Abstract
Biofilms are ubiquitous surface-associated microbial communities embedded in an extracellular polymeric (EPS) matrix, which gives the biofilm structural integrity and strength. It is often reported that biofilm-grown cells exhibit enhanced tolerance toward adverse environmental stress conditions, and thus there has been a growing interest in recent years to use biofilms for biotechnological applications. We present a time- and locus-resolved, noninvasive, quantitative approach to study biofilm development and its response to the toxic solvent styrene. Pseudomonas sp. strain VLB120ΔC-BT-gfp1 was grown in modified flow-cell reactors and exposed to the solvent styrene. Biofilm-grown cells displayed stable catalytic activity, producing (S)-styrene oxide continuously during the experimental period. The pillar-like structure and growth rate of the biofilm was not influenced by the presence of the solvent. However, the cells experience severe membrane damage during styrene treatment, although they obviously are able to adapt to the solvent, as the amount of permeabilized cells decreased from 75 to 80% down to 40% in 48 h. Concomitantly, the fraction of concanavalin A (ConA)-stainable EPS increased, substantiating the assumption that those polysaccharides play a major role in structural integrity and enhanced biofilm tolerance toward toxic environments. Compared to control experiments with planktonic grown cells, the Pseudomonas biofilm adapted much better to toxic concentrations of styrene, as nearly 65% of biofilm cells were not permeabilized (viable), compared to only 7% in analogous planktonic cultures. These findings underline the robustness of biofilms under stress conditions and its potential for fine chemical syntheses.
Biofilms are ubiquitous surface-associated microbial communities embedded in an extra cellular polymeric (EPS) matrix. Numerous studies have reported that bacterial cells in biofilms or cells artificially entrapped in a matrix are more resistant to environmental stresses (e.g., antimicrobial substances, heavy metals, toxic chemicals, organic acids, etc.) than their planktonic or freely swimming counterparts (7, 9, 16, 19, 29, 40). There has been growing interest in recent years in the exploitation of these robust catalysts for biotechnological applications (e.g., biotransformations) to produce value-added chemicals (10, 11, 12, 27). These studies have shown that the use of biofilms may overcome major bottlenecks in classical biocatalysis processes, such as substrate/product toxicity and short-term biocatalytic stability. Catalyst robustness becomes especially interesting regarding the application of organic solvents. Organic solvents are involved in biocatalysis, either as a biotransformation substrate or as a second carrier phase for toxic or poorly water-soluble substrates and products. These organic solvents may affect cell viability through membrane permeabilization and membrane potential dissipation, damaging cellular metabolism, which may result in reduced biocatalytic efficiency. Organic solvents with a log POW value (logarithm of the partition coefficient of the target compound in a mixture of octanol and water) between 1.5 and 3 are extremely toxic to microorganisms (25, 32, 33, 35, 37). Mainly Pseudomonas species have been reported to tolerate organic solvents. Different adaptation mechanisms, such as the excretion of solvents through efflux pumps, membrane alteration, lower cell membrane permeability, and changes in membrane rigidity and vesicle formation have been observed and elucidated (32, 35, 36, 37).
Until now, solvent tolerance has been investigated mainly in planktonic cells. Studies regarding the solvent tolerance, inactivation, and adaptation mechanisms of biofilms, especially for technological applications, are sparse. Quantifying and describing the solvent toxicity mechanism in biofilms is crucial for further developing these interesting catalysts for biotechnological applications. In a recent study, Li and coworkers reported that biofilm-grown Zymomonas mobilis cells showed increased resistance to toxic benzaldehyde, and the concept of biofilm factories had been extended to continuous fine chemical production, benefiting self immobilization, regeneration, and long-term stability (27). In previous studies, it has been shown that Pseudomonas sp. strain VLB120ΔC biofilms are promising catalysts for the long-term conversions of the toxic substrate styrene to (S)-styrene oxide in a continuous process (10, 11, 12). However, biofilm cellular integrity, spatial distributions of live and dead cells, inactivation, adaptation, and enhanced solvent tolerance mechanisms have not been studied, characterized, or elucidated so far.
Pseudomonas sp. strain VLB120 is a biofilm-forming organism able to grow on styrene as the sole source of carbon and energy. A gene deletion in the styrene degradation pathway resulted in Pseudomonas sp. strain VLB120ΔC, a promising catalyst for the synthesis of (S)-styrene oxide. This strain has been studied extensively in conventional stirred-tank reactors (31) and also has been used in novel biofilm reactors (10, 11, 12). To address the question of how catalytic biofilms adapt to toxic environments and to explore its ability as an alternative biocatalyst to its planktonic counterparts, this study presents a time-resolved, noninvasive, quantitative approach to investigate biofilm development and its response to the toxic solvent styrene. As a model system, biofilm of a chromosomally integrated gfp variant, Pseudomonas sp. strain VLB120ΔC-BT-gfp1, was grown in a modified flow-cell reactor designed to perform long-term experiments under solvent-saturated conditions.
