Abstract
The molecular epidemiology of 545 Salmonella enterica serovar Typhimurium isolates collected between 1977 and 2009 from cattle in Hokkaido, Japan, was investigated using pulsed-field gel electrophoresis (PFGE). Nine main clusters were identified from 116 PFGE patterns. Cluster I comprised 248 isolates, 243 of which possessed a sequence specific to definitive phage type 104 (DT104) or U302. The cluster I isolates were dominant in 1993 to 2003, but their numbers declined beginning in 2004. Beginning in 2002, an increase was observed in the number of cluster VII isolates, consisting of 21 PFGE patterns comprising 165 isolates. A total of 116 isolates representative of the 116 PFGE profiles were analyzed by multilocus variable-number tandem-repeat analysis (MLVA). Other than two drug-sensitive isolates, 19 isolates within cluster VII were classified in the same cluster by MLVA. Among the cluster VII isolates, an antibiotic resistance type showing resistance to ampicillin, chloramphenicol, streptomycin, sulfonamides, tetracycline, kanamycin, cefazolin, and sulfamethoxazole-trimethoprim and a resistance type showing resistance to ampicillin, streptomycin, sulfonamides, tetracycline, and kanamycin were found in 23 and 125 isolates, respectively. In the 19 isolates representative of cluster VII, the blaTEM-1 gene was found on a Salmonella serotype Typhimurium virulence plasmid, which was transferred to Escherichia coli by electroporation along with resistance to two to four other antimicrobials. Genomic analysis by subtractive hybridization and plasmid analysis suggested that the blaTEM-1-carrying virulence plasmid has a mosaic structure composed of elements of different origin. These results indicate an emerging multidrug-resistant S. Typhimurium clone carrying a virulence-resistance plasmid among cattle in Hokkaido, Japan.
Salmonella enterica serovar Typhimurium is a common cause of salmonellosis in humans and animals. Detailed characterization of this bacterium is necessary for studying the epidemiology of outbreaks and determining the source of contamination to avoid recurrence. Phage typing is a commonly used method for epidemiological surveillance of S. Typhimurium infection (2), but it requires special reagents and a specialized laboratory and fails to reflect the evolutionary relationships of bacterial strains. Over the last decade, new techniques in molecular biology have been developed, and new approaches have become available. Pulsed-field gel electrophoresis (PFGE) is now the gold standard for discriminating among strains at the DNA level (32). However, multiple-locus variable-number tandem-repeat analysis (MLVA), based on amplification of a variable number of tandem repeat areas, is considered to have greater discriminatory power than PFGE and has been proposed as an alternative for genotyping highly clonal groups of bacteria (18, 19). Molecular subtyping of S. Typhimurium isolates by standard procedures and development of a DNA fingerprinting database of these isolates would assist in identifying Salmonella epidemics and would enable monitoring of changes in epidemic patterns. Therefore, a sensitive surveillance system based on a molecular subtyping technique such as PFGE or MLVA is needed for early detection of such epidemics. PulseNet, a national molecular subtyping network for surveillance of food-borne diseases, is a successful example (32).
S. enterica epidemics often involve rapid dissemination of the predominant epidemic strains over a large geographic area. For example, definitive phage type 104 (DT104) has emerged and has spread to many countries. DT104 was first detected in the United Kingdom and emerged nearly simultaneously in North America, Europe, and Asia (4, 11, 28, 36, 37). The organism has a core pattern of resistance to ampicillin, chloramphenicol, streptomycin, sulfonamides, and tetracycline (6, 26); this resistance is encoded by a chromosomally located locus containing class 1 integron structures (3, 23, 29). In 1992, we observed an apparent increase in the incidence of bovine salmonellosis, particularly in adult cattle, caused by S. Typhimurium in Hokkaido, the northern island of Japan, where dairy farming is one of the main agro-industries (34). Historic surveillance data showed that the incidence was stable until 1991 but increased over the next 3 years, with cases stabilizing or declining after 1995 (34). Although the reasons for these trends are unclear, previous studies using PFGE have revealed an increased frequency of bovine DT104 isolates in Hokkaido since 1992 (27, 34).
In the present study, we investigated the molecular epidemiology of 545 S. Typhimurium isolates collected between 1977 and 2009 from cattle in Hokkaido using PFGE. We found that a non-DT104 multidrug-resistant clone has disseminated among cattle in this region since 2002. We also performed genome analysis for a detailed study of the disseminated clone by genomic subtractive hybridization and characterization of multidrug-resistance plasmids.
MATERIALS AND METHODS
Bacterial strains.
The 545 S. Typhimurium strains were isolated from diseased cattle by the staff of local Animal Hygiene Centers over the period of 1977 to 2009 in Hokkaido, Japan.
DNA isolation.
The strains were grown aerobically at 37°C on Luria-Bertani (LB) agar plates. A generous loopful of each culture was suspended in 400 μl of distilled water. For DNA extraction, the cell suspensions were boiled for 5 min and then immediately cooled on ice for 5 min and centrifuged (13,000 × g for 5 min) at 4°C; the supernatant was used as the DNA template for PCR. Total genomic DNA for subtractive hybridization was isolated from cells scraped from the LB agar plates. The harvested cells were suspended in 0.2 ml of a solution of 150 mM NaCl, 1 mM EDTA, and 10 mM Tris-hydrochloride at pH 8.0. After lysis with sodium dodecyl sulfate (1.0% final concentration), RNase A was added (final concentration, 48 μg ml−1), and the mixture was incubated at 37°C for 30 min to degrade the cellular RNA. The released nucleic acid was deproteinated by adding proteinase K (final concentration, 200 μg ml−1) and then incubated at 55°C for 30 min. The DNA was further purified by extraction with phenol-chloroform-isoamyl alcohol, followed by two extractions with chloroform-isoamyl alcohol. The nucleic acid was recovered by precipitation with ethanol, dried, and dissolved in 100 to 200 μl of 10 mM Tris-hydrochloride and 1 mM EDTA at pH 8.0. The plasmid DNA was isolated using the alkaline lysis method described previously (34). Plasmid sizes were estimated relative to a BAC-Tracker Supercoiled DNA Ladder (Epicentre Biotechnologies, Madison, WI).
Plasmid transformation.
Plasmid DNA was used to transform Escherichia coli DH5α cells (Takara Co. Ltd., Kyoto, Japan) by electroporation (at 1.25 kV, 100 Ω, and 25 μF). The cells were plated on LB agar plates containing 50 μg ml−1 ampicillin or 30 μg ml−1 cefazolin. The transformants were then collected, and their plasmid and antimicrobial susceptibility profiles were examined.
Antimicrobial susceptibility testing.
The susceptibility of the isolates to antimicrobial agents was determined by the disk diffusion test on Mueller-Hinton agar (Difco, Detroit, MI) according to the standards and interpretive criteria of the National Committee for Clinical Laboratory Standards (22). The following antibiotic disks were used (Becton Dickinson Microbiology Systems, Cockeysville, MD): ampicillin (10 μg), chloramphenicol (30 μg), streptomycin (10 μg), sulfonamides (250 μg), tetracycline (30 μg), kanamycin (30 μg), sulfamethoxazole-trimethoprim (23.75 and 1.25 μg, respectively), cefazolin (30 μg), ceftazidime (30 μg), cefotaxime (30 μg), nalidixic acid (30 μg), gentamicin (10 μg), and ciprofloxacin (5 μg). The reference strains used were E. coli ATCC 25922 and Staphylococcus aureus ATCC 29213.
PCR amplification.
