of 30 min at 70°C, the mean percentage of viable cells and VBNC cells varied from 4.6% to 71.7%. The in vitro differences in heat sensitivity were in agreement with the observed efficacy of preventive and corrective heating measures used to control water contamination. These results suggest that Legionella strains can become heat resistant after heating treatments for a long time and that flow cytometry could be helpful to check the efficacy of heat treatments on Legionella spp. and to optimize the decontamination processes applied to water systems for the control of Legionella proliferation.
Legionella spp. are widespread in natural and human-made aquatic habitats. Approximately one-third of Legionella species have been associated with a severe pneumonia in humans, Legionnaires’ disease (14, 32). Sources of contamination are aerosols from showerheads, spas, air-cooling towers, or other systems distributing hot water. In order to prevent outbreaks, surveillance of Legionella environmental contamination is recommended for hot sanitary water systems of collective settings such as hospitals, hotels, or thermal institutes (4). In France, environmental surveillance of hot water is mandatory for hospitals; to minimize the risk of Legionella infection, recommended concentrations of Legionella pneumophila must be at least under 1,000 CFU per liter and under the detection threshold (<250 CFU/liter) for immunocompromised patients. A disinfection is required for bacterial loads higher than 10,000 CFU/liter (13, 28). Many disinfection methods have been proposed to control Legionella proliferation in hot-water systems, including thermal treatments (delivery of water at 55°C or heat shocks at 70°C) and chemical procedures (continuous or shock chlorination and use of continuous chlorine dioxide, monochloramine, ozone, or aldehydes) (20). However, these disinfection procedures performed with hospital hot water systems (8, 10, 33) often have short-term efficacy, with recolonization occurring after only weeks or months. Indeed, L. pneumophila cell populations have been shown to survive as free organisms for long periods by maintaining metabolic activity but temporarily losing culturability under strict environmental conditions and requiring resuscitation by ingestion by amoebas (15, 25, 34). In a previous work using a flow cytometric assay (FCA) (2), we confirmed the existence of viable but not culturable (VBNC) Legionella cells (5, 26) in environmental samples.
The aim of this study was to evaluate the added value of FCA for estimating the efficacy of heat treatment procedures used for Legionella disinfection in water systems using strains collected prospectively during the environmental surveillance of the water circuits of a university hospital. For each collected strain, FCA profiles were obtained before and after different times of heat shock at 70°C. The results were compared to the decontamination procedures applied to the water circuits from which the strains had been recovered.
MATERIALS AND METHODS
Setting.
The University Hospital of Saint-Etienne is composed of several buildings located on two sites and supplied with hot water through independent circuits. From 1992 to 1994, a nosocomial legionellosis outbreak (6, 17) occurred due to water circuit contamination. At that time, there was no mandatory environmental surveillance of hospital hot water systems and no disinfection procedures. In 1995, an environmental surveillance procedure for the whole water system was set up. Sites and frequency of hot water sampling were defined for each building by the staff of the infection control unit and engineers, according to the complexity of the water system and the potential exposure of patients at risk. Hot water samples were collected from showers or hot tap water. The three water circuits of the University Hospital were designated circuits A to C. In 2005, a new hospital located on the same site was opened; its water circuit was designated circuit D. The pipes were made of copper for circuits A to C and mainly of cross-linked polyethylene and polyvinyl chloride for circuit D.
Legionella strain recovery.
Thirty-nine Legionella strains isolated from 1992 to 2010 and stored at −80°C in Cryobank tubes (Mast Diagnostic, Amiens, France) were used for this study. They were isolated and identified according to French Association Française de Normalisation (AFNOR) standard NF T90-431 (3, 19). Legionella colonies from frozen samples were recovered by plating onto BCYEα agar medium (buffered activated charcoal yeast extract medium, including Legionella charcoal yeast extract agar base and SR0110 supplement; Oxoid, Dardilly, France) and after incubation for 3 days at 36°C ± 2°C before the experiment.
AP-PCR typing.