MATERIALS AND METHODS
All chemicals used in this study were purchased either from Sigma-Aldrich (Steinheim, Germany) or Carl Roth GmbH (Karlsruhe, Germany) unless stated otherwise. The chemicals were of the highest purity available and used without further purification. Luria-Bertani (LB) medium was used for the precultures, and M9 medium (34), supplemented with 0.5% (wt/vol) glucose as a carbon source, Uwe Sauer (US*) trace elements (6), and kanamycin (25 μg ml−1), was used for shake flask and flow-cell reactor experiments.
Construction of Pseudomonas sp. strain VLB120ΔC-BT-gfp1.
The strains and plasmids used in this study are listed in the Table 1. The gfp variant of Pseudomonas sp. strain VLB120ΔC was constructed using the Tn7 transposon technique as described previously (21, 26). In short, the DNA sequences containing the gfp gene are located in the delivery plasmid pBK-mini Tn7-gfp1. It is flanked by Tn7 sequences (Tn7L, Tn7R) and integrated at the attTn7 side, which is located directly downstream of glmS in Pseudomonas spp. (26). The five genes needed for transpositions (tnsA to tnsE) are located on the helper plasmid pUX-BF13. Delivery and helper plasmids were isolated using commercially available kits (peqGOLD miniprep kit I; Peqlab Biotechnologie GmBH, Erlangen, Germany) and introduced into Pseudomonas sp. strain VLB120ΔC by electroporation (2,500 V; Easyject Prima EquiBio; Thermo Fisher Scientific Inc., Waltham, MA). The resulting gfp variant, Pseudomonas sp. strain VLB120ΔC-BT-gfp1, was verified by the corresponding antibiotic resistance genes and constitutive gfp expression, which was measured in a multimode multiplate fluorescence reader (excitation, 480 nm; emission, 515 nm; Tecan Trading AG, Maennedorf, Switzerland).
TABLE 1.
Bacterial strains and plasmids used in this studya
| No. | Strain and plasmid | Characterization | Reference or source |
|---|---|---|---|
| Strains | |||
| 1 | Pseudomonas sp. strain VLB120ΔC | Mutant carrying a deletion in styCb | 31 |
| 2 | Pseudomonas sp. strain VLB120ΔC-BT-gfp1 | gfp tagged | This study |
| 3 | E. coli XL1-Blue | Carrying pBK-mini Tn7-gfp1 | 21 |
| 4 | E. coli SM10 λ pir | Carrying pUX-BF13 | 1 |
| 5 | E. coli HB101 | Carrying pRK600 | 18 |
| Plasmids | |||
| 6 | PBK-mini Tn7-gfp1 | pUC19-based delivery plasmid for miniTn7-gfp1, Kmr, Cmr, Apr, mob+ | 21 |
| 7 | pUX-BF13 | R6K replicon-based helper plasmid, 12.8 kb, Apr, Tn7 transposon function in trans, pir gene in trans required for replication, RP4 tra functions | 1 |
| 8 | pRK600 | Rk2 helper plasmid, Cmr, Kmr, mob+, tra+, derivative of RK2013 | 18 |
The strains and plasmids (3 to 8) were kindly gifted by Claus Sternberg (Denmark Technical University).
styC encodes an isomerase in the degradation pathway of styrene. The mutant strain is not able to grow on styrene anymore and accumulates styrene oxide.
Development of a modified flow-cell system as a technical basis for studying biofilm response to organic solvents.
