Abstract
The survival of enteric bacteria in 10 freshly collected sheep fecal samples on pastures was measured in each of four seasons. Ten freshly collected feces were placed on pasture, and concentrations of Escherichia coli, enterococci, and Campylobacter spp. were monitored until exhaustion of the fecal samples. In all four seasons, there was an increase in enterococcal concentrations by up to 3 orders of magnitude, with peak concentrations recorded between 11 and 28 days after deposition. E. coli concentrations increased in three out of four seasons by up to 1.5 orders of magnitude, with peak concentrations recorded between 8 and 14 days after deposition. The apparent growth of E. coli and enterococci was strongly influenced by the initial water content of the feces and the moisture gained during periods of rehydration following rainfalls. Conversely, the results suggested that dehydration promoted inactivation. Campylobacter spp. did not grow and were rapidly inactivated at a rate that tended to be faster at higher temperatures. Pulsed-field gel electrophoresis (PFGE) of a selection of Campylobacter spp. suggested that these survival data are applicable to a range of Campylobacter spp., including the most frequently isolated PFGE genotype from sheep in New Zealand, and to genotypes previously observed to cause disease in humans. The results of this study are currently being incorporated into a fecal microbe reservoir model that is designed to assist water managers' abilities to estimate microbial loads on pastures grazed by sheep, including the influence of factors such as rainfall and temperature.
In New Zealand 32.4 million sheep graze on open pasture (21). While there is significant evidence of the direct impact of cattle on rural waterways (6, 9, 11, 31), sheep have nevertheless been implicated as contributors to bacterial indicator and pathogen loadings in rivers and streams (8, 10, 18, 20). It has been suggested that the total number of Escherichia coli per hectare of pasture are higher for sheep than for cattle (30).
Sheep harbor a range of microbial pathogens, including Campylobacter spp. (2, 19, 23), Giardia spp. (4, 27), and Cryptosporidium spp. (4, 17, 19, 24). Salmonella spp. carriage rates appear to be low (9), and there is evidence that many of the ovine Giardia spp. and Cryptosporidium genotypes may not be zoonotic (26). In New Zealand, most of the information on ovine carriage of potential zoonotic agents is for Campylobacter spp. (3, 10), although some of this is derived from liver and meat tissues rather than fecal samples (7, 32).
To gauge the size of the microbial “reservoir” in feces from sheep on pasture, information is required on microbial concentrations in fresh feces and their survival rates after deposition. Although survival rates of microbes in cattle feces have been reported (1, 15, 16, 28, 29), there are relatively few studies on the survival of enteric microorganisms in sheep feces. The survival of enteric bacteria within individual deposits of feces from sheep on pasture in different seasons has not been reported.
This paper describes a field study of the comparative survival of two bacterial indicators and one pathogen in ovine feces on pasture over four seasons. The selected indicators were E. coli and enterococci, which are recommended by the Ministry for the Environment in New Zealand for monitoring fresh and marine recreational waters, respectively (20). The selected pathogen was Campylobacter spp., because New Zealand has a high annual incidence of campylobacteriosis (166.3 per 100,000 people) (12). The data generated in this study will be used to develop a fecal microbe reservoir model for water managers, into which they can enter the season, number of sheep, and details of rainfall/irrigation to determine the present and future microbial loading from sheep in a catchment.
MATERIALS AND METHODS
Field site.
The study was conducted at a 10-m by 7-m, unshaded, experimental site at Lincoln, 10 km south of Christchurch, New Zealand. The site pasture, which was a typical New Zealand sward of rye grass and white clover, was hand mown throughout the study to ensure ease of access to, and prevent shading of, the samples.
Meteorological data.
Data detailing rainfall (in mm), air temperature (°C), and soil temperature at 10 cm were obtained from a weather station operated by Plant and Food Research NZ, which was situated 200 m from the site for the period of the study (March 2007 to April 2008).
Experimental setup.
Four experiments were conducted, starting in January, March, June, and October 2007, corresponding to the Southern hemisphere's summer, autumn, winter, and spring seasons, respectively.
For each experiment, the rectal-anal junction (RAJ) was collected from 11 sheep selected randomly from the same flock at an abattoir during evisceration. Samples were transported to the laboratory in a cooled, dark container. Using a sterile scalpel, fecal material from the RAJ was extracted, and this provided an individual deposited fecal sample for the survival experiments.
Ten fecal samples (approximately 32 g) were placed on the pasture at the field site, in separate, pegged plots, and the exact location was marked with a plastic hoop (10-cm diameter). The 11th sample was set aside for temperature measurements.
Sample collection.
Immediately after placement on the pasture, six pellets (approximately 3 g in total) were removed from each fecal sample by using sterile tweezers and placed in sterile, screw-cap pots. This procedure was repeated 2 and 4 days later and then typically weekly, and subsequently fortnightly, until the feces had disintegrated and/or were indistinguishable from the underlying soil. Samples were transported to the laboratory in a cooled, dark container.
Laboratory methods.
The moisture content of one pellet in each fecal sample was determined by drying at 103 to 105°C for 18 h (5). All microbial concentrations were then expressed per g dry weight.
A further five pellets per fecal sample were placed in a sterile 40-ml tube containing buffered peptone water (Oxoid, United Kingdom), and they were homogenized. A 20-ml volume was removed and used to create a series of 10-fold dilutions in 9 ml of peptone water (0.1%; Fort Richard Laboratories, Otahuhu, New Zealand).