PCR was used to amplify the antimicrobial resistance genes (blaTEM-1, blaCMY-2, and floR), integrase (intI), class 1 integrons, and Salmonella virulence plasmid genes (repFIIA, spvA, spvB, spvC, traT, rck, and pefA) using published primer sequences (5, 13, 17, 25, 31, 33). PCR amplification of an internal segment of the 16S-23S spacer region of bacterial rRNA genes was used to identify DT104, as previously described (24). The primer sets shown in Table S1 in the supplemental material were designed to detect four DNA fragments (SN-11, PU302L-2, ST104-1, and ST64B-1) selected from a subtractive hybridization library (see Table S2). Oligonucleotide primers were synthesized by Greiner Bio-One (Tokyo, Japan). The genes or sequences tested, respective primers, and expected amplicon sizes are listed in Table S1. Standard PCR was carried out in a 50-μl mixture containing 1× PCR buffer with 0.2 mM MgCl2, 2.5 U of AmpliTaq Gold DNA polymerase (Applied Biosystems, Inc., Foster City, CA), 0.2 mM deoxynucleotide triphosphates, 0.5 μM primers, and 50 ng of genomic DNA. PCR amplification was performed with an initial denaturation cycle at 95°C for 5 min, followed by 30 cycles each of 30 s of denaturation at 94°C and 30 s of annealing at 55°C, with 1 min of extension at 72°C. Incubation for 3 min at 72°C followed to complete the extension.
PFGE.
PFGE was performed using a CHEF DR-II apparatus (Bio-Rad Laboratories, Hercules, CA) following the standard protocol of PulseNet with the restriction enzyme XbaI (Takara) (32). XbaI-digested DNA from S. enterica serovar Braenderup H9812 was used as a molecular reference marker. Image normalization and construction of similarity matrices were carried out using BioNumerics, version 5.1 (Applied Maths, Sint-Martens-Latem, Belgium). Bands were assigned manually, and dendrograms were generated using the unweighted pair group method with arithmetic mean (UPGMA) based on the Dice similarity index, utilizing an optimization parameter of 1% band position tolerance. A profile that differed by at least one clear band was considered a distinct profile.
MLVA.
MLVA was performed using the primers (STTR3-F, STTR3-R, STTR5-F, STTR5-R, STTR6-F, STTR6-R, STTR9-F, STTR9-R, STTR10pl-F, and STTR10pl-R) for amplification of five loci described previously (18, 19) (see Table S1 in the supplemental material). PCR was carried out as described in the preceding section. To confirm the number of tandem repeats, the PCR product was sequenced. The amplified product was purified with QIAquick PCR purification columns (Qiagen, Hilden, Germany) and sequenced in both directions using the same primers employed in the PCR. DNA sequencing was performed with an automated DNA sequencer (ABI 3130; Applied Biosystems) using an ABI Prism BigDye Terminator, version 3.1, Cycle Sequencing Kit (Applied Biosystems) according to the manufacturer's instructions. The number of tandem repeats at each locus was manually determined using Genetyx, version 10.0 (Genetyx, Tokyo, Japan). The motif copy numbers in the tandem array were imported into the BioNumerics software, and a minimum-spanning tree was generated using the categorical coefficient of the software.
Subtractive hybridization.
Genomic DNA was extracted from S. Typhimurium KT262 (tester) and strain LT2 (driver) as described in the previous sections. Subtractive hybridization was carried out using a PCR-Select Bacterial Genome Subtraction Kit (BD Clontech, Palo Alto, CA) following the manufacturer's instructions. The PCR products obtained from the subtraction procedure were ligated into the pCRII vector using a TA Cloning Kit (Invitrogen Corp., San Diego, CA). The subtractive hybridization library was constructed by transforming the ligation mixture to E. coli DH5α cells with ampicillin (50 μg ml−1) and 5-bromo-4-chloro-3-indolyl-d-galactopyranoside (X-Gal) selection and screening on LB agar plates. Individual colonies were collected and grown overnight at 37°C in LB broth with ampicillin (50 μg ml−1) selection. The plasmid DNA was isolated using methods described previously (27). The cloned PCR products were sequenced by using M13 forward and reverse primers.
Southern hybridization analysis.
The PCR products of blaTEM-1, blaCMY-2, and spvC were purified using a QIAquick Gel Extraction Kit (Qiagen) and labeled with digoxigenin (DIG)-11-dUTP by random priming using a DIG High Prime Labeling Kit (Roche Diagnostics Co., Indianapolis, IN) following the manufacturer's instructions. Plasmid DNA was separated on an 0.8% (wt/vol) agarose gel and transferred to a positive membrane (Roche) by the capillary method. Prehybridization (>30 min) and hybridization (>16 h) with Easy Hyb solution (Roche) under high-stringency conditions and DIG detection of hybrids were carried out using a DIG Luminescent Detection Kit (Roche) following the manufacturer's instructions. Hyper MP film (GE Healthcare Limited, Buckinghamshire, United Kingdom) was exposed to the membranes for 1 to 10 min at room temperature in an X-ray film processor (Fuji Film Co., Tokyo, Japan).
Nucleotide sequence accession numbers.
The nucleotide sequences of the 25 S. Typhimurium KT262 fragments listed in Table S2 in the supplemental material have been submitted to the GenBank under accession numbers AB458414 to AB458443.
RESULTS AND DISCUSSION
PFGE analysis and MLVA.
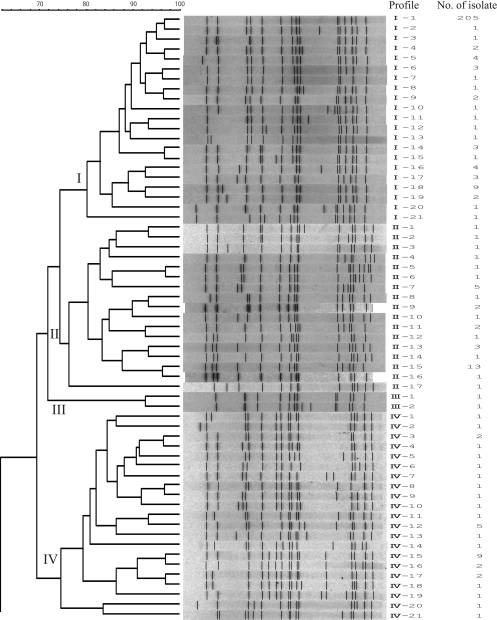
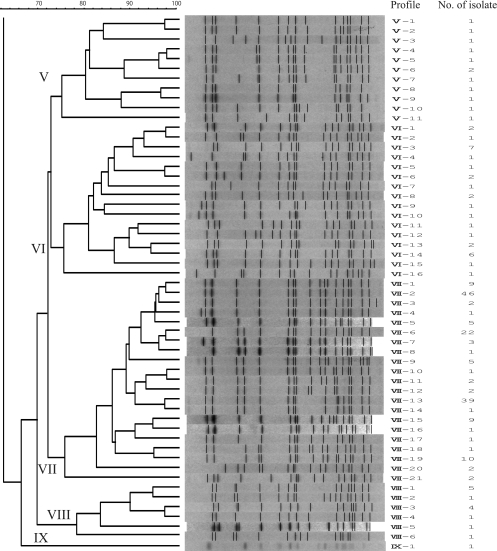
Molecular characterization of S. Typhimurium isolates obtained from cattle from 1977 to 2009 in Hokkaido, Japan, was carried out by PFGE analysis and MLVA. In the 33-year history of endemic Salmonella infection in this area, S. Typhimurium has become the major serovar causing bovine salmonellosis. We observed 116 PFGE patterns among the 545 isolates after digestion of the DNA with XbaI. To analyze the clonal relationships among the isolates according to the XbaI PFGE patterns, a dendrogram was generated using UPGMA algorithms with Dice coefficients (Fig. 1). Nine distinct clusters (I to IX) with greater than 74% similarity were designated, five of which were major clonal groups (I, II, IV, VI, and VII) (Fig. 1). Cluster I consisted of 21 patterns comprising 248 isolates. Profile I-1 was found in 205 (83%) of these isolates. The cluster I isolates were detected in isolates collected since 1986. From 1993 to 2003, cluster I was the dominant cluster in terms of the number of isolates, but the numbers declined after 2003 (Table 1). Thirty-seven isolates belonging to cluster II were associated with 17 PFGE patterns. Cluster IV was composed of 21 PFGE patterns, accounting for 36 isolates. Cluster VI included 31 isolates with 16 PFGE patterns. Clusters IV and/or VI were the dominant clusters from 1985 to 1992, just before cluster I became prevalent. Cluster VII consisted of 21 PFGE patterns with 165 isolates. Profile VII-2 was found in 46 (28%), VII-6 in 22 (13%), VII-13 in 39 (24%), VII-19 in 10 (6.0%), and the remaining profiles in 48 (29%) of these isolates. The cluster VII isolates became more prevalent after 2001 (Table 1). Clusters III, V, VIII, and IX were minor clusters, consisting of 2, 11, 6, and 1 pattern(s) of 2, 12, 13, and 1 isolate(s), respectively.