Flow cells offer the possibility of the direct and on-line microscopic examinations of biofilms and are relatively simple in design (2). However, commercially available flow cells are not suited for aggressive solvents such as styrene, due to their restricted resistance to organic solvents. In addition, the poor solubility of solvents limits the direct mixing into the aqueous phase. To overcome these issues, the flow cell used in this study was fabricated out of a single block of borosilicate glass (for further details and figures, refer to the supplemental material), incorporating a silicone membrane in the middle for the addition of the solvent to the biofilm. The use of a gfp-expressing variant of Pseudomonas sp. strain VLB120ΔC made it possible to directly monitor live cells in the biofilm. Biofilms were cultivated in a custom-made flow-cell system, which allowed the real-time fluorescence-based optical analysis of cell physiology and the direct addition of the solvent styrene to the biofilm based on a diffusion-controlled substrate delivery mechanism using a permeable silicone membrane (12). A schematic view of the setup is shown in the figure in the supplemental material. The flow-cell reactor had an aqueous-phase volume of 0.65 ml. A silicone membrane (1.0 mm inner diameter and 0.4 mm wall thickness; Deutsch & Neumann, VWR, Langenfeld, Germany) was horizontally embedded in the middle of the flow cell. The biotransformation substrate styrene diffused from this silicone tube into the aqueous phase, in which the biofilm was cultivated. The reaction product, styrene oxide, was concurrently extracted back into the organic phase via the silicone membrane. This substrate reservoir thereby concurrently served as a product sink (the concept of in situ substrate feed and in situ product recovery). The Reynolds number in the flow-cell reactor at the operated medium flow rate of 70 μl min−1 was 1.8, and the hydrodynamic conditions are considered to be in the laminar region. Medium transport occurs through a peristaltic pump (ISM 930; Ismatec, Wertheim-Mondfeld, Germany) via two inlet channels. The styrene reservoir was filled using a syringe. Outside of the flow cell, the silicone tube was insulated with a PTFE tube to avoid any loss of styrene. The experimental procedure and the respective readouts are depicted in the figure in the supporting information.
Preculture cultivation.
Precultures of Pseudomonas sp. strain VLB120ΔC-BT-gfp1 were grown overnight in 10 ml M9 medium (0.5% glucose) using baffled 100-ml Erlenmeyer flasks in a horizontal shaker (30°C and 200 rpm; Multitron; Infors HT, Bottmingen, Switzerland).
Biofilm cultivation in the flow-cell reactor.
The assembled flow-cell reactor was sterilized by pumping 70% ethanol overnight and flushed with sterile deionized water for 2 to 3 h. Subsequently, the deionized water was exchanged for M9 medium (0.5% glucose), and the system was rinsed for 2 h before inoculation with an overnight culture of Pseudomonas sp. strain VLB120ΔC-BT-gfp1 (4 to 5 ml, diluted to an optical density at 450 nm [OD450] of 0.8 to 1.0) either under standard conditions (no styrene) or in a styrene-saturated flow-cell environment. During inoculation, the medium pump was stopped. After inoculation, the flow cell was kept idle without medium supply for 2 to 3 h to enable the initial attachment of the cells to the glass substratum. Medium feed was started with a flow rate of 70 μl min−1 (dilution rate, 7.0 h−1). The temperature in the flow-cell reactor was maintained at 30°C.
Shake flask experiments with planktonic cells.
Precultures of Pseudomonas sp. strain VLB120ΔC-BT-gfp1 were transferred to 250-ml baffled Erlenmeyer flasks at an initial OD450 of 0.5. The shake flasks were saturated with styrene prior to inoculation, and the liquid-phase styrene concentration was kept at 2.1 to 2.6 mM (styrene solubility limits in aqueous solution). These cultures were incubated at 30°C in a horizontal shaker (200 rpm; Multitron; Infors HT, Bottmingen, Switzerland). Sampling was done at 24-h intervals for quantifying the ratios of live to dead/permeabilized cells, cell dry weight, and styrene and styrene oxide concentrations. Live-to-dead/permeabilized cell quantification was performed by using an InfiniteM200 multimode microplate reader (excitation at 488 nm and emission at 515 nm for gfp-tagged cells, excitation at 535 nm and emission at 617 nm for the propidium iodide (PI)-stained dead/permeabilized cells; Tecan Trading AG, Maennedorf, Switzerland).
Staining techniques.
PI (Invitrogen, OR) was used to stain dead/permeabilized cells. For the visualization of extracellular polymeric substances (EPS), concanavalin A tetramethylrhodamine conjugate (ConA) (Invitrogen, OR) was used (5). ConA binds to specific sugar residues as α-mannuronate and α-l-guluronate of the polysaccharide fraction of the EPS and can be excited at 535 nm. One ml of the stock solution of the respective dye (0.2 mg ml−1 in 0.1 M sodium hydrogen carbonate) was injected directly into the flow cell. The flow cell was incubated under nonflow conditions for 30 min before the unbound ConA was washed out by the medium flow, and image acquisition was performed. To ensure that the biofilm did not experience significant detachment due to the nonflow conditions, control experiments have been conducted in which the staining compound was directly added to the medium, so that it was not necessary to stop the pumps for this experiment. No difference was observed when the pumps have been arrested.
