Abstract
Reducing the available water in food is a long-established method for controlling bacterial growth in the food industry. Nevertheless, food-borne outbreaks of salmonellosis due to consumption of dry foods have been continuously reported. Previous studies showed that dried Salmonella cells acquire high tolerance to heat and ethanol. In order to examine if dehydration also induces tolerance to other stressors, dried Salmonella enterica serotype Typhimurium cells were exposed to multiple stresses, and their viability was assessed. Indeed, desiccated S. Typhimurium acquired higher tolerance to multiple stressors than nondesiccated cells. The dried cells were significantly more resistant to most stressors, including ethanol (10 to 30%, 5 min), sodium hypochlorite (10 to 100 ppm, 10 min), didecyl dimethyl ammonium chloride (0.05 to 0.25%, 5 min), hydrogen peroxide (0.5 to 2.0%, 30 min), NaCl (0.1 to 1 M, 2 h), bile salts (1 to 10%, 2 h), dry heat (100°C, 1 h), and UV irradiation (125 μW/cm2, 25 min). In contrast, exposure of Salmonella to acetic and citric acids reduced the survival of the dried cells (1.5 log) compared to that of nondesiccated cells (0.5 log). Three other S. enterica serotypes, S. Enteritidis, S. Newport, and S. Infantis, had similar stress responses as S. Typhimurium, while S. Hadar was much more susceptible and gained tolerance to only a few stressors. Our findings indicate that dehydration induces cross-tolerance to multiple stresses in S. enterica, demonstrating the limitations of current chemical and physical treatments utilized by the food industry to inactivate food-borne pathogens.
Salmonella enterica is a common food-borne pathogen which causes high morbidity and economic losses worldwide. International and national outbreaks of salmonellosis linked to consumption of contaminated food are frequently reported. Salmonella is an environmentally persistent pathogen capable of surviving and proliferating in diverse niches, including many environmental ecosystems, such as food production and processing plants and the intestinal tracts of host animals (19, 47, 48, 51). Most outbreaks of salmonellosis result from consumption of animal products, such as meat, eggs, and poultry (4, 47). Due to the ability of the pathogen to survive for prolonged periods on stainless steel, glass, and plastic surfaces, the risk of cross-contamination via such surfaces has also been recognized (14, 26, 34). Reducing the available water in food and on surfaces is a long-established method for controlling bacterial growth (16). However, some large international salmonellosis outbreaks have been associated with foods that have low water content, such as chocolate, snacks, almonds, peanut butter, paprika, and dried infant milk formulas (5, 28, 32, 35, 40, 44, 50). In fact, only recently, red and black dry pepper were involved in a multistate outbreak in the United States (17, 18). Salmonella has occasionally been detected in granola, pistachios, and corn flakes (6, 7, 8), supporting the notion that the pathogen can persist in dry industrially processed food items.
Considering the fact that the vast majority of these products undergo thermal processing before packaging, it has been suggested that there is a correlation between the low water content of the product and the increased thermal resistance of the pathogen (9, 21, 24, 39, 43). Using laboratory models, it was demonstrated that reducing water activity either by desiccation (24, 31) or by addition of solutes (2, 12, 30, 37, 38) resulted in increased thermal tolerance. Furthermore, low-water-activity medium (30) and dry environment (24) were found to enhance Salmonella tolerance to sodium hypochlorite and ethanol, respectively.
Although exposure to a single stressor is commonly associated with the development of cross-tolerance to other stressors (1, 11, 13, 26, 33), data on the development of cross-tolerance in Salmonella following desiccation are still scarce. In the present study, we have examined the potential cross-tolerance of desiccated S. enterica serovar Typhimurium to a wide range of stressors that the pathogen may encounter in the food-processing environment or inside the host.
MATERIALS AND METHODS
Bacterial strains and inoculum preparation.
Salmonella enterica serotypes Enteritidis, Hadar, and Infantis were clinical isolates provided by the Israeli Ministry of Health (Central Laboratories, Jerusalem, Israel). S. enterica serotypes Newport and Typhimurium (SL1344 Smr) were provided by M. Brandl (USDA, ARS, WRRC). The clinical isolates were chosen because they are among the serotypes most frequently isolated in Israel, while the S. Newport strain was originally isolated from alfalfa seeds following an outbreak of salmonellosis (27). S. Typhimurium (SL1344) is commonly used in numerous studies, and its use therefore enables comparison of the results among different laboratories.
Cultures were prepared by inoculation of a single colony grown on xylose-lysine-deoxycholate (XLD) agar (Difco Laboratories, Sparks, MD) into 5 ml of Luria-Bertani (LB) broth (Lennox; Difco Laboratories) and incubation overnight at 37°C with aeration. Cells were washed three times in sterile deionized water (SDW) by centrifugation (3,800 × g, 5 min) at room temperature, and the final pellet was resuspended in 10 ml SDW to a concentration of about 2.0 × 109 CFU/ml.
Preparation of desiccated bacteria.
Aliquots (50 μl) containing 108 CFU of washed Salmonella cell were placed into each well of a 96-well polystyrene plate (Greiner Bio-One, Frickenhausen, Germany) and air dried in a biosafety cabinet for 22 h at 25°C under ca. 40% relative humidity. These conditions were chosen because measurements of water activity (aw) (see below) revealed maximal dehydration at 20 h, where the aw level reached a value of 0.53. No substantial changes in aw occurred following dehydration for an additional length of time.
For analysis of viable counts following desiccation, 100 μl of SDW was added to each well and the plates were incubated for 5 min at room temperature, before the bacteria were resuspended by pipetting (10 times). Bacterial suspensions were serially diluted (1:10) and plated on LB agar, and colony counts were recorded following incubation for 24 h at 37°C. For heat challenge experiments, desiccation was carried out in capped, flat-bottom glass vials under the same conditions described above. Enumeration of viable bacteria was performed following resuspension of the cells in 1 ml SDW for 5 min at room temperature and serial dilution, as described above.
aw measurement.