E. coli/enterococci.
E. coli and enterococci were enumerated using the Colilert and Enterolert systems (Idexx Laboratories Inc., Westbrook, ME), respectively, according to the manufacturer's instructions, except for the use of an elevated incubation temperature (44.5°C) (33). Briefly, one ampoule of reagent (Colilert/Enterolert) was dissolved in 99 ml of sterile distilled water, 1 ml as a diluted sample was added, and the suspension was mixed well, poured into a Quanti-Tray, and sealed using a Quanti-Tray sealer. For Colilert analysis, the Quanti-Tray was placed in a waterproof container and submerged in a 44.5°C water bath for 24 h. Enterolert Quanti-Trays were incubated at 42°C for 24 h prior to analysis. The limit of detection for the Enterolert and Colilert most probable number (MPN) systems is 1 MPN per 100 ml. As a dilute sample was always analyzed, the limit of detection was determined to be 10 MPN per g of fecal sample. Yellow Colilert wells that fluoresced when viewed under UV light were enumerated as E. coli. Wells that fluoresced on Enterolert Quanti-Trays when viewed under UV light were enumerated as enterococci.
Campylobacter spp.
Campylobacter spp. were enumerated by a three-by-five MPN procedure, using 30-ml vials of Preston's broth, all of which were plated onto m-Exeter agar and incubated at 37°C for a minimum of 4 h under microaerophilic conditions (10% CO2), followed by transfer to a 42°C incubator for the remainder of a 48-h total incubation period. Suspect Campylobacter spp. colonies (typically three per plate) were confirmed using biochemical tests (oxidase, catalase), colony morphology, Gram staining, and a multiplex PCR, as described by Wong et al. (32). This PCR procedure allows for isolates to be classified as Campylobacter jejuni, Campylobacter coli, or thermotolerant Campylobacter spp.
Calculation of T90 and k values.
To characterize and compare the increase and decrease phases, regression analyses were performed on the loge-transformed data. One regression line (for the increase phase, where present) was fitted to the data up to and including the peak count, and a second line (for the decrease phase) was fitted to the data after and including the peak count. These regressions were used to derive increase and decrease coefficients (k values, in day−1). In addition, the T90 values (times to 90% inactivation) were calculated for the decrease phase only and for all data points from the time of deposition. For the former, this was calculated as 2.303/k. For the latter, the decrease phase regression equation was solved for the time of intersection with the x axis, at a nominal extinction level (on the y axis) of 0.01%. A second regression line was then drawn through the x axis intersection and the 100% y axis value (i.e., at time of deposition), and the T90 was then calculated as 2.303/k. The latter approach was required because sampling ceased (due to the loss of fecal material) before concentrations naturally reached a realistic extinction level.
Determination of variability.
An experiment was conducted to establish the natural variability of E. coli, enterococci, and Campylobacter spp. in individual pellets from a single sheep. Three fecal samples were collected from a sheep slaughter line. For one sample, E. coli and enterococci were enumerated in each fecal pellet (n = 40), and Campylobacter spp. were enumerated in the pellets of the remaining two fecal samples (n = 30), as described above.
RESULTS
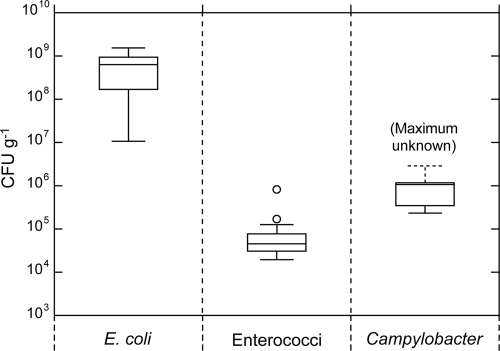
The results of the interfecal pellet variability testing are presented as box plots in Fig. 1. Overall, E. coli demonstrated the highest degree of variability, with an interquartile distance of almost 1 order of magnitude, and with 2 orders of magnitude between the maximum and minimum concentrations. The maximum count for Campylobacter spp. could not be determined because four concentrations exceeded the detection threshold, and the maximum count was assumed to be equivalent to the detection threshold. Thus, the quartile and median values should be regarded as probable underestimates.
FIG. 1.
Variability associated with individual fecal pellets collected from the rectal-anal junction from a single sheep. The cross-pieces of each box plot represent (from top to bottom) the maximum, upper quartile, median, lower quartile, and minimum values. The outliers (open circles) were defined as points whose values were either above the upper quartile, or below the lower quartile, by 1.5 times the interquartile distance. The maximum value is unknown for Campylobacter spp., because four concentrations exceeded the detection threshold of the MPN procedure.