The genomic diversities of the L. pneumophila serogroup 1 strains and of Legionella anisa were analyzed by arbitrarily primed PCR (AP-PCR) using primer Eric 2 (5′-AAGTAAGTGACTGGGGTGAGC-3′) and primer G (5′-GGTGGTGGCT-3′), as previously described (17).
Preparation of calibrated suspensions of Legionella colonies.
All suspensions were prepared by suspending Legionella colonies into sterile normal saline (0.9% NaCl) to achieve an optical density of 0.2 at 600 nm (Biomate TM3; Avantec, Illkirch, France), which corresponds to a final concentration of 108 CFU/ml.
In vitro heat shock treatments.
Calibrated suspensions of strains collected were diluted into sterile normal saline to 106 CFU/ml and then incubated in a water bath set at 70°C for 0, 10, 30, and 60 min.
Flow cytometric assay.
For the FCA, each strain was used at a concentration of 105 CFU/ml. FCA profiles were obtained by using a combination of two fluorescent dyes staining nucleic acids, Syto9 for cells with intact membranes and propidium iodide (PI) for cells with damaged membranes, as previously described (2). Flow cytometric measurements were performed by using a BD FACSCalibur instrument (Becton Dickinson Biosciences, Le Pont-de-Claix, France) equipped with an air-cooled argon laser (488-nm emission; 20 mW). The green fluorescent emission from Syto9 was collected in the fluorescence 1 (FL1) channel (500 to 5600 nm), and the red fluorescence from PI was collected in the FL3 channel (>670 nm). A threshold was applied onto the FL1 channel to eliminate background signals. Analyses were performed at a low-flow-rate setting. Results were analyzed with Cell Quest Pro software (Becton Dickinson Biosciences) as previously described (2).
RESULTS
Presentation of the different water circuits contaminated by Legionella.
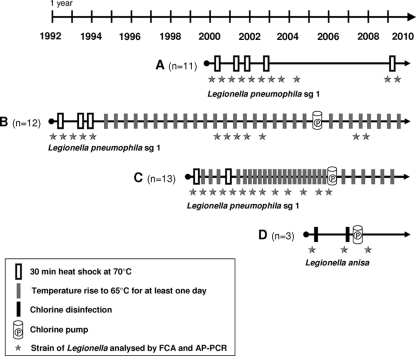
Figure 1 illustrates the follow-up of the Legionella contamination of the four water circuits taken into consideration in this study and the main control measures that were applied through time. Circuits A to C were found to be contaminated with Legionella pneumophila serogroup 1, whereas circuit D was contaminated with L. anisa. Circuit A illustrates the efficacy of punctual heat shock treatments leading to the drastic reduction of the Legionella load over time. In contrast, circuit C illustrates the inability of a rise of the temperature to control the Legionella reservoir, leading to the setup of a chlorine pump in 2006. In comparison to the two above-described circuits, circuit B represents an intermediate situation, with an initial control of Legionella growth by a combination of heat shock and temperature rise measures, followed by a resurgence of Legionella contamination after 2000, leading in 2005 to the setup of a chlorine pump. Circuit D, contaminated by L. anisa, was treated directly by chlorine disinfection because the pipe material, including polyvinyl chloride, did not support high temperatures.
FIG. 1.
Chronology of physical treatments applied to four hospital water circuits chronically contaminated with Legionella. Rectangles correspond to the different decontamination measures described. Cylinder figures indicate the initiation of a continuous treatment using a chlorine pump. The Legionella strains used in the study are represented by stars positioned at the time of their isolation.
AP-PCR and FCA patterns of Legionella strains.