To measure the aw of the drying bacterial cells through the desiccation period, a 96-well plate was cut into several pieces, with each piece containing 4 intact wells. Inoculated wells were placed into an aw meter (model ms1; Novasina, Lahen, Switzerland), and measurements were performed in triplicate (3 pieces) every 2 h during 26 h of dehydration. Additional measurements were performed after 36 and 48 h.
Preparation of nondesiccated bacteria.
Two milliliters of washed bacterial suspension (2.0 × 109 CFU/ml) was incubated for 22 h at 25°C in a 15-ml polypropylene screw-cap tube (Labcon, Petaluma, CA). Under these conditions, essentially no evaporation occurred. Enumeration of bacteria was performed by plating serial dilutions, as described above. Prior to each challenge, the bacterial suspension was aliquoted (50 μl per well) into a 96-well plate. For heat challenge, 1 ml bacterial suspension (1.0 × 108 CFU) was placed in glass vials.
Challenge experiments.
Desiccated Salmonella cells in 96-well plates were resuspended in 50 μl of SDW and immediately mixed with 50 μl of double-strength stressor solution to achieve the indicated concentration in a final volume of 100 μl. Nondesiccated cells (50 μl) were similarly treated with 50 μl of stressor solution. The cells were then incubated at room temperature in the presence of the following stressors for the indicated times: (i) 0.1 to 1 M NaCl for 2 h (Bio Lab Ltd., Jerusalem, Israel), (ii) 1 to 10% bile salts (oxgall) for 2 h (Difco Laboratories), (iii) 10 to 40% ethanol for 5 min (Bio Lab Ltd.), (iv) 0.5 to 2% hydrogen peroxide for 30 min (Merck, Darmstadt, Germany), (v) 10 to 100 ppm sodium hypochlorite for 10 min (Johnson Diversey Israel, Yavne, Israel), (vi) 0.05 to 0.25% didecyl dimethyl ammonium chloride (DDAC) for 5 min (Johnson Diversey Israel), (vii) citric acid (50 mM, pH 3.0) for 2 h (Bio Lab Ltd.), and (viii) acetic acid (80 mM pH 3.0) for 2 h (Bio Lab Ltd.).
The selected stressors and challenge conditions were chosen on the basis of findings from preliminary studies (NaCl; data not shown), as well as previous publications (bile salts, ethanol, hydrogen peroxide, organic acids, and sodium hypochlorite) (3, 24, 29, 42, 46), except that for ethanol, hydrogen peroxide, and organic acids, the exposure time was extended. DDAC treatment was performed under the conditions recommended by the manufacturer for decontamination of surfaces (Johnson Diversey Israel).
Following exposure, Salmonella cells were promptly neutralized by 10-fold serial dilution with SDW, and viable bacteria were enumerated, as described above. Sodium hypochlorite was neutralized by adding 200 μl of 0.1 M Na2S2O3 for 30 s, before serial dilution, while DDAC was neutralized by 200 μl of 3.0% polysorbate 80, 3.0% saponin, 0.3% lecithin, 0.1% l-histidine, 0.5% Na2S2O3, 0.1% tryptone, and 0.85% sodium chloride, as described by the manufacturer (Johnson Diversey Israel). In other experiments, 96-well plates containing desiccated and nondesiccated bacteria were exposed to UV irradiation (125 μW/cm2) for 25 min.
For heat challenge experiments, the following treatment sets were used: (i) desiccated bacteria, (ii) desiccated bacteria that were rehydrated for 5 min in 1 ml SDW prior to challenge, and (iii) nondesiccated cells (1 ml). Bacteria were placed in tightly capped flat-bottom glass vials and then exposed to dry heat (60 to 100°C) for 1 h in an air oven (M.R.C. Ltd., Holon, Israel). The temperature inside the vials reached the target temperatures of 60, 80, and 100°C within 9, 12, and 15 min, respectively. In a separate set of experiments, the inactivation kinetics of Salmonella at 100°C were examined for up to 60 min. Following heat treatment, the vials were cooled on ice for 5 min, the desiccated bacteria were resuspended in 1 ml SDW, and viable counts were determined.
To examine the effect of acid prechallenge on survival during desiccation stress, Salmonella cells were incubated in citric acid (50 mM, pH 3.0) for 1 h, as described above, except that the incubation was performed in 1.7-ml polypropylene microtubes (Axygen, Union City, CA). After exposure, the cells were washed three times and resuspended in SDW. The washed acid-exposed bacteria were desiccated in 96-well plates and enumerated, as described above. Control experiments included preexposure of cells to SDW instead of acid.
Statistical analysis.
Survival was calculated as the mean percentage of the inoculum (considered 100%) or as the absolute number of surviving cells. All experiments were performed in triplicate and were repeated independently at least twice on different days. Comparisons between survival percentages and viable counts were made by one-way analysis of variance (ANOVA) and the Tukey-Kramer multiple-comparisons test using Instat (version 3) software (GraphPad Software Inc., La Jolla, CA). Differences were considered significant for P values of <0.01.
RESULTS
Survival of Salmonella following desiccation.
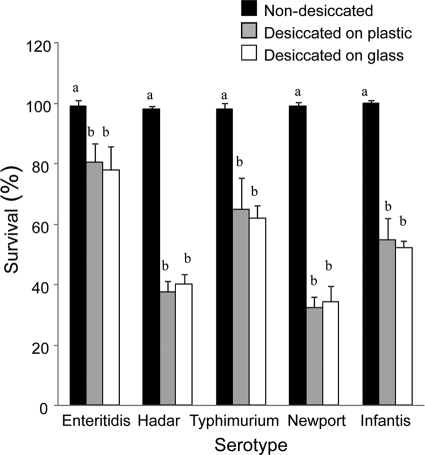
S. enterica cells were dried on 96-well plates and on glass surfaces, and the number of surviving cells was recorded. The rates of survival of the different serotypes varied, with S. Enteritidis having the highest survival value (80% ± 9%) and S. Newport having the lowest (36% ± 3%). Survival of control (nondesiccated) cells incubated in SDW for the same period of time was ∼98% for all strains (Fig. 1). No significant differences were observed among the two tested surfaces.
FIG. 1.