FIG. 1.
Dendrograms of 116 PFGE types obtained by XbaI PFGE of the 545 S. Typhimurium isolates. Nine clusters (I to IX) are marked, and the different PFGE profiles and corresponding numbers of isolates are indicated.
TABLE 1.
PFGE clusters identified from 545 S. Typhimurium isolates from cattle between 1977 and 2009
| Yr of isolation | No. of isolates by PFGE cluster |
Total no. of isolates | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | VIII | IX | ||
| 1977 | 1 | 1 | ||||||||
| 1980 | 2 | 2 | ||||||||
| 1981 | 1 | 1 | ||||||||
| 1982 | 4 | 4 | ||||||||
| 1983 | 1 | 1 | ||||||||
| 1984 | 1 | 1 | 2 | 4 | ||||||
| 1985 | 1 | 6 | 3 | 10 | ||||||
| 1986 | 1 | 1 | 7 | 1 | 10 | |||||
| 1987 | 3 | 5 | 1 | 9 | ||||||
| 1988 | 6 | 1 | 7 | |||||||
| 1989 | 2 | 5 | 2 | 9 | ||||||
| 1990 | 4 | 1 | 5 | |||||||
| 1991 | 1 | 10 | 2 | 13 | ||||||
| 1992 | 4 | 1 | 5 | 1 | 6 | 17 | ||||
| 1993 | 27 | 3 | 30 | |||||||
| 1994 | 38 | 1 | 1 | 40 | ||||||
| 1995 | 22 | 2 | 24 | |||||||
| 1996 | 12 | 1 | 13 | |||||||
| 1997 | 21 | 21 | ||||||||
| 1998 | 19 | 1 | 2 | 1 | 23 | |||||
| 1999 | 28 | 28 | ||||||||
| 2000 | 9 | 2 | 1 | 1 | 13 | |||||
| 2001 | 13 | 1 | 1 | 1 | 1 | 17 | ||||
| 2002 | 13 | 3 | 1 | 10 | 27 | |||||
| 2003 | 18 | 5 | 12 | 35 | ||||||
| 2004 | 6 | 3 | 19 | 28 | ||||||
| 2005 | 3 | 1 | 2 | 42 | 48 | |||||
| 2006 | 5 | 32 | 37 | |||||||
| 2007 | 10 | 3 | 44 | 57 | ||||||
| 2008 | 1 | 2 | 3 | 6 | ||||||
| 2009 | 2 | 2 | 1 | 5 | ||||||
| Total | 248 | 37 | 2 | 36 | 12 | 31 | 165 | 13 | 1 | 545 |
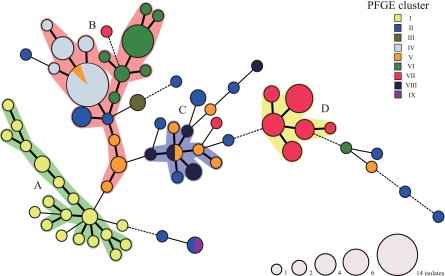
Representative isolates of the 116 PFGE patterns were subjected to MLVA based on five variable tandem repeat (VNTR) loci and were distinguished into 68 MLVA profiles (Fig. 2). The MLVA profiles were used for categorical clustering in BioNumerics, and a minimum-spanning tree was constructed (Fig. 2). MLVA clustering was achieved if neighbors differed in no more than one of the five VNTR loci. As shown in Fig. 2, the MLVA profiles were classified into four main clusters (A, B, C, and D). Eighteen of the 21 isolates belonging to PFGE cluster I were classified into MLVA cluster A, and 19 of the 21 isolates belonging to PFGE cluster VII were assigned to MLVA cluster D.
FIG. 2.
Minimum-spanning tree based on MLVA allelic profiles showing the phylogenetic relationships between representative isolates of the 116 PFGE patterns. Each node represents a unique MLVA profile. The nodal size is proportional to the number of isolates per MLVA. The colors indicate PFGE clusters. The different lines indicate the distance between the circles, where a thick line represents a closer distance than a thin line, and a thin line denotes a closer distance than a dotted line. The MLVA clusters differing by 0 or 1 VNTR locus are regarded as a group and are highlighted (MLVA cluster A, green; cluster B, pink; cluster C, blue; cluster D, yellow).
Antimicrobial susceptibility.
Forty-one of the 545 studied isolates were susceptible to all of the tested antimicrobials. A total of 423 isolates (78%) exhibited resistance to at least five of the tested antimicrobials. The disk diffusion tests showed that 483 isolates (89%) had resistance phenotypes to two or more agents. The bovine isolates displayed resistance most often to sulfonamides (90%), tetracycline (84%), streptomycin (83%), ampicillin (81%), and chloramphenicol (55%) and to a lesser extent to kanamycin (36%), sulfamethoxazole-trimethoprim (5.5%), cefazolin (5.3%), nalidixic acid (3.7%), and gentamicin (0.2%). No isolate was resistant to ciprofloxacin. Forty-three distinct antimicrobial resistance patterns were observed among the 545 isolates (Table 2). The majority of the PFGE cluster I isolates (91%) exhibited resistance to ampicillin, chloramphenicol, streptomycin, sulfonamides, and tetracycline, which is the typically observed DT104 pentaresistance pattern.
TABLE 2.