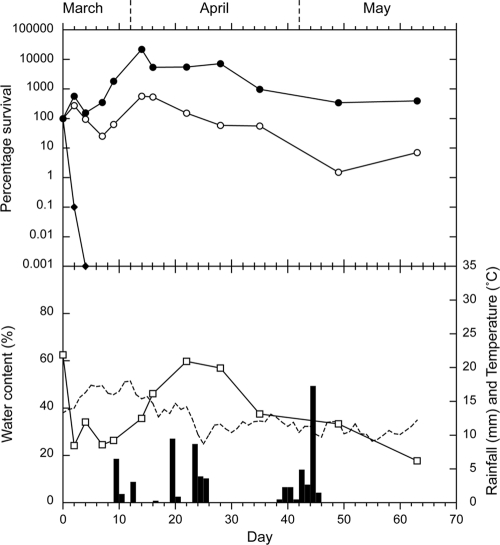
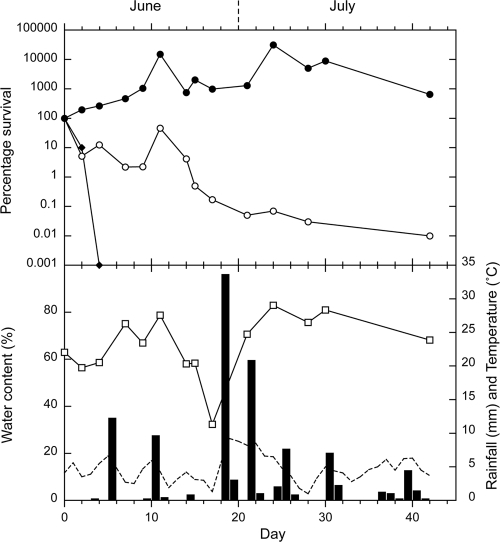
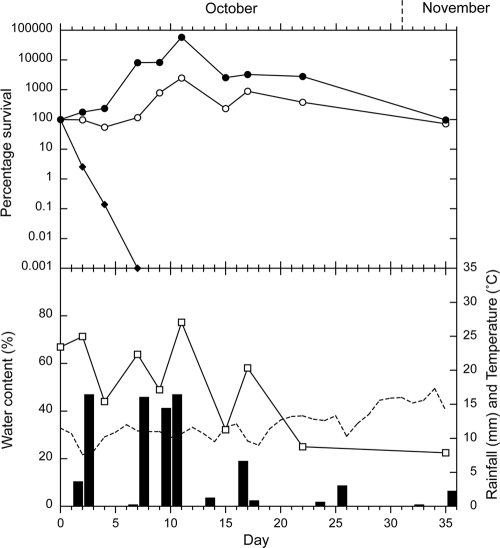
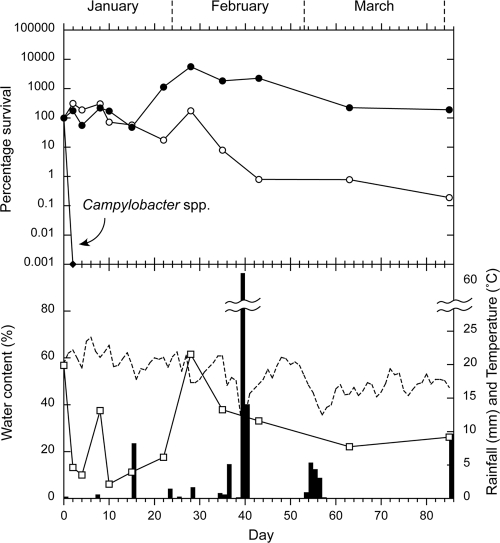
The survival curves for E. coli, enterococci, and Campylobacter spp. in the sheep feces on pasture are presented for autumn, winter, spring, and summer in Fig. 2, 3, 4, and 5, respectively. In each figure, the survival curves are compared with the data regarding water content of the feces at each sampling, rainfall, and soil temperature (at 10 cm), which was recorded hourly. An initial increase in concentrations occurred, which was attributed to growth of the microorganisms, followed by a decrease in all four seasons for enterococci and during two seasons for E. coli (summer and autumn). The increases in concentrations ranged from 2 to 3 orders of magnitude for enterococci and from <1 to >2 orders of magnitude for E. coli. The peak values and the day on which they were reached are presented in Table 1, together with the initial and final concentrations. Campylobacter spp. showed no increase in numbers and were rapidly inactivated in all seasons (Table 1). All Campylobacter spp. isolates tested were identified as C. jejuni.
FIG. 2.
Autumn experiment. Survival of E. coli (○), enterococci (•), and Campylobacter spp. (⧫), compared with rainfall (▪), the water content of the sheep feces (□), and soil temperature (dashed line). Each bacterial data point is the median of 10 samples.
FIG. 3.
Winter experiment. Survival of E. coli (○), enterococci (•), and Campylobacter spp. (⧫), compared with rainfall (▪), the water content of the sheep feces (□), and soil temperature (dashed line). Each bacterial data point is the median of 10 samples, with the exception of the peak enterococci value, which was obtained from a single sample.
FIG. 4.
Spring experiment. Survival of E. coli (○), enterococci (•), and Campylobacter spp. (⧫), compared with rainfall (▪), the water content of the sheep feces (□), and soil temperature (dashed line). Each bacterial data point is the median of 10 samples.
FIG. 5.
Summer experiment. Survival of E. coli (○), enterococci (•), and Campylobacter spp. (⧫), compared with rainfall (▪), the water content of the sheep feces (□), and soil temperature (dashed line). Each bacterial data point is the median of 10 samples.
TABLE 1.