Survival of dehydrated and nondehydrated S. enterica serotypes on polystyrene and glass surface. Bars represent the average percentage of surviving cells (+ standard deviation) in three independent experiments. For each serovar, means followed by different letters indicate a significant difference between desiccated and nondesiccated cells (P < 0.01).
Exposure to NaCl and bile salts.
Salmonella was exposed to increasing concentrations of NaCl and bile salts for 2 h (Fig. 2). Higher survival percentages were observed for desiccated cells than for nondesiccated cells. Furthermore, the desiccated cells were able to maintain their viability in 1 to 5% bile salts and 0.1 to 0.5 M NaCl, while the number of the nondesiccated cells continuously declined in a dose-dependent manner.
FIG. 2.
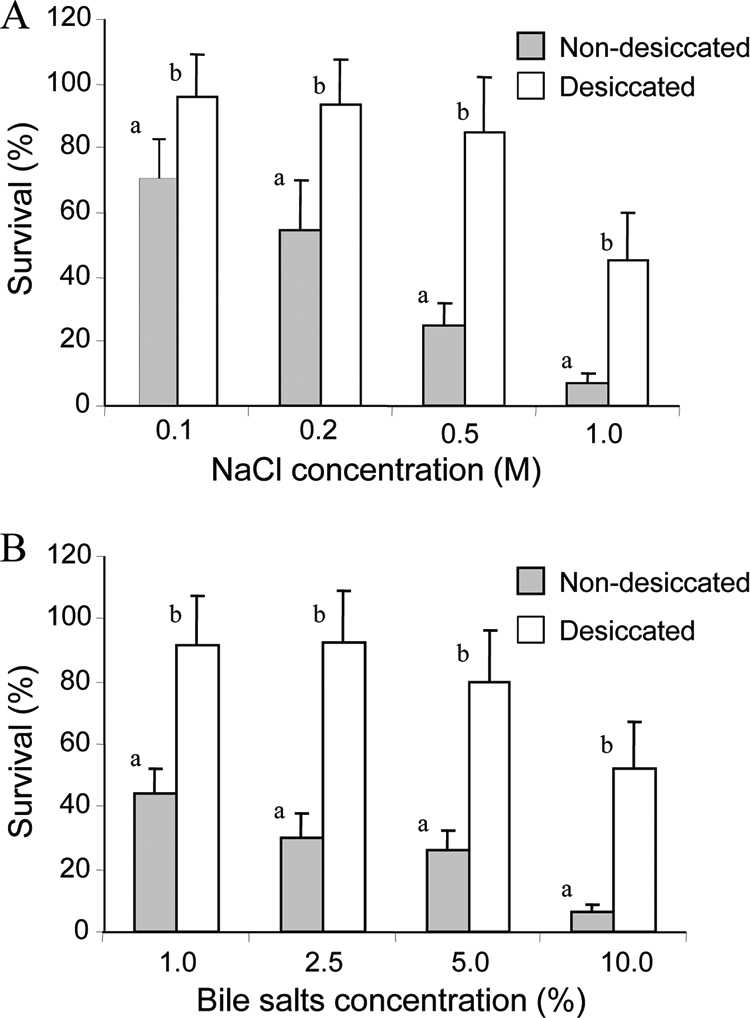
Survival of S. Typhimurium following exposure to increasing concentrations of NaCl (A) and bile salts (B). Bacterial cells were exposed to each salt for 2 h. The bars represent the average percentage of surviving cells (+ standard deviation) in three independent experiments. For each treatment, means followed by different letters indicate significant differences between desiccated and nondesiccated cells (P < 0.01).
Exposure to various disinfecting agents.
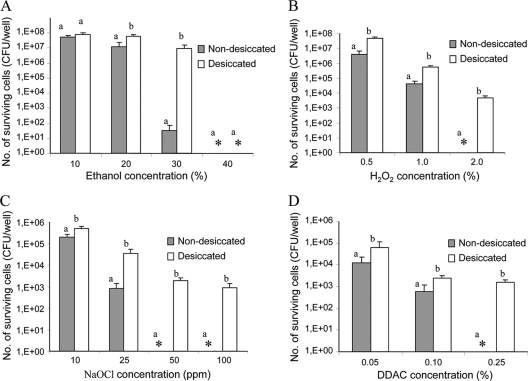
Salmonella cells were exposed to increasing concentrations of ethanol, hydrogen peroxide, sodium hypochlorite, and quaternary ammonium chloride (DDAC) for the indicated times (see “Challenge experiments” above; Fig. 3). Desiccated cells demonstrated significantly higher tolerance to all the disinfectants. Moreover, desiccated cells still survived under conditions where nondesiccated cells were undetectable, such as exposure to 2% hydrogen peroxide for 30 min, 50 to 100 ppm sodium hypochlorite for 5 min, and 0.25% DDAC for 5 min. Desiccated cells survived exposure to 30% ethanol for 5 min, with only a 1.0-log reduction, whereas nondesiccated cells showed a 6.5-log decrease. However, both populations' numbers declined below the detection limit (100 CFU) following exposure to 40% ethanol for 5 min.
FIG. 3.
Effects of disinfecting agents on the survival of desiccated and nondesiccated S. Typhimurium. Bacterial cells were exposed to different concentrations of ethanol for 5 min (A), hydrogen peroxide for 30 min (B), sodium hypochlorite for 10 min (C), and DDAC for 5 min (D). The average numbers (+ standard deviations) of surviving cells from at least two independent experiments are presented. For each treatment, means followed by different letters indicate a significant difference between desiccated and nondesiccated cells (P < 0.01). Asterisks, Salmonella counts below the detection limit (for H2O2 and ethanol, <100 CFU; for NaHOCl3 and DDAC, <30 CFU).
Effects of organic acids.
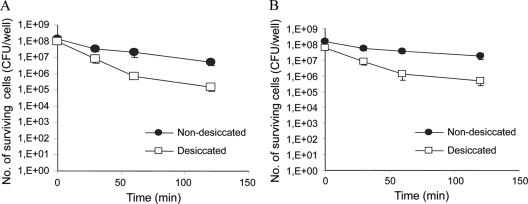
In contrast to the effects of all other stressors, exposure of Salmonella to acetic or citric acid at pH 3.0 had the opposite effect on desiccated cells. In both cases, desiccated cells were more susceptible than nondesiccated cells to these acidic conditions (Fig. 4).