Antimicrobial resistance patterns and PFGE cluster of 545 S. Typhimurium isolates
| Antimicrobial resistance patterna | No. of isolates by PFGE cluster |
Total no. of isolates | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | VII | VIII | IX | ||
| AMP, CHL, STR, SUL, TET, KAN, CFZ, SXT | 23 | 23 | ||||||||
| AMP, CHL, STR, SUL, TET, KAN, CFZ, CTX | 1 | 1 | ||||||||
| AMP, CHL, STR, SUL, TET, KAN, CFZ | 1 | 1 | 2 | |||||||
| AMP, CHL, STR SUL, TET, KAN, NAL | 3 | 3 | ||||||||
| AMP, STR, SUL, TET, KAN, CFZ, SXT | 1 | 1 | ||||||||
| AMP, CHL, STR, SUL, TET, KAN, SXT | 1 | 1 | ||||||||
| AMP, STR, SUL, TET, KAN, CFZ | 1 | 1 | ||||||||
| AMP, CHL, STR, SUL, TET, KAN | 1 | 1 | 5 | 1 | 9 | 2 | 19 | |||
| AMP, CHL, STR, SUL, TET, NAL | 5 | 1 | 1 | 7 | ||||||
| AMP, STR, SUL, TET, KAN, GEN | 1 | 1 | ||||||||
| AMP, STR, SUL, TET, KAN, SXT | 4 | 4 | ||||||||
| AMP, CHL, SUL, TET, NAL | 1 | 1 | ||||||||
| AMP, STR, TET, CFZ, NAL | 1 | 1 | ||||||||
| AMP, CHL, STR, SUL, KAN | 1 | 1 | ||||||||
| AMP, CHL, STR, SUL, TET | 218 | 6 | 2 | 226 | ||||||
| AMP, CHL, SUL, TET, KAN | 3 | 1 | 4 | |||||||
| AMP, STR, SUL, TET, KAN | 1 | 125 | 126 | |||||||
| AMP, CHL, SUL, TET, SXT | 1 | 1 | ||||||||
| AMP, CHL, SUL, TET | 3 | 3 | 6 | |||||||
| AMP, STR, SUL, TET | 2 | 1 | 1 | 1 | 5 | |||||
| AMP, STR, TET, KAN | 1 | 1 | ||||||||
| AMP, SUL, TET, KAN | 1 | 1 | ||||||||
| CHL, SUL, TET, KAN | 1 | 1 | ||||||||
| STR, SUL, TET, NAL | 1 | 2 | 3 | |||||||
| SUL, TET, KAN, NAL | 1 | 1 | ||||||||
| AMP, CHL, SUL | 1 | 1 | ||||||||
| AMP, STR, TET | 1 | 1 | ||||||||
| AMP, TET, KAN | 1 | 1 | ||||||||
| STR, SUL, TET | 5 | 1 | 1 | 1 | 8 | |||||
| STR, TET, KAN | 1 | 1 | ||||||||
| CHL, SUL, TET | 1 | 1 | ||||||||
| AMP, SUL | 1 | 1 | 1 | 1 | 4 | |||||
| AMP, TET | 1 | 1 | ||||||||
| CHL, SUL | 1 | 1 | ||||||||
| STR, SUL | 13 | 13 | ||||||||
| STR, TET | 1 | 1 | 2 | |||||||
| SUL, KAN | 1 | 1 | ||||||||
| SUL, NAL | 1 | 2 | 3 | |||||||
| SUL, TET | 3 | 1 | 4 | |||||||
| NAL | 1 | 1 | ||||||||
| STR | 1 | 1 | 2 | |||||||
| SUL | 4 | 5 | 5 | 3 | 17 | |||||
| TET | 1 | 1 | ||||||||
| Sensitive | 4 | 18 | 2 | 7 | 5 | 3 | 2 | 41 | ||
| Total | 248 | 37 | 2 | 36 | 12 | 31 | 165 | 13 | 1 | 545 |
AMP, ampicillin; CHL, chloramphenicol; STR, streptomycin; SUL, sulfonamides; TET, tetracycline; KAN, kanamycin, SXT, sulfamethoxazole-trimethoprim; CFZ, cefazolin; CTX, cefotaxime; NAL, nalidixic acid; GEN, gentamicin.
A total of 162 of the 165 isolates from cluster VII showed ampicillin resistance, and 26 isolates showed cefazolin resistance. Among the cluster VII isolates, an antibiotic resistance type showing resistance to ampicillin, chloramphenicol, streptomycin, sulfonamides, tetracycline, kanamycin, cefazolin, and sulfamethoxazole-trimethoprim and a resistance type showing resistance to ampicillin, streptomycin, sulfonamides, tetracycline, and kanamycin were found in 23 and 125 isolates, respectively. Three isolates with PFGE profiles VII-17 and VII-21 were sensitive to all of the antibiotics and did not belong to MLVA cluster D. Only two isolates resistant to cefazolin were collected before 2000, and an increased occurrence of cefazolin-resistant isolates was noted after 2000. Therefore, these results indicate the presence of a novel multidrug-resistant clone of S. Typhimurium among cattle in Hokkaido, Japan.
PCR detection of common genes in DT104.
To investigate the genetic backgrounds of the isolates, the 545 isolates were tested using PCR for the presence of the integron marker gene intI and the florfenicol resistance gene floR, both of which are commonly associated with DT104 (5), and a sequence of the 16S-23S spacer region, which is specific to DT104 and its closely related phage type, U302 (Table 3). PCR was also conducted for the sequence of the chromosomal virulence genes invA (33) and spvC (14), which are encoded on the 94.7-kb serovar-specific virulence plasmid. Of the 248 isolates belonging to PFGE cluster I, 243 (98%) possessed the sequence of the 16S-23S spacer region of DT104 (Table 3). This sequence was not detected in the isolates belonging to the other PFGE clusters. A total of 227 of the 248 isolates (92%) belonging to cluster I and 26 of the 165 isolates (16%) belonging to cluster VII showed the presence of floR, whereas this gene was not detected in the isolates of the other clusters (Table 3). The intI gene was found in 98% (244/248), 61% (22/36), and 97% (160/165) of the cluster I, IV, and VII isolates (Table 3), respectively. Of the 96 isolates assigned to the other clusters, only five contained the intI gene. Taken together, these results suggest that almost all of the cluster I isolates are related to DT104. In fact, two of the cluster I isolates submitted for phage typing were assigned as DT104.
TABLE 3.
Prevalence of DT104-related genes or sequence, blaTEM-1, and blaCMY-2 in S. Typhimurium isolates
| PFGE cluster | No. of isolates (%) with: |
||||||
|---|---|---|---|---|---|---|---|
| invA | floR | spvC | intI | 162-bp amplicona | blaTEM-1 | blaCMY-2 | |
| I | 248 (100) | 227 (92) | 247 (99.6) | 244 (98) | 243 (98) | NTb | NT |
| II | 37 (100) | 0 (0) | 30 (81) | 1 (3) | 0 (0) | NT | NT |
| III | 2 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NT | NT |
| IV | 36 (100) | 0 (0) | 0 (0) | 22 (61) | 0 (0) | NT | NT |
| V | 12 (100) | 0 (0) | 6 (50) | 0 (0) | 0 (0) | NT | NT |
| VI | 31 (100) | 0 (0) | 2 (6.5) | 3 (9) | 0 (0) | NT | NT |
| VII | 165 (100) | 26 (16) | 155 (94) | 160 (97) | 0 (0) | 162 (98) | 26 (16) |
| VIII | 13 (100) | 0 (0) | 11 (85) | 0 (0) | 0 (0) | NT | NT |
| IX | 1 (100) | 0 (0) | 1 (100) | 1 (100) | 0 (0) | NT | NT |
Specific sequence of 16S-23S spacer region of phage type DT104 and its related phage type, U302.
NT, not tested.
Subtraction of the genomic DNA of the KT262 isolate (profile VII-6) with DNA from the LT2 strain.
Beginning in 2002, we observed an increase in bovine-origin S. Typhimurium isolates that were classified as PFGE cluster VII. Almost all of these isolates showed multidrug resistance and were assigned to the same cluster by MLVA. To identify and characterize the specific sequences of these isolates, genomic subtractive hybridization was performed. We selected a representative isolate (KT262) from isolates showing cefazolin resistance (PFGE profile VII-6) as a tester and used the LT2 strain as a driver. The subtractive hybridization library resulted in 88 different DNA fragments, 25 of which were not found in the S. Typhimurium LT2 genome using BLASTN analysis (see Table S2 in the supplemental material). After BLASTN analysis through GenBank, these 25 fragments were divided into five groups showing sequence similarity to either the S. enterica serovar Newport SL254 plasmid pSN254 (n = 14), S. Typhimurium plasmid pU302L (n = 4), S. Typhimurium DT104 bacteriophage ST104 (n = 2), S. Typhimurium DT64 bacteriophage STB64B (n = 3), or avian pathogenic E. coli (APEC) strain O1 (n = 2) (see Table S2). The presence of four DNA fragments (SN-11, PU302-2, ST104-1, and ST64B-1) showing high sequence similarities to portions of pSN254 (38), pU302L (7), ST104 (35), and STB64 (21), respectively, was tested in 29 isolates, including 21 representative isolates of the 21 PFGE profiles assigned to cluster VII by PCR. Fragments SN-11 and PU302L-2 were present in almost all of the representative isolates of cluster VII but were absent in those of the other clusters (Table 4).