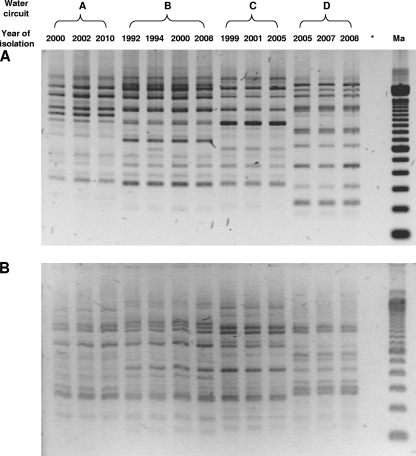
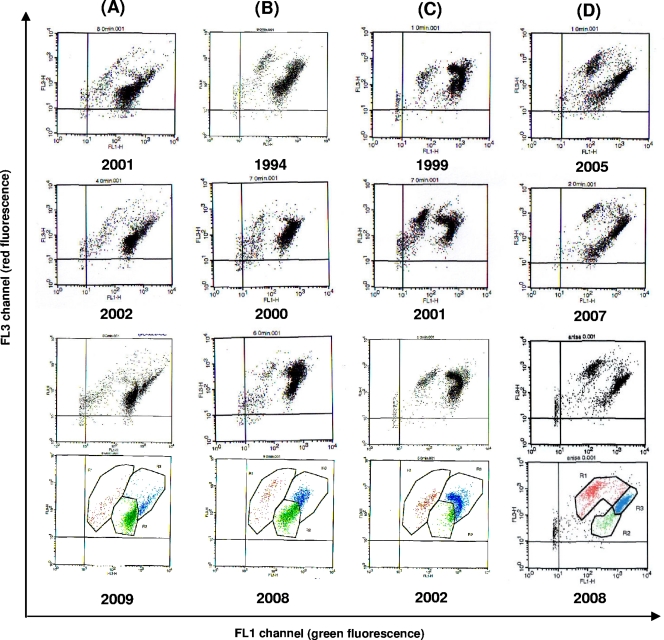
Legionella strains isolated from the four water circuits described above and illustrated by stars in Fig. 1 were used to study their temperature sensitivity through time by using FCA. First, in order to verify their clonal character, they were all tested by AP-PCR: strains from the same water circuit were shown to share the same profile, whereas each circuit was contaminated by a different clone (Fig. 2). These results demonstrate the persistence over time of the same Legionella strain in each independent circuit, even for a long period of time (at least 16 years for circuit B). For each strain displayed in Fig. 1, the respective percentages of viable and culturable (VC), viable but not culturable (VBNC), and dead (D) cells were then evaluated by FCA. The cytograms of representative strains are displayed in Fig. 3. Interestingly, as shown for the AP-PCR profiles, the distributions among the three cell categories (VC, VBNC, and D) were very similar between strains isolated over time from the same circuit but different from one circuit to another.
FIG. 2.
Representative AP-PCR profiles of strains of Legionella isolated from four different water circuits over an 18-year period with primers G (A) and Eric 2 (B). Ma, size marker; *, negative control.
FIG. 3.
Representative flow cytometry patterns of Legionella strains from circuits A, B, C (L. pneumophila sg1), and D (L. anisa) after 3 days of culture on BCYE medium. The number under each panel represents the year of isolation of the corresponding strain. Windows allowing determinations of viable (green points), VBNC (blue points), and dead (red points) cell percentages are depicted for strains recovered in 2009, 2008, 2002, and 2008 from circuits A, B, C, and D, respectively.
FCA analysis of Legionella susceptibility to temperature.