FIG. 4.
Effects of organic acids on survival of desiccated and nondesiccated S. Typhimurium. The cells were exposed for 30 to 120 min to 50 mM citric acid (A) or 80 mM acetic acid (B), both at pH 3.0. The average numbers (+ standard deviations) of surviving cells from at least two independent experiments are presented.
Effect of UV irradiation.
Exposure to UV irradiation (125 μW/cm2) for 25 min resulted in complete eradication of nondesiccated cells, whereas a 3-log reduction in survival occurred for desiccated cells (Fig. 5).
FIG. 5.
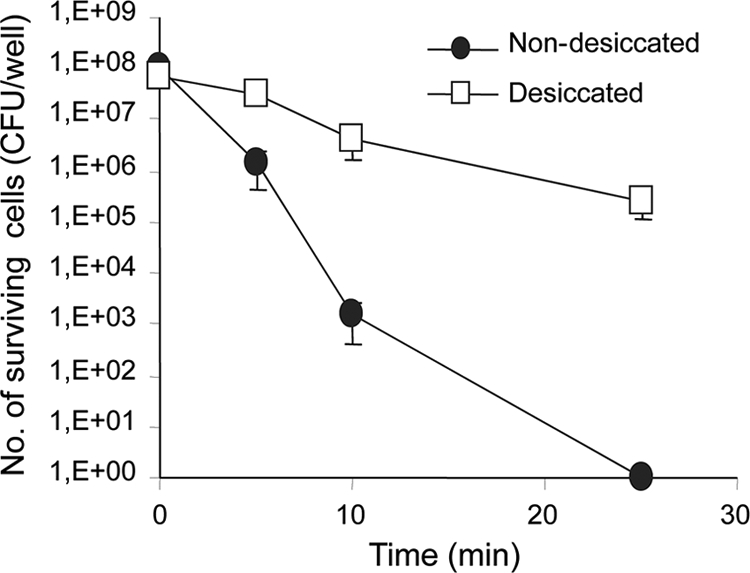
Effect of UV irradiation on survival of desiccated and nondesiccated S. Typhimurium. The cells were exposed for 5 to 25 min to UV irradiation (125 μW/cm2). The average numbers (+ standard deviations) of surviving cells from three independent experiments are presented.
Effect of dry heat.
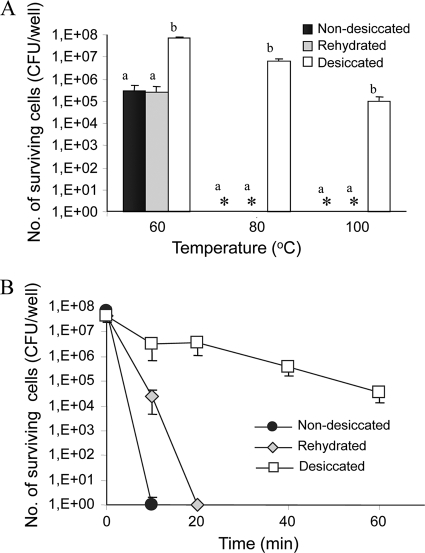
Desiccated cells have demonstrated high tolerance to a 1-h exposure to dry heat, with no substantial change in their viable counts at 60°C compared to their initial, prechallenge count and 1.5- and 3.1-log reductions at 80 and 100°C, respectively. In contrast, nondesiccated cells were highly susceptible, with as much as a 3-log reduction occurring at 60°C and an 8-log reduction (under the detection limit) occurring at 80 and 100°C. To examine if desiccated bacteria could still maintain heat tolerance after rehydration, desiccated cells were rehydrated with SDW immediately before exposure to heat. No significant difference was found between rehydrated and nondesiccated bacteria (Fig. 6A). Since exposure to 80 and 100°C for 1 h resulted in complete eradication of nondesiccated Salmonella, we have also investigated the killing kinetics at 100°C. While nondesiccated cells were completely inactivated within 10 min and rehydrated cells were killed after 20 min, the desiccated cells remained viable, with only a 3-log CFU reduction occurring at 60 min (Fig. 6B).
FIG. 6.
Effect of desiccation on thermal tolerance of S. Typhimurium. Desiccated, nondesiccated, and rehydrated cells were exposed to temperatures of 60, 80, and 100°C for 1 h (A) or to 100°C for up to 60 min (B). The average numbers of surviving cells (+ standard deviations) from three independent experiments are presented. For each temperature, means followed by different letters indicate a significant difference between desiccated and nondesiccated cells (P < 0.01). Asterisks, Salmonella counts below the detection limit (<10 CFU).
Effect of preexposure to organic acid on tolerance to desiccation.
Salmonella cells prechallenged with citric acid (50 mM, pH 3.0) for 1 h have a survival pattern similar to that of SDW-exposed cells (control). Acid preexposure was more detrimental than SDW to bacterial survival following desiccation (Fig. 7).
FIG. 7.
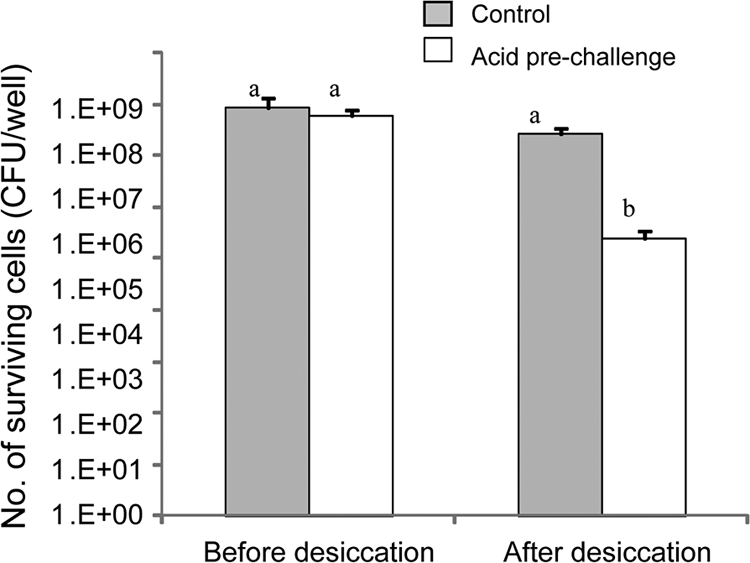
Effect of preexposure to organic acid on tolerance of S. Typhimurium to desiccation. Bacterial cells were exposed to 50 mM citric acid at pH 3.0 for 1 h prior to desiccation. Cells incubated in SDW were used as a control. The average numbers (+ standard deviations) of surviving cells from three independent experiments are presented. Means followed by different letters indicate a significant difference between desiccated and nondesiccated cells (P < 0.01).