Initial, peak, and final counts of indicators and pathogens in sheep feces placed on pasture in each seasona
| Season, count, and time point | Count (MPN/g [dry wt] of feces) and days on pasture |
||
|---|---|---|---|
| E. coli | Enterococci | Campylobacter spp. | |
| Autumn | |||
| Initial count (range) | 1.1 × 108 (1.1 × 106-1.5 × 109) | 4.0 × 104 (2.9 × 103-7.0 × 105) | 1.4 × 105 (0-2.7 × 107) |
| Peak (range), time point | 6.2 × 108 (3.9 × 107-5.2 × 109), day 14 | 8.8 × 106 (2.1 × 104-5.8 × 109), day 14 | —c |
| Final countb (range), time point | 7.6 × 106 (3.6 × 105-4.1 × 107), day 63 | 1.6 × 105 (6.9 × 102-1.4 × 106), day 63 | 0 (—), day 4 |
| Winter | |||
| Initial count (range) | 8.1 × 106 (9.0 × 103-9.5 × 108) | 6.2 × 104 (3.8 × 103-8.8 × 106) | 90 (0-1.1 × 105) |
| Peak (range), time point | — | 3.7 × 107 (—),d day 244 | — |
| Final countb (range), time point | 4.6 × 102 (1.4 × 102-2.0 × 105), day 42 | 4.0 × 105 (1.8 × 103-8.9 × 108), day 42 | 0 (—), day 4 |
| Spring | |||
| Initial count (range) | 1.5 × 108 (1.0 × 107-3.1 × 109) | 1.4 × 106 (3.8 × 104-9.7 × 106) | 8.5 × 103 (0-1.4 × 106) |
| Peak (range), time point | 3.8 × 109 (1.4 × 108-1.3 × 1010), day 11 | 8.2 × 108 (2.8 × 107-2.6 × 109), day 11 | — |
| Final countb (range), time point | 1.1 × 108 (1.4 × 107-7.1 × 108), day 35 | 1.4 × 106 (3.7 × 105-3.1 × 108), day 35 | 0 (—), day 7 |
| Summer | |||
| Initial count (range) | 6.1 × 107 (2.05 × 105-4.2 × 109) | 7.6 × 103 (2.4 × 102-1.8 × 106) | 2.3 × 103 (0-1.1 × 104) |
| Peak (range), time point | 1.8 × 108 (1.1 × 107-6.3 × 108), day 8 | 4.3 × 105 (2.0 × 103-4.9 × 106), day 28 | — |
| Final countb (range), time point | 1.2 × 105 (8.2 × 103-2.7 × 106), day 85 | 1.4 × 104 (1.8 × 103-2.0 × 105), day 85 | 0 (—), day 2 |
Data summarize the initial, peak, and final counts of indicators and pathogens in sheep feces placed on pasture in each season. The time point (day) on which the count was recorded is also reported. Except for the peak enterococci count in winter, each count represents the median of 10 samples (with each sample consisting of five pellets from each feces). Peak counts are given only where they were above the initial count. The date of deposition was designated day zero.
For Campylobacter spp., this was the first count below the level of detection. For E. coli and enterococci, this was the count on the last day on which fecal material was available for analysis.
—, the initial concentration was the highest value recorded.
No range was calculated; the peak is based on a single sample.
The increase (growth) and decrease (inactivation) phase coefficients and the associated T90 values are presented in Table 2. In addition, a comparison of the smaller perturbations between sequential samplings in the E. coli and enterococci survival curves in Fig. 2 to 5 produced a 77% match in terms of positive or negative slopes.
TABLE 2.
Growth and decrease phase coefficients and T90 values for E. coli, enterococci, and Campylobacter in sheep feces on pasturea
| Season | Indicator or pathogen | Increase coefficient, kI (day−1) | R2 | Decrease coefficient, kD (day−1) | R2 | T90 for decrease phase (days)c | T90 from deposition (days) |
|---|---|---|---|---|---|---|---|
| Winter | E. coli | —d | — | −0.22 | 0.74 | (10.5) | 10.5 |
| Enterococci | 0.17 | 0.73 | −0.20 | 0.95 | 11.6 | 24.3 | |
| Campylobacter | — | — | −2.88 | 0.99 | (0.8) | 0.8 | |
| Spring | E. coli | 0.29 | 0.95 | −0.12 | 0.85 | 18.9 | 26.8 |
| Enterococci | 0.17 | 0.73 | −0.20 | 0.95 | 11.6 | 18.8 | |
| Campylobacter | — | — | −1.63 | 0.99 | (1.4) | 1.4 | |
| Summer | E. coli | 0.10 | 0.58 | −0.09 | 0.71 | 24.8 | 27.6 |
| Enterococci | 0.13 | 0.96 | −0.06 | 0.94 | 37.9 | 60.1 | |
| Campylobacter | — | — | −5.76 | (1)b | (0.4) | 0.4 | |
| Autumn | E. coli | 0.06 | 0.63 | −0.11 | 0.98 | 21.0 | 27.8 |
| Enterococci | 0.35 | 0.99 | −0.08 | 0.82 | 28.6 | 46.6 | |
| Campylobacter | — | — | −2.88 | 0.99 | (0.8) | (0.8) |
Growth and decrease phase coefficients and T90 values (calculated for both the decrease phase only and for the total survival curve) for E. coli, enterococci, and Campylobacter in sheep feces on pasture are reported. The coefficient (k) was derived from a regression of the natural logarithms, i.e., n = ekt, where n = count and t = time. For the decrease phase, the T90 was calculated as 2.303/k. For the total survival curve (from time of deposition), the T90 was similarly calculated for a nominal extinction level of 0.01%.