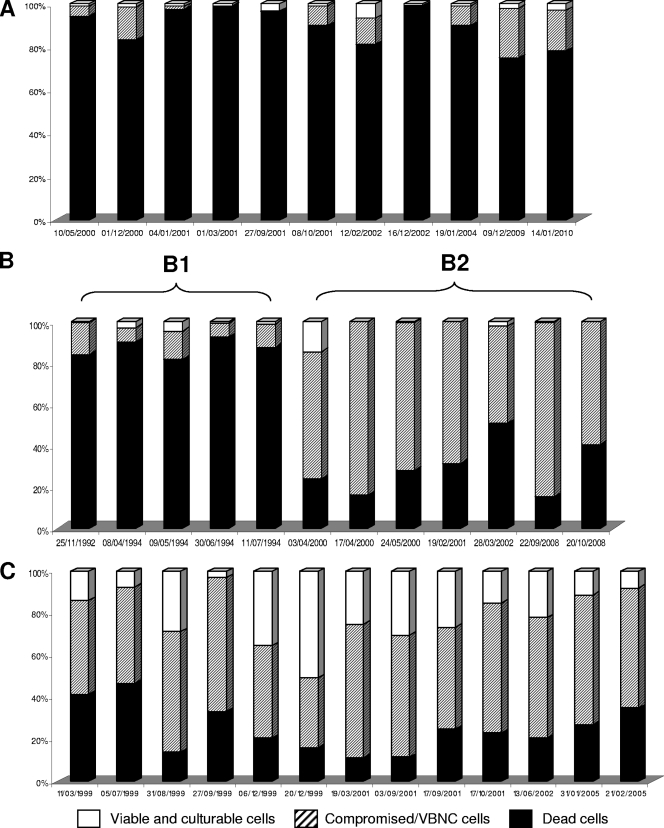
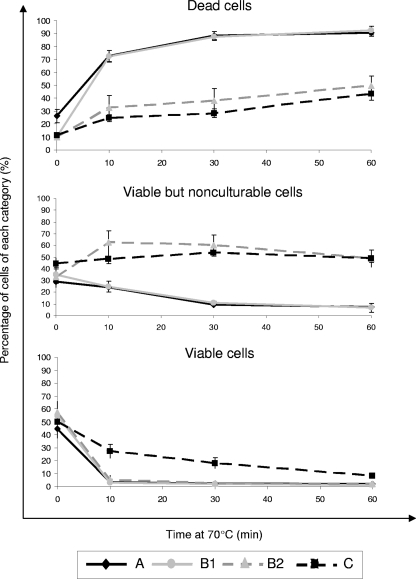
In order to analyze the temperature susceptibility of the Legionella strains shown in Fig. 1, each of them was submitted to a heat treatment at 70°C during 0, 10, 30, and 60 min. The respective percentages of VC, VBNC, and D cells were then determined by FCA. Figure 4 (individual patterns of each strain after 30 min at 70°C) and Fig. 5 (mean kinetics of inactivation at 70°C through time) illustrate the temperature susceptibility of the L. pneumophila strains isolated from circuits A to C. Cytometric analyses clearly discriminate two main profiles, a profile of high susceptibility to heating (more than 75% dead cells at 30 min), represented by all strains of circuit A and the first 5 strains of circuit B (cluster B1), and a profile of resistance to heating (less than 50% dead cells at 30 min), represented by all strains of circuit C and the 7 last strains of circuit B (cluster B2). It is worthwhile to note that, despite identical AP-PCR patterns (Fig. 2) and similar FCA profiles before heating (Fig. 3) over the entire study period, the reservoir of L. pneumophila from circuit B, which was submitted regularly to heating treatment, evolved from susceptible to resistant to heating over time. As shown in Fig. 4, the change occurred between 1994 and 2000; as no strain was kept frozen in the meantime, it was not possible to determine more precisely the moment at which this evolution took place. Table 1 synthesizes the pooled results for the heat susceptibilities of strains from each circuit before and after treatment at 70°C. As determined by FCA, the mean percentage of viable cells after 30 min at 70°C discriminates the two profiles well and is close to that obtained for viable cells after a heating time of 60 min. L. anisa strains recovered from circuit D exhibited a highly susceptible profile to heating (Table 1).
FIG. 4.
Resistance of Legionella strains to heat shock for 30 min at 70°C. Letters refer to the three water circuits contaminated by strains of L. pneumophila sg1. Bars correspond to the different strains presented in Fig. 1. For water circuit B, two successive heat resistance profiles were observed: cluster B1 for strains isolated from 1992 to 1994 and cluster B2 for strains isolated from 2000 to 2008.
FIG. 5.
Viability of L. pneumophila sg1 strains obtained by flow cytometry assay after treatment at 70°C for 0, 10, 30, and 60 min. Letters refer to the groups of strains described in the legend of Fig. 4. Results are expressed as means ± standard errors.
TABLE 1.