Effect of desiccation on cross-tolerance in other Salmonella serotypes.
To examine whether the cross-tolerance phenomenon is unique to S. Typhimurium strain SL1344, the responses of serotypes S. Enteritidis, S. Hadar, S. Infantis, and S. Newport to the same stressors were tested (Table 1). Similar to S. Typhimurium, desiccation significantly (P < 0.01) enhanced the tolerance of all four serotypes to NaCl (1 M, 2 h) bile salts (10%, 2 h), ethanol (30%, 5 min), dry heat (100°C, 1 h), and UV irradiation (125 μW/cm2, 25 min). Except for Salmonella serotype Hadar, desiccated cells of all strains survived exposure to sodium hypochlorite (100 ppm, 5 min), hydrogen peroxide (2%, 5 min), and DDAC (0.25%, 5 min), with 4- to 6-log CFU reductions, while nondesiccated cells reached undetectable levels (>7.0-log CFU reduction) under these conditions. Exposure of all strains to citric acid (50 mM, pH 3.0) resulted in significantly (P < 0.01) reduced viability (0.6- to 2.3-log CFU reductions) for desiccated cells compared to that for nondesiccated bacteria (0.2- to 0.6-log reductions), as was previously demonstrated for S. Typhimurium (Fig. 4A).
TABLE 1.
Mean reductions of desiccated and nondesiccated Salmonella serovars exposed to different stressorsa
| Salmonella serotype | Mean log CFU reduction (SD) |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NaCl |
Bile |
Ethanol |
H2O2 |
DDAC |
NaOCl |
Dry heat |
UV |
Citric acid |
||||||||||
| + | − | + | − | + | − | + | − | + | − | + | − | + | − | + | − | + | − | |
| Enteritidis | 0.4aA (0.2) | 1.0bA (0.1) | 0.7aA (0.1) | 1.6bA (0.3) | 2.0aA (0.3) | 3.4bA (0.2) | 5.5aA (0.2) | >7bA | 5.6aA (0.3) | >7bA | 5.8aA (0.2) | >7bA | 4.2aA (0.2) | >7bA | 3.3aA (0.2) | >7bA | 2.3aA (0.3) | 0.3bA,C (0.3) |
| Hadar | 0.9aB (0.2) | 1.4bB (0.3) | 1.1aB (0.3) | 2.9bB (0.4) | 1.9aA,C (0.3) | 3.2bA (0.2) | >7aB | >7aA | >7aB | >7aA | >7aB | >7aA | 7.0aB (0.4) | >7bA | 5.4aB (0.4) | >7bA | 3.3aB (0.3) | 1.2bB (0.2) |
| Typhimurium | 0.2aA,C (0.2) | 0.9bA (0.1) | 0.4aC (0.1) | 1.4bA (0.2) | 0.9aB (0.1) | 6.5bB (0.4) | 4.3aC (0.2) | >7bA | 4.4aC (0.2) | >7bA | 4.3aC (0.2) | >7bA | 3.1aC (0.2) | >7bA | 3.1aA (0.3) | >7bA | 1.5aC (0.4) | 0.5bC (0.2) |
| Newport | 0.1aC (0.2) | 0.6bC (0.1) | 0.5aA,C (0.2) | 1.1bA,C (0.3) | 1.6aC (0.2) | 4.4bC (0.2) | 6.1aD (0.1) | >7bA | 5.9aA (1.2) | >7bA | 4.9aD (0.3) | >7bA | 4.5aA (0.3) | >7bA | 4.7aC (0.3) | >7bA | 0.6aD (0.3) | 0.2bA (0.2) |
| Infantis | 0.2aA,C (0.2) | 0.8bA (0.2) | 0.4aC (0.1) | 0.9bC (0.2) | 0.6aB (0.3) | 3.8bD (0.1) | 4.3aC (0.2) | >7bA | 4.3aC (0.2) | >7bA | 4.1aC (0.2) | >7bA | 2.7aD (0.2) | >7bA | 3.0aA (0.2) | >7bA | 0.7aD (0.1) | 0.2bA (0.2) |
Desiccated (+) and nondesiccated (−) Salmonella serotypes Enteritidis, Hadar, Typhimurium, Newport, and Infantis were exposed to NaCl (1 M, 2 h), bile (10%, 2 h), ethanol (30%, 5 min), hydrogen peroxide (2%, 5 min), DDAC (0.25%, 5 min), sodium hypochlorite (100 ppm, 5 min), dry heat (100°C, 1 h), UV irradiation (125 μW/cm2, 25 min), and citric acid (50 mM, pH 3.0, 2 h). Log reduction is calculated by subtraction of the log number of CFU after challenge from the log number of CFU before challenge. Values are presented as means of at least two independent experiments, each performed in triplicate. For each stressor, means followed by different lowercase letters indicate a significant difference between desiccated/nondesiccated cells (P < 0.01). Means followed by different capital letters indicate significant difference between serotypes (the same column) exposed to the same stressor (P < 0.01).