TABLE 4.
Characteristics of representative isolates of S. Typhimurium
| Isolate | PFGE pattern | MLVA clustera | Antibiotic resistance profileb | Plasmid profile (kb) | Target for PCRe |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| floR | 162-bp ampliconc | Class I integron (bp) | ST104-1 | ST64B-1 | SN-11 | PU302L-2 |
spvC |
blaTEM-1 |
blaCMY-2 |
||||||||
| PCR detection | Plasmid size (kb)d | PCR detection | Plasmid size (kb)d | PCR detection | Plasmid size (kb)d | ||||||||||||
| KT20 | I-1 | A | AMP, CHL, STR, SUL, TET | 94 | + | + | 1,000 | + | + | − | − | + | 94 | − | − | ||
| 1,200 | |||||||||||||||||
| IS18-33 | II-15 | NT | — | 94 | − | − | − | − | + | − | − | + | 94 | − | − | ||
| TST49 | III-1 | NT | AMP, STR, SUL, TET | None | − | − | − | + | + | − | − | − | + | − | |||
| N48 | IV-15 | B | AMP, CHL, STR, SUL, TET, KAN | 190, 145 | − | − | 2,000 | − | − | − | − | − | − | − | |||
| TST161 | V-3 | B | AMP, CHL, STR, SUL, TET, KAN | 130 | − | − | − | − | − | − | − | − | − | − | |||
| TST178 | VI-3 | B | AMP, CHL, STR, SUL, TET, KAN | 190 | − | − | − | − | − | − | − | − | + | 190 | − | ||
| TST205 | VII-1 | D | AMP, STR, SUL, TET, KAN | 130 | − | − | 1,000 | − | + | + | + | + | 130 | + | 130 | − | |
| KT261 | VII-2 | D | AMP, STR, SUL, TET, KAN | 130 | − | − | 1,000 | − | + | + | + | + | 130 | + | 130 | − | |
| R18-1 | VII-3 | D | AMP, STR, SUL, TET, KAN | 130, 96 | − | − | 1,000 | − | + | + | + | + | 130 | + | 130 | − | |
| 07SY9 | VII-4 | D | AMP, STR, SUL, TET, KAN | 130 | − | − | 1,000 | − | + | + | + | + | 130 | + | 130 | − | |
| TST228 | VII-5 | D | AMP, STR, SUL, TET, KAN | 130, 78 | − | − | 1,000 | − | + | + | + | + | 130 | + | 130 | − | |
| KT262 | VII-6 | D | AMP, CHL, STR, SUL, TET, KAN CFZ, SXT | 130 | + | − | 1,000 | + | + | + | + | + | 130 | + | 130 | + | |
| KT291 | VII-7 | D | AMP, STR, SUL, TET, KAN | 130 | − | − | 1,000 | + | + | + | + | + | 130 | + | 130 | − | |
| KT302 | VII-8 | D | AMP, CHL, STR, SUL, TET, KAN CFZ, CAZ | 130, 95 | + | − | 1,000 | + | + | + | + | + | 130 | + | 130 | + | |
| TST207 | VII-9 | D | AMP, CHL, STR, SUL, TET, KAN, CFZ, CAZ, CTX | 120, 110 | + | − | 1,000 | + | + | + | + | + | 110 | + | 110 | + | 120 |
| KT165 | VII-10 | D | AMP, STR, SUL, TET, KAN | 110 | − | − | 1,000 | + | + | + | + | + | 110 | + | 110 | − | |
| NST78 | VII-11 | D | AMP, STR, SUL, TET, KAN | 110 | − | − | 1,000 | + | + | + | + | + | 110 | + | 110 | − | |
| KT158 | VII-12 | D | AMP, STR, SUL, TET, KAN | 110 | − | − | 1,000 | + | + | + | + | + | 110 | + | 110 | − | |
| KT161 | VII-13 | D | AMP, STR, SUL, TET, KAN | 110 | − | − | 1,000 | + | + | + | + | + | 110 | + | 110 | − | |
| NST110 | VII-14 | D | AMP, TET, KAN | 97 | − | − | − | + | + | − | + | + | 97 | + | 97 | − | |
| TST233 | VII-15 | D | AMP, STR, SUL, TET, KAN | 120, 95 | − | − | 1,000 | − | + | + | − | − | 120 | + | 120 | − | |
| 07IB1 | VII-16 | D | AMP, STR, SUL, TET, KAN | 95 | − | − | 1,000 | − | + | + | − | − | 95 | + | 95 | − | |
| NST95 | VII-17 | NT | — | 94 | − | − | − | − | + | − | − | + | 94 | − | − | ||
| HD | VII-18 | D | AMP, STR, SUL, TET, KAN | 130 | − | − | 1,000 | + | + | + | + | + | 130 | + | 130 | − | |
| TST31 | VII-19 | D | AMP, STR, SUL, TET, KAN | 110 | − | − | 1,000 | + | + | + | + | + | 110 | + | 110 | − | |
| KT271 | VII-20 | D | AMP, CHL, STR, SUL, TET, KAN, CFZ, SXT | 130 | + | − | 1,000 | + | + | + | + | + | 130 | + | 130 | + | |
| IS19-6 | VII-21 | NT | — | 94 | − | − | − | − | + | − | − | + | 94 | − | − | ||
| TST181 | VIII-1 | C | AMP, STR, TET,CFZ, NAL | 94, 8.4 | − | − | − | + | + | − | − | + | 94 | + | 8.4 | − | |
| TST164 | IX-1 | NT | AMP, CHL, STR, SUL, TET, KAN, CFZ | 94 | − | − | 2,000 | + | − | − | − | + | 94 | + | − | ||
NT, nontypeable.
AMP, ampicillin; CHL, chloramphenicol; STR, streptomycin; SUL, sulfonamides; TET, tetracycline; KAN, kanamycin; CFZ, cefazolin; CAZ, ceftazidime; CTX, cefotaxime; SXT, sulfamethoxazole-trimethoprim; NAL, nalidixic acid; —, sensitive.
Sequence of 16S-23S spacer region which is specific to DT104 and its related phage type, U302.
Detection of the genes located on the plasmids by Southern hybridization.
+, presence of an amplification product from the primers directed at the indicated gene or fragment; −, absence of amplification product of PCR.
Characterization of multidrug resistance plasmids from isolates belonging to PFGE cluster VII.