Measure of percentages of viable cells (VC and VBNC populations) by flow cytometry assay before and after treatment at 70°C
| Group of strainsa (Legionella species) | No. of strains | Mean % of viable cells (range) |
||
|---|---|---|---|---|
| Before treatment | After 30 min at 70°C | After 60 min at 70°C | ||
| A (L. pneumophila) | 11 | 73.9 (35.5-92.3) | 11.7 (0.8-32.4) | 9.1 (0.3-21.6) |
| B1 (L. pneumophila) | 5 | 89.9 (86.3-94.1) | 12.7 (7.3-18.4) | 7.7 (1.5-20.2) |
| B2 (L. pneumophila) | 7 | 90.2 (80.1-95.0) | 70.5 (49.1-84.7) | 57.5 (40.5-67.5) |
| C (L. pneumophila) | 13 | 84.4 (42.5-95.1) | 71.7 (41.8-88.7) | 59.9 (39.4-84.8) |
| D (L. anisa) | 3 | 70.9 (58.8-79.1) | 4.6 (1.4-11.8) | 2.4 (0.4-5.8) |
As defined in the legend of Fig. 4.
DISCUSSION
This study was conducted on water circuits from two French hospitals that were found to be chronically contaminated with Legionella species, from 5 years for circuit D to at least 18 years for circuit B (Fig. 1). The environmental surveillance was started following the occurrence of a nosocomial outbreak (6, 17), a few years before it became recommended by French legislation. The main hygienic measures taken to circumvent the contamination are depicted in Fig. 1. No further cases of hospital-acquired legionellosis have been recorded for a period of 18 years in our setting. These results plead for the usefulness of environmental surveillance, as recommended by several European guidelines (7, 9, 13) and in accordance with the results of the Allegheny County Health Department in the United States (1, 31). Convinced of the efficacy of environmental monitoring, we undertook the present study to evaluate FCA as a refined tool to measure Legionella susceptibility to heating in the context of our hospital setting.
FCA was first tested to analyze the relative distribution of cells of different viabilities within the same Legionella strain population in the absence of any treatment. It was concluded that FCA profiles were relatively similar for different strains contaminating the same circuit but different for strains isolated from distinct circuits (Fig. 3). Interestingly, these results were in accordance with AP-PCR typing data depicted in Fig. 2, using two different primers. Also, the clonal character of Legionella strains contaminating the same circuit was shown by previous works (12, 24, 27, 30). The present study documents water circuit contamination by the same strain of Legionella for a period of up to 18 years (circuit B).
The main objective of the study was to evaluate the ability of FCA to measure the susceptibility of Legionella strains recovered from the environment to heating treatment. The use of FCA was based on the fact that this technique is a performing tool to demonstrate the existence of VBNC in Legionella populations (2). By now, increasing the temperature is considered one of the best ways to control the contamination of water circuits by Legionella (21, 22, 35) and constitutes the rationale for several guidelines. The minimum temperature for Legionella thermal disinfection is 60°C, since the times required to obtain 1-log kill (90% reduction) at 45°C, 50°C, 60°C, and 70°C were 2,500, 380, <5, and < 1 min, respectively (18, 20-22). In contrast, one of the major findings of the present study was that by using FCA, some strains of Legionella submitted to superheating in the environment for a long time were shown to develop resistance to high temperatures. This phenomenon was demonstrated by the high proportion of culturable cells and not culturable but viable cells still present after a 30-min treatment at 70°C (Fig. 4 and 5 and Table 1). The percentage of VBNC Legionella cells in environmental samples is likely to be associated with variations of biotic or abiotic factors affecting the ecosystem in which Legionella agents proliferate (2, 15, 26, 33).