DISCUSSION
Bacteria evoke a common stress response when encountering diverse environmental stresses. Usually, exposure to a single stress may result in cross-tolerance to heterogeneous stresses (1, 23, 33, 49). Cross-tolerance was also reported in Salmonella. For example, carbon starvation induces a protective effect against low pH, hyperosmolarity, heat, polymyxin B, and peroxides (45), while exposure to mild acidic conditions induces tolerance to lower pH, heat, NaCl (2.5 M), crystal violet, and polymyxin B (36). Exposure to acetic acid was shown to induce protection against high concentrations of NaCl and KCl (22), as well as against low temperature (4°C) (51a).
Food items usually undergo several processing stages aimed at limiting growth and survival of pathogenic and spoilage microorganisms. Furthermore, sanitation of food-contact surfaces involves the usage of a variety of antimicrobial agents. Nevertheless, Salmonella is occasionally found in dry food (6, 7, 8) and is sporadically involved in outbreaks linked to consumption of dry food items (5, 28, 32, 35, 40, 44, 50). We therefore hypothesized that desiccation stress might also induce tolerance to multiple stressors that the pathogen may encounter during food preparation and processing. In agreement with this notion, others have demonstrated that desiccation increases Salmonella tolerance to heat (24, 31) and ethanol (24).
In the present study we have examined the tolerance of desiccated and nondesiccated Salmonella cells to a number of common stresses, including NaCl, bile salts, organic acids at pH 3.0, dry heat, UV irradiation, and various sanitation agents. Desiccated S. Typhimurium demonstrated significantly higher levels of tolerance to 9 of the 10 tested stressors. Thermal processing of food is commonly utilized to inactivate microorganisms. Our study implies that Salmonella present on dry surfaces is in fact tolerant to inactivation by dry heat (100°C, 1 h). Comparable heat tolerance was previously reported in Salmonella present in high-fat, low-water-activity food (peanut butter) (43), as well as in nonfat dry milk (39) and on model surfaces (24, 31). Hiramatsu et al. suggest that heat tolerance in desiccated bacteria is a physical property associated with inhibition of protein denaturation due to a lack of water (24). However, we observed that desiccated cells that were rehydrated just prior to the heat challenge were still more resistant to dry heat (100°C) at 10 min of exposure than nondesiccated cells (Fig. 6B). This finding implies that heat tolerance is not merely a physical property associated with a lack of water but, rather, is a more complex phenomenon.
It must be noted that both desiccated and nondesiccated cells were suspended in SDW and therefore experienced identical prechallenge conditions, i.e., starvation and hypo-osmolarity during the 22 h of incubation. However, desiccation took place in 96-well plates (50 μl/well), while the nondesiccated cells were kept in 15-ml tubes (2 ml/tube) prior to the challenge experiments. Under these conditions, nondesiccated cells may have encountered reduced oxygen conditions compared to those for the desiccated cells. One might hypothesize that exposure to these conditions has sensitized bacteria to subsequent challenges, thus enhancing killing. To overrule this possibility, we have also repeated the challenge experiments with freshly prepared (no 22-h preincubation) nondesiccated bacteria. Both fresh and 22-h-incubated bacteria had similar stress responses (data not shown), negating the possibility that reduced oxygen might have specifically sensitized the nondesiccated cells.
Acid adaptation was previously shown to provide cross-protection against various stresses, such as high salts, heat, and peroxides (26, 36, 51a), suggesting the presence of a common stress response mechanism that is responsible for the development of cross-tolerance to other stressors potentially found in the same environment. Similarly, preexposure to desiccation induced cross-tolerance to multiple stresses in S. enterica. In contrast, desiccated S. Typhimurium cells were more susceptible to acetic and citric acids (pH 3.0). To explore the association between organic acids and desiccation stress, we have also tested desiccation tolerance following preexposure to the organic acids. Interestingly, acid-adapted cells did not mount tolerance to desiccation and, in fact, were more susceptible to this stress. Likewise, exposure of Salmonella-contaminated tomato or apple slices to acids (ascorbic and citric acids) also reduced Salmonella survival following dehydration (15, 52). Thus, prechallenge to a single stress does not necessarily result in the development of tolerance to another stress. It is likely that different regulatory pathways are responsible for mounting tolerance to organic acids and dehydration.
The response of desiccated S. Typhimurium (SL1344) to the various stresses is not unique. We show that at least three other serotypes (S. Enteritidis, S. Newport, and S. Infantis) respond similarly. However, unlike the other strains, Salmonella serotype Hadar seems to be more susceptible to desiccation, as well as to the majority of the tested stressors. In contrast, Salmonella serotype Newport, which also displayed relatively low survival following desiccation, showed higher resistance to multiple stresses. Thus, it is likely that variations in tolerance to desiccation and subsequent cross-tolerance to other stresses might occur among the heterogeneous population of S. enterica serotypes. Nevertheless, since only a single strain from each serotype was tested, it is possible that variation in stress tolerance also takes place among different isolates of the same serotype. Presumably, those strains exhibiting high cross-tolerance will more likely be involved in contamination of foods undergoing industrial processing. This notion should be further studied in future studies.
Desiccated Salmonella cells have, on the one hand, higher susceptibility to acid but, on the other hand, higher tolerance to bile salts compared to nondesiccated cells. Since the first barriers that food-borne pathogens encounter in the human host are stomach acidity and bile salts, the outcomes of these seemingly opposing responses are not clear. It has previously been reported that although high infectious doses (>106 CFU) are required to cause an infection (10), some outbreaks caused by consumption of low-water-activity food items (peanut butter and chocolate) apparently involved much lower infectious doses (20, 41). The relative contribution of stomach acidity and bile salts to Salmonella infection is yet to be determined.
Finally, heat, UV irradiation, and sanitizing agents are among the main antimicrobial barriers used in the food industry. We have demonstrated that desiccated Salmonella cells acquire tolerance to these stressors and are resistant to conditions lethal to nondesiccated cells. In light of the recent salmonellosis outbreaks associated with food items of low water content (5, 17, 18, 28, 50), our findings indicate that the chemicals utilized for sanitation of food-contact surfaces (sodium hypochlorite and DDAC), as well as thermal treatment of dry foods, might not always be sufficient to ensure efficient eradication of human pathogens. Existing sanitation protocols and processes of microbial inactivation of food items in all food sectors should be revisited and examined to confirm the anticipated results. Our findings regarding the enhanced susceptibility of dried Salmonella cells to the organic acids might be useful in devising improved decontamination methods for dry surfaces.