Plasmids pSN254 and pU302L harbor the β-lactamase genes blaCMY-2 and blaTEM-1, respectively (7, 38). All but three isolates grouped into cluster VII contained the blaTEM-1 gene, and 26 isolates contained blaCMY-2 (Table 3). Salmonella serovar Typhimurium carries a 94.7-kb Salmonella virulence plasmid (14). Recently, serovar-specific virulence plasmids of various sizes have been found in Salmonella; some of these virulence-resistance plasmids, each encoding different resistance genes, have been detected in Salmonella isolates collected in various countries (8, 9, 12, 13, 20). To examine the presence of virulence-resistance plasmids in cluster VII isolates, the same 29 representative isolates were examined for plasmid content and the presence and location of blaCMY-2, blaTEM-1, and spvC using both PCR and Southern hybridization. Twenty-one isolates grouped into cluster VII harbored plasmids ranging in size from approximately 78 kb to 130 kb (Table 4). PCR and Southern hybridization analysis showed that the blaTEM-1 gene was present in all 19 ampicillin-resistant isolates grouped into cluster VII (Table 4; see also Fig. S1 in the supplemental material). In 16 of these 19 isolates, the blaTEM-1 gene was carried on a 130-kb or 110-kb plasmid on which virulence gene spvC was located. The 97-kb plasmid DNA isolated from NST110, which showed resistance to ampicillin, tetracycline, and kanamycin, also harbored both blaTEM-1 and spvC. The 120- and 95-kb plasmids isolated from TST233 and O7IB1, respectively, carried blaTEM-1 (Table 4; see also Fig. S1). Although PCR analysis determined that spvC was absent in these two isolates, Southern hybridization analysis showed that they carried spvC (Table 4; see also Fig. S1). This PCR failure may have resulted from a primer-hybrid mismatch due to template variation. The 19 blaTEM-1-positive isolates of PFGE cluster VII listed in Table 4 were tested for the specific blaTEM-1 gene by sequencing the PCR amplification products. All of the PCR products from the 19 blaTEM-1-positive isolates were 100% identical to the blaTEM-1 sequence in GenBank (GenBank accession number AM234722).
The cefotaxime-resistant isolate of TST207 harbored 110- and 120-kb plasmids on which the blaTEM-1 and blaCMY-2 genes were located, respectively (Table 4; see also Fig. S1 in the supplemental material). Although the 110-kb blaTEM-1-carrying plasmid from TST207 carried spvC, this gene was not detected in the 120-kb blaCMY-2-carrying plasmid by Southern hybridization (Table 4; see also Fig. S1). DNA sequence analysis of the PCR amplicon of the blaCMY-2 gene from KT262, KT302, TST207, and KT271 demonstrated 100% homology to blaCMY-2 (GenBank accession number Y16784).
These blaTEM-1- and blaCMY-2-carrying plasmids were further identified as being multidrug-resistant by analyzing the antimicrobial resistance profiles of E. coli DH5α transformants. Transformation of plasmid DNAs isolated from the 19 cluster VII isolates into E. coli DH5α was carried out on a selective medium containing ampicillin or cefazolin. As expected, the transformant obtained with a large plasmid from each of the 19 isolates resulted in the isolation of ampicillin-resistant E. coli containing the large plasmid (blaTEM-1- or blaCMY-2-carrying plasmid) (Table 5). However, a cefazolin-resistant E. coli transformant was obtained only by transformation of the blaCMY-2-carrying plasmid DNA (120 kb) isolated from TST207. When the resistance phenotypes of the transformants were evaluated by disk diffusion assay, all but one of the blaTEM-1-carrying plasmid transformants demonstrated resistance to ampicillin, streptomycin, sulfonamides, tetracycline, and kanamycin (Table 5). The transformant carrying a 97-kb plasmid from NST110 was resistant to ampicillin, tetracycline, and kanamycin but sensitive to streptomycin and sulfonamides (Table 5). On the other hand, the cefazolin-resistant transformant with the blaCMY-2-carrying plasmid was resistant to ampicillin, chloramphenicol, streptomycin, sulfonamides, and tetracycline (Table 5).
TABLE 5.
Characterization of E. coli transformants with multidrug resistance plasmids from isolates of PFGE cluster VII
| Transformant | PFGE pattern of donor | Antibiotic resistance patterna | Plasmid size (kb) | Target for PCRb |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| intI | Class I integron (bp) | froR | SN-11 | PU302-2 | repFIIA | spvA | spvB | spvC | rck | traT | pefA | ||||
| TF:TST205 | VII-1 | AMP, STR, SUL, TET, KAN | 130 | + | 1,000 | − | + | + | + | + | + | + | + | + | − |
| TF:KT261 | VII-2 | AMP, STR, SUL, TET, KAN | 130 | + | 1,000 | − | + | + | + | + | + | + | + | + | − |
| TF:R18-1 | VII-3 | AMP, STR, SUL, TET, KAN | 130 | + | 1,000 | − | + | + | + | + | + | + | + | + | − |
| TF:07SY9 | VII-4 | AMP, STR, SUL, TET, KAN | 130 | + | 1,000 | − | + | + | + | + | + | + | + | + | − |
| TFTST228 | VII-5 | AMP, STR, SUL, TET, KAN | 130 | + | 1,000 | − | + | + | + | + | + | + | + | + | − |
| TF:KT262 | VII-6 | AMP, STR, SUL, TET, KAN | 130 | + | 1,000 | − | + | + | + | + | + | + | + | + | − |
| TF:KT291 | VII-7 | AMP, STR, SUL, TET, KAN | 130 | + | 1,000 | − | + | + | + | + | + | + | + | + | − |
| TF:KT302 | VII-8 | AMP, STR, SUL, TET, KAN | 130 | + | 1,000 | − | + | + | + | + | + | + | + | + | − |
| TF:TST207-1 | VII-9 | AMP, CHL, STR, SUL,TET, CFZ | 120 | − | − | + | + | − | − | − | − | − | − | − | − |
| TF:TST207-4 | VII-9 | AMP, STR, SUL, TET, KAN | 110 | + | 1,000 | − | + | + | + | + | + | + | − | + | − |
| TF:KT165 | VII-10 | AMP, STR, SUL, TET, KAN | 110 | + | 1,000 | − | + | + | + | + | + | + | + | + | − |
| TF:NST78 | VII-11 | AMP, STR, SUL, TET, KAN | 110 | + | 1,000 | − | + | + | + | + | + | + | + | + | − |
| TF:KT158 | VII-12 | AMP, STR, SUL, TET, KAN | 110 | + | 1,000 | − | + | + | + | + | + | + | + | + | − |
| TF:KT161 | VII-13 | AMP, STR, SUL, TET, KAN | 110 | + | 1,000 | − | + | + | + | + | + | + | + | + | − |
| TF:NST110 | VII-14 | AMP, TET, KAN | 97 | − | − | − | − | + | + | + | + | + | + | + | − |
| TF:TST233 | VII-15 | AMP, STR, SUL, TET, KAN | 120 | + | 1,000 | − | + | − | + | + | + | − | + | + | − |
| TF:07IB1 | VII-16 | AMP, STR, SUL, TET, KAN | 95 | + | 1,000 | − | + | − | + | + | + | − | + | + | − |
| TF:HD | VII-18 | AMP, STR, SUL, TET, KAN | 130 | + | 1,000 | − | + | + | + | + | + | + | + | + | − |
| TF:TST31 | VII-19 | AMP, STR, SUL, TET, KAN | 110 | + | 1,000 | − | + | + | + | + | + | + | + | + | − |
| TF:KT271 | VII-20 | AMP, STR, SUL, TET, KAN | 130 | + | 1,000 | − | + | + | + | + | + | + | + | + | − |
AMP, ampicillin; CHL, chloramphenicol; STR, streptomycin; SUL, sulfonamides; TET, tetracycline; KAN, kanamycin; CFZ, cefazolin.
+, presence of an amplification product from the primers directed at the indicated gene or fragment; −, absence of amplification product of PCR.
To compare the restriction fragment length polymorphisms of the blaTEM-1-carrying plasmids, plasmid DNA from the E. coli transformants was isolated and digested with PstI. As shown in Fig. 3, complete identity was not observed between any of the plasmids. However, many fragments were shared among the blaTEM-1-carrying plasmids from these transformants, suggesting that the plasmids share a highly related plasmid backbone.
FIG. 3.