Only two data points were available.
When an increase phase was absent, the T90 reported is the same as the T90 from deposition and is thus reported in parentheses.
—, no increase was recorded.
The increases and decreases in water content between sequential samplings were also compared with the slope directions of the E. coli and enterococci curves. For both indicators, there was a 68% match with water content curve slope direction. The probabilities of a match at or above this level occurring by chance were 1.68% and 0.81% for E. coli and enterococci, respectively, with the differences in the number of slopes and signs of the perturbations accounting for the dissimilarities in probabilities. This suggests that rises and falls in water contents of the feces were correlated with, respectively, growth and inactivation of the indicators. Fecal water content at the time of sampling was not strongly related to preceding rainfall events, although moisture retention and/or increased levels appeared to be associated with periods of relatively frequent rainfall, e.g., in the first half of the spring experiment (Fig. 4) and the second half of the winter experiment (Fig. 3).
A fecal sample was set aside for temperature measurements, but tests with a temperature probe inserted in a fecal sample showed the internal temperature very closely followed soil temperature at 10 cm at the nearby weather station. Accordingly, this soil value was used as a temperature reference and was recorded hourly.
There was no consistent relationship between soil temperature and inactivation or regrowth of either E. coli or enterococci. However, a period of high soil temperatures (>20°C) at the start of the summer experiment (Fig. 5) corresponded with rapid fecal dehydration and flat E. coli and enterococci curves. The fastest inactivation of Campylobacter spp. also occurred during this period, and the slowest Campylobacter spp. inactivation occurred during a period of high fecal hydration combined with low temperature (spring [Fig. 4]).
DISCUSSION
The major finding from this study was the significant initial increase, in every season, in concentrations of enterococci in the deposited sheep feces. Similarly, although less marked, increases were also recorded for E. coli. These results suggest that both indicators can grow in sheep feces when they are deposited on pasture. Furthermore, the largely (77%) concordant rises and falls in E. coli and enterococci concentrations suggest that the growth/regrowth and inactivation phases of these two indicators were influenced by the same or similar factors. The probability of a match at or above this level occurring by chance is 0.01%, based on a Bernoulli trial, using the observed frequencies of positive or negative slopes. The days on which the peak counts were recorded also matched in three of the four seasons, and there was a match between the E. coli peak and a high enterococci count in the remaining season (winter [Fig. 3]).
Previous studies have identified the key factors which influence the survival of bacteria within animal feces. These include moisture content, temperature, pH, nutrients, the animal's diet, and the bacterial strain (28, 29). Solar radiation may also inactivate bacteria on the surface of the fecal pellets. The 68% match in curve direction between the indicator concentration and water content suggests that indicator growth, particularly for enterococci, was strongly influenced by the initial moisture content of the sheep feces and the moisture gained during periods of rehydration. Conversely, the results suggest that dehydration promoted inactivation.
These results are similar to those recorded in our earlier study of cow pats (28), where increases in concentrations of these indicators occurred in the first 1 to 3 weeks after deposition and for as long as the pat water content remained above 80%. Thereafter, concentrations decreased, but increases in both E. coli and enterococci concentrations were recorded during periods of rehydration of the pats. However, whereas the initial water content of the cow pats in our earlier investigation was around 80%, it was around 60 to 70% in the sheep feces in the present study. Similarly, whereas E. coli and enterococci inactivation in the cow pats tended to occur when the water content fell from 80% to 40%, inactivation of these bacteria in the sheep feces appeared to be associated with far lower water concentrations (20 to 30%). Even in winter, when water content tended to remain between 60 and 80%, a decrease in indicator concentrations between days 11 and 17 was associated with a decline in water content to around 30%.
This study also suggests that increases in enterococci concentrations in sheep feces can occur when there is a lower water content than in cow pats, possibly as low as 40 to 60%, but the reasons for this difference are unclear. E. coli regrowth was less pronounced than for enterococci and appeared to require a higher moisture content, generally 60% or more. Indicator inactivation became more consistent after 15 to 20 days, although in winter (Fig. 3), when water content largely remained above 60%, indicator inactivation became evident earlier for E. coli and later for enterococci. We speculate that a combination of nutrient exhaustion and leaching through rainfall determined the point at which the inactivation phases became more permanent.
Although moisture retention and/or increased moisture levels appeared to be associated with periods of relatively frequent rainfall, e.g., in the first half of the spring experiment and the second half of the winter experiment, the association between periods of rainfall and rehydration of feces, overall, was less obvious than in our earlier study of cow pats (28). This difference is probably attributable to the physical characteristics of the feces. Each of the cow feces investigated by Sinton et al. (28) was deposited as a single pat with a diameter of 30 cm, which formed a crust after 2 to 3 days of sunlight exposure. Although this crust appeared to initially deflect rainfall, once moistened, the pats were slow to dehydrate and water content measured at the time of sampling was influenced by rainfall up to the 5 days previous. In contrast, the individual pellets that comprised a single deposition of sheep feces in this experiment appeared to be both rapidly hydrated and dehydrated. Thus, we assume that the recorded growth of E. coli and enterococci occurred soon after the rapid hydration periods.