More studies are necessary to optimize chemical and physical treatments of low-water-activity foods and dry surfaces in order to better control microbial contamination.
Acknowledgments
This study was partially supported by the United States-Israel Binational Agricultural Research and Development (BARD) Fund, grant IS-4267-09.
Footnotes
Published ahead of print on 7 January 2011.
REFERENCES
- 1.Abee, T., and J. A. Wouters. 1999. Microbial stress response in minimal processing. Int. J. Food Microbiol. 50:65-91. [DOI] [PubMed] [Google Scholar]
- 2.Aljarallah, K. M., and M. R. Adams. 2006. Mechanisms of heat inactivation in Salmonella serotype Typhimurium as affected by low water activity at different temperatures. J. Appl. Microbiol. 102:153-160. [DOI] [PubMed] [Google Scholar]
- 3.Alt, E., et al. 1999. Hydrogen peroxide for prevention of bacterial growth on polymer biomaterials. Ann. Thorac. Surg. 68:2123-2128. [DOI] [PubMed] [Google Scholar]
- 4.Altekruse, S. F., M. L. Cohen, and D. L. Swerdlow. 1997. Emerging foodborne diseases. Emerg. Infect. Dis. 3:285-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anonymous. 2007. Multistate outbreak of Salmonella serotype Tennessee infections associated with peanut butter—United States, 2006-2007. MMWR Morb. Mortal. Wkly. Rep. 56:521-524. [PubMed] [Google Scholar]
- 6.Anonymous. 2008. Cornflakes product recalls. Food and Drug Administration, Rockville, MD. http://www.fda.gov/Safety/Recalls/MajorProductRecalls/Cornflakes/default.htm.
- 7.Anonymous. 2009. Pistachio product recalls. Food and Drug Administration, Rockville, MD. http://www.fda.gov/Safety/Recalls/MajorProductRecalls/Pistachio/default.htm.
- 8.Anonymous. 2010. Granola bars product recalls. Food and Drug Administration, Rockville, MD. http://www.fda.gov/Safety/Recalls/MajorProductRecalls/Granola/default.htm.
- 9.Archer, J. E., T. Jervis, J. Bird, and J. E. Gaze. 1998. Heat resistance of Salmonella Weltevreden in low-moisture environments. J. Food Prot. 61:969-973. [DOI] [PubMed] [Google Scholar]
- 10.Blaser, M. J., and L. S. Newman. 1982. A review of human salmonellosis. I. infective dose. Rev. Infect. Dis. 4:1096-1106. [DOI] [PubMed] [Google Scholar]
- 11.Boor, K. J. 2006. Bacterial stress responses: what doesn't kill them can make them stronger. PLoS Biol. 4:e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiewchan, N., W. Pakdee, and S. Devahastin. 2007. Effect of water activity on thermal resistance of Salmonella Krefeld in liquid medium and on rawhide surface. Int. J. Food Microbiol. 114:43-49. [DOI] [PubMed] [Google Scholar]
- 13.Chowdhury, R., G. K. Sahu, and J. Das. 1996. Stress response in pathogenic bacteria. J. Biosci. 21:149-160. [Google Scholar]
- 14.de Cesare, A., B. W. Sheldon, K. S. Smith, and L. A. Jaykus. 2003. Survival and persistence of Campylobacter and Salmonella species under various organic loads on food contact surfaces. J. Food Prot. 66:1587-1594. [DOI] [PubMed] [Google Scholar]
- 15.DiPresio, P. A., P. A. Kendall, M. Calicioglu, and J. N. Sofos. 2003. Inactivation of Salmonella during drying and storage of apple slices treated with acidic or sodium metabisulfite solutions. J. Food Prot. 66:2245-2251. [DOI] [PubMed] [Google Scholar]
- 16.Fennema, O. R. 1985. Food chemistry, 2nd ed., revised and expanded, p. 46-50. Marcel Dekker, New York, NY.
- 17.Flynn, D. 2010. Black pepper positive for Salmonella. In Food safety news. http://www.foodsafetynews.com/2010/01/black-pepper-positive-for-outbreak-strain/.
- 18.Flynn, D. 2010. Red pepper suspected in Salmonella outbreak. In Food safety news. http://www.foodsafetynews.com/2010/02/red-pepper-suspecte-in-salmonella-outbreak/.
- 19.Foster, J. W., and M. P. Spector. 1995. How Salmonella survive against the odds. Annu. Rev. Microbiol. 49:145-174. [DOI] [PubMed] [Google Scholar]
- 20.Gill, O. N., et al. 1985. Outbreak of Salmonella Napoli infection caused by contaminated chocolate bars. Lancet 321:574-577. [DOI] [PubMed] [Google Scholar]
- 21.Goepfert, J. M., and R. A. Biggie. 1968. Heat resistance of Salmonella Typhimurium and Salmonella Senftenberg 775W in milk chocolate. Appl. Microbiol. 16:1939-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenarce, E. J., and T. F. Brocklehurst. 2006. The acetic acid tolerance response induces cross-protection to salt stress in Salmonella Typhimurium. Int. J. Food Microbiol. 112:62-65. [DOI] [PubMed] [Google Scholar]
- 23.Hecker, M., and U. Volker. 2001. General stress response of Bacillus subtilis and other bacteria. Adv. Microb. Physiol. 44:35-91. [DOI] [PubMed] [Google Scholar]
- 24.Hiramatsu, R., M. Matsumoto, K. Sakae, and Y. Miyazaki. 2005. Ability of Shiga toxin-producing Escherichia coli and Salmonella spp. to survive in a desiccation model system and in dry foods. Appl. Environ. Microbiol. 71:6657-6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reference deleted.