A further factor that fosters the survival and dissemination of Legionella in aquatic environments is the biofilm (23, 29). Even if it is not clear whether the pipe material (37) or the type of disinfection influences the development of a biofilm, it was demonstrated previously that the presence of a biofilm reduces the efficacy of disinfection treatments (36). For example, the incorporation of natural noncultivable L. pneumophila into potable-water biofilms provides a protective niche against chlorination stress (16). Moreover, if Legionella cells are present at a high density, they may communicate in a way that enables them to better survive within a stressful environment (25, 38). In close relation to our study and by using a similar approach, Chang et al. demonstrated recently that starvation enhances significantly the resistance of Legionella to superheating or chlorination (11). Those authors postulated that the stabilization of the cell membrane and/or the induction of proteins and other gene products may explain the resistance of starved strains to heat stress (11). Our results obtained by using FCA illustrate for the first time the ability of repetitive heat shocks to generate heat-resistant Legionella strains in the environment. The intensity of the heating treatment seems to act as a factor of selection pressure: whereas strains from circuit A or D, submitted to a low frequency of heat shocks (circuit A) or to chlorine treatment due to the material of the pipes (circuit D), were shown to remain susceptible to superheating through time, strains from circuit C, submitted to an intensive program of heating, became rapidly heat resistant. Circuit B offers an intermediate situation, since the same clone, initially highly susceptible to heating, became heat resistant after several years of intensive heat shock procedures (Fig. 1 and 4). Genotypic changes similar to those invoked by Chang et al. (11) may explain this spontaneous evolution. Additional experiments are in progress in our laboratory to document the in vitro acquisition of heat resistance by Legionella strains submitted to various stresses, including heat shock, chlorine treatment, or starvation in sterile tap water. Further studies should also be undertaken to elucidate the molecular mechanisms involved in the passage from heat susceptibility to heat resistance; actually, genetic analyses comparing clade B1 and B2 strains could help in an understanding of the phenotypic changes mentioned above.
From a practical point of view, this study indicates that FCA could be useful to rapidly evaluate the heat susceptibility of Legionella strains in order to optimize the measures used to control their proliferation. Actually, the determination of the proportions of VC and VBNC cells by FCA after a 30-min heating at 70°C (Fig. 4) could help to predict the effectiveness of thermal treatment of a water circuit contaminated by Legionella. Many years before the setup of a chlorine pump, this measure could have been a determinant for deciding for which circuits this preventive measure was necessary and the pursuit of repetitive heat shocks was useless. Before FCA may be recommended as an additional tool for the monitoring of the contamination of water circuits by Legionella, these observational data need to be confirmed for a larger number of environmental situations.
Acknowledgments
This project was supported financially in part by the Agence Française de Sécurité Sanitaire de l'Environnement et du Travail (AFSSET) and by Microbiodetection SARL (Commercy, France).
We are indebted to the following contributors of the University Hospital of Saint-Etienne: the medical and nursing staff of the infection control unit for the collection of samples and the monitoring of water circuit treatment; the engineers, notably François Chord, who conducted the control measures to decrease Legionella contamination of the water systems; and the technical staff of the Laboratory of Bacteriology-Virology-Hygiene for the isolation and collection of strains, particularly Julie Rissoan and Horia Tuzet, who performed the AP-PCR experiments. We also acknowledge Thibaut Epalle and Thomas Ros for skillful technical assistance.
Footnotes
Published ahead of print on 23 December 2010.
REFERENCES
- 1.Allegheny County Health Department. 1997. Approaches to prevention and control of Legionella infection in Allegheny County health care facilities, 2nd ed. Allegheny County Health Department, Pittsburgh, PA.
- 2.Allegra, S., et al. 2008. Use of flow cytometry to monitor Legionella viability. Appl. Environ. Microbiol. 74:7813-7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Association Française de Normalisation. 2003. Water quality—detection and enumeration of Legionella spp and Legionella pneumophila. Method by direct culture inoculation and after concentration by membrane filtration or centrifugation, NF T90-431. Association Française de Normalisation, Paris, France.