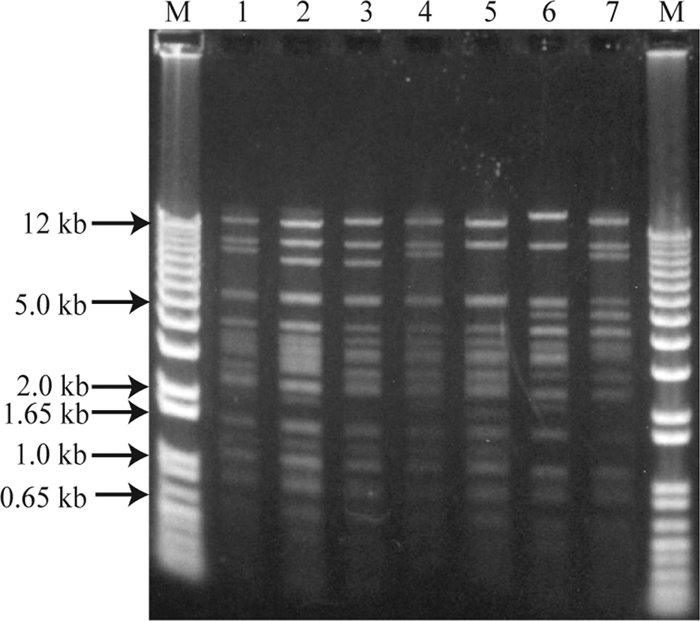
PstI-digested plasmid DNA isolated from the E. coli DH5α transformants (TF). Lane M, molecular size marker; lane 1, TF:KT261 (130-kb plasmid); lane 2, KT262 (130-kb plasmid); lane 3, TF:TST207-4 (110-kb plasmid); lane 4, TF:KT161 (110-kb plasmid); lane 5, TF:NST110 (97-kb plasmid); lane 6, TF:TST233 (120-kb plasmid); lane 7, 07IB1 (95-kb plasmid).
When these blaTEM-1-carrying plasmids were tested by PCR for virulence plasmid genes (repFIIA [plasmid incompatibility group FII replicons]; traT [conjugative transfer]; spvA, spvB, and spvC [Salmonella plasmid virulence]; rck [resistance to complement killing]; and pefA [plasmid-encoded fimbrial]), the expected amplicons were generated from almost all of the blaTEM-1-carrying plasmids with all of the primers except those for pefA (Table 5). Almost all of the blaTEM-1-carrying plasmids contained sequences (SN-11 and/or PU302L-2) present in two multidrug-resistant plasmids, pN254 isolated from S. enterica serovar Newport and pU302L from S. Typhimurium, suggesting a mosaic structure composed of elements of different origin (Table 5). Therefore, the large blaTEM-1-carrying plasmid may have been derived from genetic recombination between the 94.7-kb S. Typhimurium-specific virulence plasmid and another nonvirulence plasmid. Furthermore, by sequencing analysis, 1.0-kb class I integron conserved-segment amplicons bearing addA for streptomycin resistance were confirmed to be present in all but one of the cluster VII isolates harboring blaTEM-1-carrying plasmids (Tables 4 and 5), indicating a mechanism for generation of multidrug resistance.
Recently, in the Pacific Northwest of the United States, an increased frequency of a novel multidrug-resistant clone of S. Typhimurium, WA-TYP035/187, with no recognized phage type, has been reported (1, 10). Some isolates showing similar PFGE profiles to those of WA-TYP035/187 were observed among the PFGE cluster VII isolates (data not shown). Furthermore, three MLVA profiles for 13 isolates belonging to PFGE cluster VII were the same as those reported for WA-TYP035/187 (1) (see Table S3 in the supplemental material). We examined the phage type of certain isolates belonging to this cluster but found that they could not be typed (data not shown). These results suggest that an epidemic clone similar to WA-TYP035/187 disseminated among cattle in Japan.
A significant increase in the prevalence of ceftazidime-resistant isolates among WA-TYP035/187 has been observed, and the presence of blaCMY-2-carrying plasmids in these isolates has been reported (1, 10). The blaCMY-2 gene was identified in all 26 cefazolin-resistant isolates grouped into PFGE cluster VII; however, only four isolates were resistant to ceftazidime (data not shown). Furthermore, the blaCMY-2-carrying plasmid was detected in only one isolate, which was resistant to cefotaxime, suggesting that this gene is located on the chromosome of most cefazolin-resistant isolates. Therefore, although the epidemic clones of multidrug-resistant S. Typhimurium in the United States and Japan appear to be similar, the proportion of isolates resistant to third-generation cephalosporins differs. In Japan, extended-spectrum β-lactamase-producing E. coli (30) and S. enterica serovar Senftenberg (15) have been obtained from cattle and broilers, respectively. Therapeutic use of the extended-spectrum cephalosporin ceftiofur in cattle may promote an increase in third-generation cephalosporin resistance of S. Typhimurium in Japan, as has been observed in the United States.
Conclusions.
A previous study indicated that the proportion of DT104-related S. Typhimurium isolates from cattle decreased from 71.9% in the period from 1999 to 2001 to 30.8% in the period from 2002 to 2005 in Japan (16). In agreement with this report, our results show that the proportion of PFGE cluster I isolates, including DT104 isolates, decreased from 82% in the period from 1990 to 1999 to 27% in the period from 2000 to 2009. On the other hand, we observed an increase beginning in 2002 in bovine-origin S. Typhimurium isolates of PFGE cluster VII, an emerging multidrug resistance clone carrying a virulence-resistance plasmid, suggesting that clonal replacement is occurring. The use of standardized subtyping methods such as PFGE and MLVA allows comparison of isolates from different areas, and routine and long-term epidemiological surveillance with such methods enables early recognition of epidemic Salmonella clones.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Ministry of Agriculture, Forestry, and Fisheries of Japan (research project for ensuring food safety from farm to table, LP-5201).