Temperature appeared to be less important than water content in determining E. coli and enterococcal growth and inactivation in the sheep feces. There was no consistent relationship between soil temperature and inactivation or regrowth of either indicator organism. Significant increases in enterococcal concentrations occurred at both low (≤10°C [Fig. 3 and 4]) and high (≥15°C [Fig. 2 and 5]) temperatures. Similarly, inactivation of both E. coli and enterococci occurred at temperatures ranging from <5°C to >15°C. However, some indirect temperature effects were likely. For example, a period of high soil temperatures (>20°C) at the start of the summer experiment corresponded with rapid fecal dehydration and no change in E. coli and enterococcal concentrations. In a comparative study of the survival of E. coli in cow pats in pasture and under laboratory conditions, Van Kessel et al. (29) also concluded that while temperature was a major factor in the survival of E. coli in the laboratory study, many other factors affected its survival on pasture and that laboratory die-off rates have to be corrected under field conditions. The assumption is made that the reductions in numbers of detectable bacteria in the experiments were due to inactivation (which includes loss of the ability to grow on the selective medium as a result of injury). However, decreases in counts in the samples may also have been due to other factors, particularly the transport of bacteria from the fecal samples into the underlying soil.
The Campylobacter spp. results were also similar to those recorded in our earlier study of cow pats (28), with no evidence of growth, rapid inactivation in every season, and no consistent relationship between decreases in concentrations and fecal water content. Although inactivation occurred during periods of dehydration in summer and autumn, it also occurred during periods of high moisture content in spring and winter. While the relationship was not as consistent as was recorded in cow pats (28), Campylobacter spp. survival appeared to be more related to temperature, with the highest inactivation occurring in summer, when the soil temperature was around 20°C, and lower in the other seasons, when temperatures ranged from 5 to 12°C.
A selection of 31 Campylobacter spp. isolates collected during the winter season were analyzed by pulsed-field gel electrophoresis (PFGE) using the restriction enzymes SmaI and KpnI and following the methods of Gilpin et al. and Ribot et al. (13, 25). By using an indistinguishable criterion of 100% (using Dice similarity coefficients with an optimization of 1.0% and position tolerance of 1.5%), nine different PFGE patterns were identified (results not shown). Six of the isolates were of a genotype that was the most frequently recorded in ovine offal and fecal samples collected from five regions throughout New Zealand, and six of the nine genotypes identified have also been recovered from human clinical isolates (unpublished data from the Institute of Environmental Science and Research Ltd. [ESR], Christchurch, New Zealand). The wide range of distinct PFGE patterns, including the genotype most frequently isolated from ovine sources in New Zealand, indicates that the Campylobacter spp. recorded in this study are typical of naturally occurring strains in ovine feces throughout New Zealand. The presence of six genotypes that were indistinguishable from human clinical isolates suggests that the survival data generated by this study will be applicable to zoonotic Campylobacter strains.
Initial E. coli concentrations were equal to or higher than the equivalent results for cow pats presented by Sinton et al. (28), and all but one of the enterococcal concentrations were higher. A similar comparison of peak concentrations showed that all of the indicator concentrations in the sheep feces were equal to or greater than in the cow pats. A comparison of final concentrations is not meaningful, because of the comparatively higher loss of sheep feces material associated with sampling (resulting in earlier termination of the experiments). Similarly, a comparison of Campylobacter spp. concentrations would be invalid, because the pats studied by Sinton et al. (28) were seeded with the pathogen. However, a comparison with the naturally occurring Campylobacter spp. concentrations recorded in fresh cow pats in the experiment of Gilpin et al. (during the summer season) (14) shows that the initial concentrations in sheep feces in all seasons were equal to or greater than the median value (around 102) in cow pats. The times to extinction in both studies were within similar ranges (2 to 9 days).
Overall, the variable microbial concentrations and the physical size and structure of the sheep feces were problematic in terms of experimental design and interpretation. First, the individual fecal pellets that typically formed in the sheep intestines contained highly variable concentrations of enteric microbes, with a maximum-minimum range of around 2 orders of magnitude for E. coli and enterococci. This was probably higher for Campylobacter spp.: the samples collected from the two sheep at slaughter ranged from functionally zero (less than 1 per g−1) to over 106 per g−1. Thus, this variability required the collection of six pellets from each fecal sample per sampling occasion. The small size of the sheep feces (on average, 30 g each) led to the second problem, i.e., the sampling process removed fecal material at a rate that functionally exceeded the rate of inactivation of the indicators. Thus, in each season, the available fecal material was exhausted well before the indicator concentrations fell below the detection threshold of the MPN method. Indeed, in spring, the concentrations (on day 35) in the final pellets remained very high, at 108 for E. coli and 106 for enterococci.
Early loss of sample created a third problem, i.e., although it was possible to establish inactivation coefficients (k values) for the decrease phase of the E. coli and enterococcal survival curves, the T90 values from time of deposition were estimates based on a nominated extinction concentration (0.01% of the initial concentration). If the nominated extinction level is set lower, the times to extinction and the derived T90 value from deposition estimates will rise. Thus, the T90 values from time of deposition may be valid comparative parameters (between seasons and bacteria) but are less useful as absolute values. Overall, the T90 values were higher for enterococci than E. coli. The exception was in the winter experiment, where an unusually flat E. coli curve resulted in a reverse ranking. Overall, the T90 values (from time of deposition) in our study (ranging from 10 to 28 days) (Table 2) were slightly lower than the equivalent figure of 36 days for E. coli survival in sheep feces estimated by Avery et al. (1).