- 4.Atlas, R. M. 1999. Legionella: from environmental habitats to disease pathology, detection and control. Environ. Microbiol. 1:283-293. [DOI] [PubMed] [Google Scholar]
- 5.Barer, M. R., and C. R. Harwood. 1999. Bacterial viability and culturability. Adv. Microb. Physiol. 41:93-137. [DOI] [PubMed] [Google Scholar]
- 6.Berthelot, P., F. Grattard, A. Ros, F. Lucht, and B. Pozzetto. 1998. Nosocomial legionellosis outbreak over a three-year period: investigation and control. Clin. Microbiol. Infect. 4:385-391. [DOI] [PubMed] [Google Scholar]
- 7.Best, M., et al. 1983. Legionellaceae in the hospital water-supply. Epidemiological link with disease and evaluation of a method for control of nosocomial Legionnaires’ disease and Pittsburgh pneumonia. Lancet ii:307-310. [DOI] [PubMed] [Google Scholar]
- 8.Blanc, D. S., P. Carrara, G. Zanetti, and P. Francioli. 2005. Water disinfection with ozone, copper and silver ions, and temperature increase to control Legionella: seven years of experience in a university teaching hospital. J. Hosp. Infect. 60:69-72. [DOI] [PubMed] [Google Scholar]
- 9.Boccia, S., et al. 2006. Prospective 3-year surveillance for nosocomial and environmental Legionella pneumophila: implications for infection control. Infect. Control Hosp. Epidemiol. 27:459-465. [DOI] [PubMed] [Google Scholar]
- 10.Casari, E., A. Ferrario, and A. Montanelli. 2007. Prolonged effect of two combined methods for Legionella disinfection in a hospital water system. Ann. Ig. 19:525-532. [PubMed] [Google Scholar]
- 11.Chang, C. W., Y. H. Hwang, W. Y. Cheng, and C. P. Chang. 2007. Effects of chlorination and heat disinfection on long-term starved Legionella pneumophila in warm water. J. Appl. Microbiol. 102:1636-1644. [DOI] [PubMed] [Google Scholar]
- 12.Darelid, J., S. Bernander, K. Jacobson, and S. Lofgren. 2004. The presence of a specific genotype of Legionella pneumophila serogroup 1 in a hospital and municipal water distribution system over a 12-year period. Scand. J. Infect. Dis. 36:417-423. [DOI] [PubMed] [Google Scholar]
- 13.European Working Group for Legionella Infections. 2005. European guidelines for control and prevention of travel associated Legionnaires’ disease. European Working Group for Legionella Infections, London, United Kingdom. http://www.ewgli.org/.
- 14.Fry, N. K., et al. 2007. Identification of Legionella spp. by 19 European reference laboratories: results of the European Working Group for Legionella Infections External Quality Assessment Scheme using DNA sequencing of the macrophage infectivity potentiator gene and dedicated online tools. Clin. Microbiol. Infect. 13:1119-1124. [DOI] [PubMed] [Google Scholar]
- 15.Garcia, M. T., S. Jones, C. Pelaz, R. D. Millar, and Y. Abu Kwaik. 2007. Acanthamoeba polyphaga resuscitates viable non-culturable Legionella pneumophila after disinfection. Environ. Microbiol. 9:1267-1277. [DOI] [PubMed] [Google Scholar]
- 16.Giao, M. S., S. Wilks, N. F. Azevedo, M. J. Vieira, and C. W. Keevil. 2009. Incorporation of natural uncultivable Legionella pneumophila into potable water biofilms provides a protective niche against chlorination stress. Biofouling 25:335-341. [DOI] [PubMed] [Google Scholar]
- 17.Grattard, F., et al. 1996. Molecular typing of nosocomial strains of Legionella pneumophila by arbitrarily primed PCR. J. Clin. Microbiol. 34:1595-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hruba, L. 2009. The colonization of hot water systems by Legionella. Ann. Agric. Environ. Med. 16:115-119. [PubMed] [Google Scholar]
- 19.International Organization for Standardization. 1998. Water quality—detection and enumeration of Legionella, ISO-11731. International Organization for Standardization, Geneva, Switzerland.