- 26.Humphrey, T. 2004. Salmonella, stress response and food safety. Nat. Rev. Microbiol. 2:504-509. [DOI] [PubMed] [Google Scholar]
- 27.Inami, G. B., and S. E. Moler. 1999. Detection and isolation of Salmonella from naturally contaminated alfalfa seeds following an outbreak investigation. J. Food Prot. 62:662-664. [DOI] [PubMed] [Google Scholar]
- 28.Isaacs, S., et al. 2005. An international outbreak of salmonellosis associated with raw almonds contaminated with a rare phage type of Salmonella enteritidis. J. Food Prot. 68:191-198. [DOI] [PubMed] [Google Scholar]
- 29.Joseph, B., S. K. Otta, and I. Karunasagar. 2001. Biofilm formation by Salmonella spp. on food contact surfaces and their sensitivity to sanitizers. Int. J. Food Microbiol. 64:367-372. [DOI] [PubMed] [Google Scholar]
- 30.Kieboom, J., et al. 2006. Survival, elongation, and elevated tolerance of Salmonella enterica serovar Enteritidis at reduced water activity. J. Food Prot. 69:2681-2686. [DOI] [PubMed] [Google Scholar]
- 31.Kirby, R. M., and R. Davies. 1990. Survival of dehydrated cells of Salmonella Typhimurium LT2 at high temperatures. J. Appl. Bacteriol. 68:241-246. [DOI] [PubMed] [Google Scholar]
- 32.Kirk, M., et al. 2004. An outbreak due to peanuts in their shell caused by Salmonella enterica serotypes Stanley and Newport—sharing molecular information to solve international outbreaks. Epidemiol. Infect. 132:571-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kultz, D. 2005. Molecular and evolutionary basis of the cellular stress response. Annu. Rev. Physiol. 67:225-257. [DOI] [PubMed] [Google Scholar]
- 34.Kusumaningrum, H. D., G. Riboldi, W. C. Hazeleger, and R. R. Beumer. 2002. Survival of foodborne pathogens on stainless steel surfaces and cross-contamination to foods. Int. J. Food Microbiol. 85:227-236. [DOI] [PubMed] [Google Scholar]
- 35.Lehmacher, A., J. Bockemühl, and S. Aleksic. 1995. Nationwide outbreak of human salmonellosis in Germany due to contaminated paprika and paprika-powdered potato chips. Epidemiol. Infect. 115:501-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leyer, G. J., and E. A. Johnson. 1993. Acid adaptation induces cross-protection against environmental stress in Salmonella Typhimurium. Appl. Environ. Microbiol. 59:1842-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mattick, K. L., F. Jørgensen, J. D. Legan, H. M. Lappin-Scott, and T. J. Humphrey. 2000. Habituation of Salmonella spp. at reduced water activity and its effect on heat tolerance. Appl. Environ. Microbiol. 66:4921-4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mattick, K. L., et al. 2001. Effect of challenge temperature and solute type on heat tolerance of Salmonella serovars at low water activity. Appl. Environ. Microbiol. 67:4128-4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McDonough, F. E., and R. E. Hargrove. 1968. Heat resistance of Salmonella in dried milk. J. Dairy Sci. 51:1587-1591. [DOI] [PubMed] [Google Scholar]
- 40.Rowe, B., N. T. Begg, and D. N. Hutchinson. 1987. Salmonella Ealing infections associated with consumption of infant dried milk. Lancet ii:900-903. [DOI] [PubMed] [Google Scholar]
- 41.Scheil, W., S. Cameron, C. Dalton, C. Murray, and D. Wilson. 1998. A South Australian Salmonella Mbandaka outbreak investigation using a database to select controls. Aust. N. Z. J. Public Health 22:536-539. [DOI] [PubMed] [Google Scholar]
- 42.Scher, K., U. Romling, and S. Yaron. 2005. Effect of heat, acidification, and chlorination on Salmonella enterica serovar Typhimurium cells in a biofilm formed at the air-liquid interface. Appl. Environ. Microbiol. 71:1163-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shachar, D., and S. Yaron. 2006. Heat tolerance of Salmonella enterica serovars Agona, Enteritidis, and Typhimurium in peanut butter. J. Food Prot. 69:2687-2691. [DOI] [PubMed] [Google Scholar]
- 44.Shohat, T., M. S. Green, and D. Marom. 1996. International epidemiological and microbiological study of outbreak of Salmonella agona infection from a ready-to-eat savoury snack—II: Israel. BMJ 313:1107-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spector, M. P. 1998. The starvation-stress response (SSR) of Salmonella. Adv. Microb. Physiol. 40:233-279. [DOI] [PubMed] [Google Scholar]
- 46.Sung, J. Y., E. A. Shaffer, and J. W. Costerton. 1993. Antibacterial activity of bile salts against common biliary pathogens. Effects of hydrophobicity of the molecule and in the presence of phospholipids. Dig. Dis. Sci. 38:2104-2112. [DOI] [PubMed] [Google Scholar]
- 47.Tauxe, R. V. 1991. Salmonella: a postmodern pathogen. J. Food Prot. 54:563-568. [DOI] [PubMed] [Google Scholar]
- 48.Uzzau, S., et al. 2000. Host adapted serotypes of Salmonella enterica. Epidemiol. Infect. 125:229-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vorob'eva, L. I. 2003. Stressors, stress reactions, and survival of bacteria: a review. Appl. Biochem. Microbiol. 40:217-224. [PubMed] [Google Scholar]
- 50.Werber, D., et al. 2005. International outbreak of Salmonella Oranienburg due to German chocolate. BMC Infect. Dis. 5:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winfield, M. D., and E. A. Groisman. 2003. Role of non-host environments in the lifestyles of Salmonella and Escherichia coli. Appl. Environ. Microbiol. 69:3687-3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51a.Xu, H., H. Y. Lee, and J. Ahn. 2008. Cross-protective effect of acid-adapted Salmonella enterica on resistance to lethal acid and cold stress conditions. Lett. Appl. Microbiol. 47:290-297. [DOI] [PubMed] [Google Scholar]
- 52.Yoon, Y., J. D. Stopforth, P. A. Kendall, and J. N. Sofos. 2004. Inactivation of Salmonella during drying and storage of Roma tomatoes exposed to predrying treatments including peeling, blanching and dipping in organic acid solutions. J. Food Prot. 67:1344-1352. [DOI] [PubMed] [Google Scholar]