Finally, it is important to note that, although overall enterococcal growth in the sheep feces was considerably greater than that of E. coli, subsequent rates of decreased growth were broadly similar. In addition, initial E. coli concentrations exceeded those of enterococci by up to 4 orders of magnitude. Thus, in three of four seasons, the final E. coli count still exceeded the enterococci count by 1 to 2 orders of magnitude. The results of this study are currently being incorporated into a fecal microbe reservoir model to assist water managers' estimates of microbial loads on pastures grazed by sheep.
Conclusions.
Our study suggests that enterococci counts in freshly deposited sheep feces are likely to significantly underestimate counts 2 to 3 weeks after deposition by up to 2 orders of magnitude, because of bacterial growth in the fecal material. The equivalent effect for E. coli is more dependent on season and rainfall but may also underestimate counts by up to 1 order of magnitude.
Although enterococci exhibit far greater growth rates in sheep feces (k = 0.35 days for enterococci and 0.06 for E. coli in autumn), particularly after fecal rehydration associated with rainfall, final concentrations are likely to be lower for enterococci than for E. coli (1.6 × 105 for enterooccci and 7.6 × 106 for E. coli in autumn) by the time the feces have disintegrated because of the far lower initial concentrations. Together, these findings suggest that enterococci are less suitable than E. coli as indicators of fecal contamination by sheep.
Campylobacter spp. appear to be rapidly inactivated in sheep feces on pasture, particularly in warmer weather (T90 of 0.4 days in summer) and are likely to disappear long before the feces disintegrate. Thus, although Campylobacter spp. have been detected in both the feces of lambs at slaughter and sheep on pasture in New Zealand (22), often at high concentrations, only freshly deposited feces are likely to be a significant reservoir for this pathogen.
Concentrations of E. coli, enterococci, and Campylobacter spp. appear to be higher in sheep feces than in cow pats. Whether this translates into higher loadings on pasture is yet to be determined. To this end, the results of this study, in combination with those from associated surveys of concentrations in fresh lamb and sheep feces, are being incorporated into a fecal microbe reservoir model. This model will assist water managers in assessing the impact of fresh and aged ovine feces on surface waters and facilitate predictions of the microbial burden from sheep feces on pasture under a variety of climatic conditions.
Acknowledgments
This research was funded by the New Zealand Public Good Science Fund, administered by the Foundation for Research, Science and Technology.
We gratefully acknowledge the assistance of the staff at the meatworks from where the sheep feces samples were obtained. Thanks are due also to Plant and Food Research, who provided the land for the experimental site. David Wood, Brent Gilpin, Andrew Hudson, and Hilary Michie, ESR, and the journal's referees provided valuable review comments.
Footnotes
Published ahead of print on 14 January 2011.
REFERENCES
- 1.Avery, S. M., A. Moore, and M. L. Hutchison. 2004. Fate of Escherichia coli originating from livestock faeces deposited directly onto pasture. Lett. Appl. Microbiol. 38:355-359. [DOI] [PubMed] [Google Scholar]
- 2.Bailey, G. D., et al. 2003. A study of the foodborne pathogens: Campylobacter, Listeria and Yersinia, in faeces from slaughter-age cattle and sheep in Australia. Commun. Dis. Intell. 27:249-257. [DOI] [PubMed] [Google Scholar]
- 3.Baker, M. G., B. J. Gilpin, and M. G. Savill. 2007. Statistical comparison of Campylobacter jejuni subtypes from human cases and environmental sources. J. Appl. Microbiol. 103:2113-2121. [DOI] [PubMed] [Google Scholar]
- 4.Castro-Hermida, J. A., et al. 2007. Occurrence of Cryptosporidium parvum and Giardia duodenalis in healthy adult domestic ruminants. Parasitol. Res. 101:1443-1448. [DOI] [PubMed] [Google Scholar]
- 5.Clesceri, L. S., A. E. Greenberg, and A. D. Eaton 1998. Standard methods for the examination of water and wastewater, 20th ed. American Public Health Association, Washington, DC.
- 6.Collins, R., A. M. Donnison, C. Ross, and M. McLeod. 2004. Attenuation of effluent-derived faecal microbes in grass buffer strips. N. Z. J. Mar. Freshwater Res. 47:565-574. [Google Scholar]
- 7.Cornelius, A. J., C. Nicol, and J. A. Hudson. 2005. Campylobacter spp. in New Zealand raw sheep liver and human campylobacteriosis cases. Int. J. Food Microbiol. 99:99-105. [DOI] [PubMed] [Google Scholar]
- 8.Davies, R. H., et al. 2004. National survey for Salmonella in pigs, cattle and sheep at slaughter in Great Britain (1999-2000). J. Appl. Microbiol. 96:750-760. [DOI] [PubMed] [Google Scholar]
- 9.Davies-Colley, R. J., J. W. Nagels, R. A. Smith, R. G. Young, and C. J. Philips. 2004. Water quality impact of a dairy cow herd crossing a stream. N. Z. J. Mar. Freshwater Res. 38:569-576. [Google Scholar]
- 10.Devane, M. L., et al. 2005. The occurrence of Campylobacter subtypes in environmental reservoirs and potential transmission routes. J. Appl. Microbiol. 98:980-990. [DOI] [PubMed] [Google Scholar]
- 11.Donnison, A., and C. Ross. 1999. Animal and human faecal pollution in New Zealand rivers. N. Z. J. Mar. Freshwater Res. 33:119-128. [Google Scholar]
- 12.ESR. May 2010, posting date. EpiSurv. Institute of Environmental Science and Research Limited, Wellington, New Zealand. http://www.surv.esr.cri.nz/episurv/index.php.