- 20.Kim, B. R., J. E. Anderson, S. A. Mueller, W. A. Gaines, and A. M. Kendall. 2002. Efficacy of various disinfectants against Legionella in water systems. Water Res. 36:4433-4444. [DOI] [PubMed] [Google Scholar]
- 21.Lin, Y. S., J. E. Stout, V. L. Yu, and R. D. Vidic. 1998. Disinfection of water distribution systems for Legionella. Semin. Respir. Infect. 13:147-159. [PubMed] [Google Scholar]
- 22.Muraca, P., J. E. Stout, and V. L. Yu. 1987. Comparative assessment of chlorine, heat, ozone, and UV light for killing Legionella pneumophila within a model plumbing system. Appl. Environ. Microbiol. 53:447-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murga, R., et al. 2001. Role of biofilms in the survival of Legionella pneumophila in a model potable-water system. Microbiology 147:3121-3126. [DOI] [PubMed] [Google Scholar]
- 24.Oberdorfer, K., G. Mussigbrodt, and C. Wendt. 2008. Genetic diversity of Legionella pneumophila in hospital water systems. Int. J. Hyg. Environ. Health 211:172-178. [DOI] [PubMed] [Google Scholar]
- 25.Ohno, A., N. Kato, K. Yamada, and K. Yamaguchi. 2003. Factors influencing survival of Legionella pneumophila serotype 1 in hot spring water and tap water. Appl. Environ. Microbiol. 69:2540-2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliver, J. D. 2005. The viable but nonculturable state in bacteria. J. Microbiol. 43(Spec. No.):93-100. [PubMed] [Google Scholar]
- 27.Rangel-Frausto, M. S., et al. 1999. Persistence of Legionella pneumophila in a hospital's water system: a 13-year survey. Infect. Control Hosp. Epidemiol. 20:793-797. [DOI] [PubMed] [Google Scholar]
- 28.Ricketts, K. D., and C. A. Joseph. 2006. The impact of new guidelines in Europe for the control and prevention of travel-associated Legionnaires’ disease. Int. J. Hyg. Environ. Health 209:547-552. [DOI] [PubMed] [Google Scholar]
- 29.Saby, S., A. Vidal, and H. Suty. 2005. Resistance of Legionella to disinfection in hot water distribution systems. Water Sci. Technol. 52:15-28. [PubMed] [Google Scholar]
- 30.Scaturro, M., I. Dell'eva, F. Helfer, and M. L. Ricci. 2007. Persistence of the same strain of Legionella pneumophila in the water system of an Italian hospital for 15 years. Infect. Control Hosp. Epidemiol. 28:1089-1092. [DOI] [PubMed] [Google Scholar]
- 31.Squier, C. L., et al. 2005. A proactive approach to prevention of health care-acquired Legionnaires’ disease: the Allegheny County (Pittsburgh) experience. Am. J. Infect. Control 33:360-367. [DOI] [PubMed] [Google Scholar]
- 32.Steinert, M., K. Heuner, C. Buchrieser, C. Albert-Weissenberger, and G. Glockner. 2007. Legionella pathogenicity: genome structure, regulatory networks and the host cell response. Int. J. Med. Microbiol. 297:577-587. [DOI] [PubMed] [Google Scholar]
- 33.Steinert, M., G. Ockert, C. Luck, and J. Hacker. 1998. Regrowth of Legionella pneumophila in a heat-disinfected plumbing system. Zentralbl. Bakteriol. 288:331-342. [DOI] [PubMed] [Google Scholar]
- 34.Storey, M. V., J. Winiecka-Krusnell, N. J. Ashbolt, and T. A. Stenstrom. 2004. The efficacy of heat and chlorine treatment against thermotolerant Acanthamoebae and Legionellae. Scand. J. Infect. Dis. 36:656-662. [DOI] [PubMed] [Google Scholar]
- 35.Stout, J. E., M. G. Best, and V. L. Yu. 1986. Susceptibility of members of the family Legionellaceae to thermal stress: implications for heat eradication methods in water distribution systems. Appl. Environ. Microbiol. 52:396-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tachikawa, M., M. Tezuka, M. Morita, K. Isogai, and S. Okada. 2005. Evaluation of some halogen biocides using a microbial biofilm system. Water Res. 39:4126-4132. [DOI] [PubMed] [Google Scholar]
- 37.van der Kooij, D., H. R. Veenendaal, and W. J. Scheffer. 2005. Biofilm formation and multiplication of Legionella in a model warm water system with pipes of copper, stainless steel and cross-linked polyethylene. Water Res. 39:2789-2798. [DOI] [PubMed] [Google Scholar]
- 38.Withers, H., S. Swift, and P. Williams. 2001. Quorum sensing as an integral component of gene regulatory networks in Gram-negative bacteria. Curr. Opin. Microbiol. 4:186-193. [DOI] [PubMed] [Google Scholar]