Footnotes
Published ahead of print on 14 January 2011.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Adhikari, B., et al. 2010. Multilocus variable-number tandem-repeat analysis and plasmid profiling to study the occurrence of blaCMY-2 within a pulsed-field gel electrophoresis-defined clade of Salmonella enterica serovar Typhimurium. Appl. Environ. Microbiol. 76:69-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, E. S., L. R. Ward, M. J. Saxe, and J. D. de Sa. 1977. Bacteriophage-typing designations of Salmonella typhimurium. J. Hyg. (Lond.) 78:297-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arcangioli, M.-A., S. Leroy-Sétrin, J.-L. Martel, and E. Chaslus-Dancla. 1999. A new chloramphenicol and florfenicol resistance gene flanked by two integron structures in Salmonella typhimurium DT104. FEMS Microbiol. Lett. 174:327-332. [DOI] [PubMed] [Google Scholar]
- 4.Besser, T. E., et al. 2000. Multiresistant Salmonella Typhimurium DT104 infections of humans and domestic animals in the Pacific Northwest of the United States. Epidemiol. Infect. 124:193-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolton, L. F., L. C. Kelley, M. D. Lee, P. J. Fedorka-Cray, and J. J. Maurer. 1999. Detection of multidrug-resistant Salmonella enterica serotype typhimurium DT104 based on a gene which confers cross-resistance to florfenicol and chloramphenicol. J. Clin. Microbiol. 37:1348-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briggs, C. E., and P. M. Fratamico. 1999. Molecular characterization of an antibiotic resistance gene cluster of Salmonella typhimurium DT104. Antimicrob. Agents Chemother. 43:846-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, C.-Y., G. W. Nace, B. Solow, and P. Fratamico. 2007. Complete nucleotide sequences of 84.5- and 3.2-kb plasmids in the multi-antibiotic resistant Salmonella enterica serovar Typhimurium U302 strain G8430. Plasmid 57:29-43. [DOI] [PubMed] [Google Scholar]
- 8.Chu, C., and C.-H. Chiu. 2006. Evolution of the virulence plasmids of non-typhoid Salmonella and its association with antimicrobial resistance. Microbes Infect. 8:1931-1936. [DOI] [PubMed] [Google Scholar]
- 9.Chu, C., et al. 2001. Large drug resistance virulence plasmids of clinical isolates of Salmonella enterica serovar Choleraesuis. Antimicrob. Agents Chemother. 45:2299-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis, M. A., et al. 2007. Multidrug-resistant Salmonella typhimurium, Pacific Northwest, United States. Emerg. Infect. Dis. 13:1583-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glynn, M. K., et al. 1998. Emergence of multidrug-resistant Salmonella enterica serotype Typhimurium DT104 infections in the United States. N. Engl. J. Med. 338:1333-1338. [DOI] [PubMed] [Google Scholar]
- 12.Guerra, B., I. Laconcha, S. M. Soto, M. A. González-Hevia, and M. C. Mendoza. 2000. Molecular characterisation of emergent multiresistant Salmonella enterica serotype [4,5,12:i:−] organisms causing human salmonellosis. FEMS Microbiol. Lett. 190:341-347. [DOI] [PubMed] [Google Scholar]
- 13.Guerra, B., S. Soto, R. Helmuth, and M. C. Mendoza. 2002. Characterization of a self-transferable plasmid from Salmonella enterica serotype Typhimurium clinical isolates carrying two integron-borne gene cassettes together with virulence and drug resistance genes. Antimicrob. Agents Chemother. 46:2977-2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gulig, P. A., et al. 1993. Molecular analysis of spv virulence genes of the Salmonella virulence plasmids. Mol. Microbiol. 7:825-830. [DOI] [PubMed] [Google Scholar]
- 15.Ishihara, K., et al. 2009. National surveillance of Salmonella enterica in food-producing animals in Japan. Acta Vet. Scand. 51:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawagoe, K., et al. 2007. Changes of multi-drug resistance pattern in Salmonella enterica subspecies enterica serovar Typhimurium isolates from food-producing animals in Japan. J. Vet. Med. Sci. 69:1211-1213. [DOI] [PubMed] [Google Scholar]
- 17.Levesque, C., L. Piche, C. Larose, and P. H. Roy. 1995. PCR mapping of integrons reveals several novel combinations of resistance genes. Antimicrob. Agents Chemother. 39:185-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindstedt, B.-A., E. Heir, E. Gjernes, and G. Kapperud. 2003. DNA fingerprinting of Salmonella enterica subsp. enterica serovar Typhimurium with emphasis on phage type DT104 based on variable number of tandem repeat loci. J. Clin. Microbiol. 41:1469-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindstedt, B.-A., T. Vardund, L. Aas, and G. Kapperud. 2004. Multiple-locus variable-number tandem-repeats analysis of Salmonella enterica subsp. enterica serovar Typhimurium using PCR multiplexing and multicolor capillary electrophoresis. J. Microbiol. Methods 59:163-172. [DOI] [PubMed] [Google Scholar]
- 20.Llanes, C., V. Kirchgesner, and P. Plesiat. 1999. Propagation of TEM- and PSE-type β-lactamases among amoxicillin-resistant Salmonella spp. isolated in France. Antimicrob. Agents Chemother. 43:2430-2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mmolawa, P. T., H. Schmieger, and M. W. Heuzenroeder. 2003. Bacteriophage ST64B, a genetic mosaic of genes from diverse sources isolated from Salmonella enterica serovar Typhimurium DT 64. J. Bacteriol. 185:6481-6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. 2000. Performance standards for antimicrobial disk susceptibility tests; approved standard M2-A7, 7th ed. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 23.Ng, L.-K., M. R. Mulvey, I. Martin, G. A. Peters, and W. Johnson. 1999. Genetic characterization of antimicrobial resistance in Canadian isolates of Salmonella serovar Typhimurium DT104. Antimicrob. Agents Chemother. 43:3018-3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pritchett, L. C., M. E. Konkel, J. M. Gay, and T. E. Besser. 2000. Identification of DT104 and U302 phage types among Salmonella enterica serotype Typhimurium isolates by PCR. J. Clin. Microbiol. 38:3484-3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riaño, I., et al. 2006. Detection and characterization of extended-spectrum β-lactamases in Salmonella enterica strains of healthy food animals in Spain. J. Antimicrob. Chemother. 58:844-847. [DOI] [PubMed] [Google Scholar]
- 26.Ridley, A., and E. J. Threlfall. 1998. Molecular epidemiology of antibiotic resistance genes in multiresistant epidemic Salmonella typhimurium DT 104. Microb. Drug Resist. 4:113-118. [DOI] [PubMed] [Google Scholar]
- 27.Saitoh, M., et al. 2005. The artAB genes encode a putative ADP-ribosyltransferase toxin homologue associated with Salmonella enterica serovar Typhimurium DT104. Microbiology 151:3089-3096. [DOI] [PubMed] [Google Scholar]
- 28.Sameshima, T., et al. 2000. Salmonella Typhimurium DT104 from livestock in Japan. Jpn. J. Infect. Dis. 53:15-16. [PubMed] [Google Scholar]
- 29.Sandvang, D., F. M. Aarestrup, and L. B. Jensen. 1998. Characterisation of integrons and antibiotic resistance genes in Danish multiresistant Salmonella enterica Typhimurium DT104. FEMS Microbiol. Lett. 160:37-41. [DOI] [PubMed] [Google Scholar]
- 30.Shiraki, Y., N. Shibata, Y. Doi, and Y. Arakawa. 2004. Escherichia coli producing CTX-M-2 β-lactamase in cattle, Japan. Emerg. Infect. Dis. 10:69-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skyberg, J. A., C. M. Logue, and L. K. Nolan. 2006. Virulence genotyping of Salmonella spp. with multiplex PCR. Avian Dis. 50:77-81. [DOI] [PubMed] [Google Scholar]
- 32.Swaminathan, B., T. J. Barrett, S. B. Hunter, R. V. Tauxe, and the CDC PulseNet Task Force. 2001. PulseNet: the molecular subtyping network for foodborne bacterial disease surveillance, United States. Emerg. Infect. Dis. 7:382-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swamy, S. C., H. M. Barnhart, M. D. Lee, and D. W. Dreesen. 1996. Virulence determinants invA and spvC in salmonellae isolated from poultry products, wastewater, and human sources. Appl. Environ. Microbiol. 62:3768-3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamada, Y., et al. 2001. Molecular typing and epidemiological study of Salmonella enterica serotype Typhimurium isolates from cattle by fluorescent amplified-fragment length polymorphism fingerprinting and pulsed-field gel electrophoresis. J. Clin. Microbiol. 39:1057-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanaka, K., et al. 2004. Molecular characterization of a prophage of Salmonella enterica serotype Typhimurium DT104. J. Clin. Microbiol. 42:1807-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Threlfall, E. J., J. A. Frost, L. R. Ward, and B. Rowe. 1994. Epidemic in cattle and humans of Salmonella typhimurium DT 104 with chromosomally integrated multiple drug resistance. Vet. Rec. 134:577. [DOI] [PubMed] [Google Scholar]
- 37.Villar, R. G., et al. 1999. Investigation of multidrug-resistant Salmonella serotype Typhimurium DT104 infections linked to raw-milk cheese in Washington State. JAMA 281:1811-1816. [DOI] [PubMed] [Google Scholar]
- 38.Welch, T. J., et al. 2007. Multiple antimicrobial resistance in plague: an emerging public health risk. PLoS One 2:e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.