- 13.Gilpin, B. J., et al. 2006. Application of pulsed-field gel electrophoresis to identify potential outbreaks of campylobacteriosis in New Zealand. J. Clin. Microbiol. 44:406-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilpin, B. J., B. Robson, P. Scholes, F. Nourozi, and L. W. Sinton. 2009. Survival of Campylobacter spp. in bovine faeces on pasture. Lett. Appl. Microbiol. 48:162-166. [DOI] [PubMed] [Google Scholar]
- 15.Kress, M., and G. F. Gifford. 1984. Faecal coliform release from cattle faecal deposits. Water Res. Bull. 20:61-66. [Google Scholar]
- 16.Kudva, I. T., K. Blanch, and C. J. Hovde. 1998. Analysis of Escherichia coli O157:H7 survival in ovine or bovine manure and manure slurry. Appl. Environ. Microbiol. 64:3166-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Majewska, A. C., A. Werner, P. Sulima, and T. Luty. 2000. Prevalence of Cryptosporidium in sheep and goats bred on five farms in west-central region of Poland. Vet. Parasitol. 89:269-275. [DOI] [PubMed] [Google Scholar]
- 18.McDowell, R. W. 2006. Contaminant losses in overland flow from cattle, deer and sheep dung. Water Air Soil Pollut. 174:211-222. [Google Scholar]
- 19.Milnes, A. S., et al. 2008. Intestinal carriage of verocytotoxigenic Escherichia coli O157, Salmonella, thermophilic Campylobacter and Yersinia enterocolitica, in cattle, sheep and pigs at slaughter in Great Britain during 2003. Epidemiol. Infect. 136:739-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ministry for the Environment. 2003. Microbiological water quality guidelines for marine and freshwater recreational areas. Ministry for the Environment and Ministry for Health, Wellington, New Zealand.
- 21.Ministry of Agriculture and Forestry. 2010. Livestock statistics. Ministry of Agriculture and Forestry, Wellington, New Zealand. http://www.maf.govt.nz/news-resources/statistics-forecasting/livestock-statistics.aspx.
- 22.Moriarty, E. M., et al. Incidence and prevalence of microbial pathogens in ovine faeces. N. Z. J. Agric. Res., in press.
- 23.Oporto, B., J. I. Esteban, G. Aduriz, R. A. Juste, and A. Hurtado. 2007. Prevalence and strain diversity of thermophilic campylobacters in cattle, sheep and swine farms. J. Appl. Microbiol. 103:977-984. [DOI] [PubMed] [Google Scholar]
- 24.Quilez, J., et al. 2008. Cryptosporidium genotypes and subtypes in lambs and goat kids in Spain. Appl. Environ. Microbiol. 74:6026-6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ribot, E. M., C. Fitzgerald, K. Kubota, B. Swaminathan, and T. J. Barrett. 2001. Rapid pulsed-field gel electrophoresis protocol for subtyping of Campylobacter jejuni. J. Clin. Microbiol. 39:1889-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryan, U. M., et al. 2005. Sheep may not be an important zoonotic reservoir for Cryptosporidium and Giardia parasites. Appl. Environ. Microbiol. 71:4992-4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santin, M., J. M. Trout, and R. Fayer. 2007. Prevalence and molecular characterization of Cryptosporidium and Giardia species and genotypes in sheep in Maryland. Vet. Parasitol. 146:17-24. [DOI] [PubMed] [Google Scholar]
- 28.Sinton, L. W., R. R. Braithwaite, C. H. Hall, and M. L. Mackenzie. 2007. Survival of indicator and pathogenic bacteria in bovine feces on pasture. Appl. Environ. Microbiol. 73:7917-7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Kessel, J. S., Y. A. Pachepsky, D. R. Shelton, and J. S. Karns. 2007. Survival of Escherichia coli in cowpats in pasture and in laboratory conditions. J. Appl. Microbiol. 103:1122-1127. [DOI] [PubMed] [Google Scholar]
- 30.Wilcock, R. J. 2006. Assessing the relative importance of faecal pollution in rural catchments. Environment Waikato, Hamilton, New Zealand.
- 31.Wilcock, R. J., et al. 1999. Water quality of a lowland stream in a New Zealand dairy farming catchment. N. Z. J. Mar. Freshwater Res. 33:683-696. [Google Scholar]
- 32.Wong, T., et al. 2004. Validation of a PCR method for detection on poultry packs. Br. Food J. 106:642-650. [Google Scholar]
- 33.Yakub, G. P., et al. 2002. Evaluation of Colilert and Enterolert defined substrate methodology for wastewater applications. Water Environ. Res. 74:131-135. [DOI] [PubMed] [Google Scholar]