Image acquisition and data treatment.
Image acquisition was performed using a Zeiss LSM5 Pascal confocal laser-scanning microscope (CLSM; Carl Zeiss, Jena, Germany) equipped with an argon and helium-neon laser. Images were obtained using an EC Plan-Neofluar 20×, 0.50 Ph2 M27 objective. Three-dimensional (3D) image reconstructions, quantification of biofilms, and living and dead cell distribution was done using the software packages IMARIS (Bitplane AG, Zürich, Switzerland), which is commercially available, and COMSTAT (17), which is freely available upon request.
Determination of biofilm catalytic activity.
The biofilm catalytic activity was determined by measuring the amount of styrene oxide formed as product per time unit in the organic as well as in the aqueous phase using gas chromatography (GC). Samples were prepared, treated, and analyzed according to the method described in Halan et al. (12).
RESULTS
The biofilm of model organism Pseudomonas sp. strain VLB120ΔC-BT-gfp1 was grown in modified flow-cell reactors. Biofilm development and maturation in standard (nonsolvent) and in styrene environments was monitored noninvasively in real time at regular time intervals. For comparison, the response of planktonic cells to the solvent styrene was monitored in a similar setup in shake flasks. For both flow-cell and planktonic systems, the same overnight culture was used without any preadaptation.
Biofilm-grown cells develop uniform pillar structure.
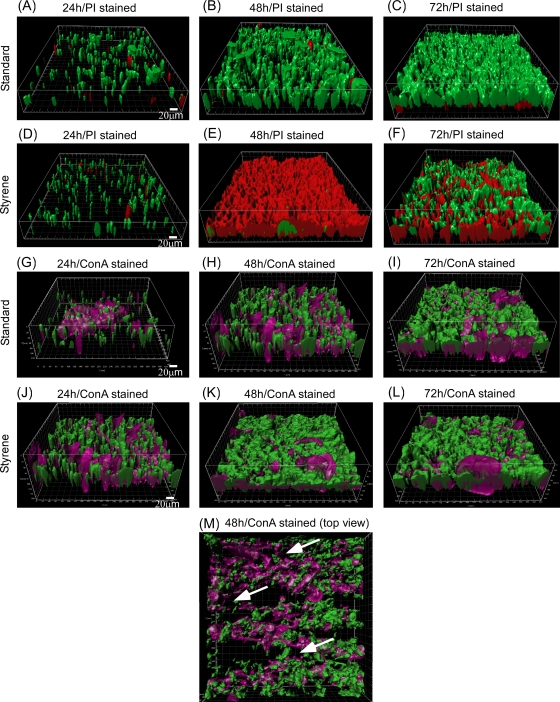
To investigate the general morphology and development stages of Pseudomonas sp. strain VLB120ΔC-BT-gfp1 biofilms, flow-cell experiments were conducted. Under standard growth conditions (without styrene) in the flowthrough system, biofilm-grown cells developed a uniform pillar structure (Fig. 1A). Initially, cells attached to the glass substratum formed microcolonies, which developed into pillars within 24 h. These pillars had an approximate maximal thickness of 50 μm and spread over the entire substratum during 72 h (Fig. 1C). The biofilm appeared densely packed and reached a maximum thickness of around 120 μm after 96 h. The pillars are interconnected by polysaccharides, which are part of the EPS and appear violet in the respective images (Fig. 1G to L). EPS is the major component of biofilms and is composed mainly of polysaccharides and proteins (28). The key function is the formation of water channels (Fig. 1M) and the stabilization of the structural integrity of the biofilm.
FIG. 1.
Confocal micrographs showing biofilm development stages under standard growth conditions (no solvents) and in the styrene environment. (A to C) gfp-expressing intact and PI-stained biofilms under normal growth conditions after 24 (A), 48 (B), and 72 (C). (D to F) gfp-expressing intact and PI-stained biofilms in the styrene environment after 24 (D), 48 (E), and 72 h (F). (G to I) ConA-stained biofilm under normal growth conditions after 24 (G), 48 (H), and 72 h (I). (J to L) ConA-stained biofilm in the styrene environment after 24 (J), 48 (K), and 72 h (L). (M) Top view of 48-h-old ConA-stained biofilm with water channels as indicated by arrows. Green color represents the intact gfp-expressing cells, red color represents PI-stained dead or permeabilized cells, and violet color (ConA) represents polysaccharides in the EPS matrix. Representative IMARIS-treated and 3D-reconstructed images from three independent experiments are shown. Scale bar, 20 μm.
Styrene inhibits planktonic cell growth and causes cell permeabilization.
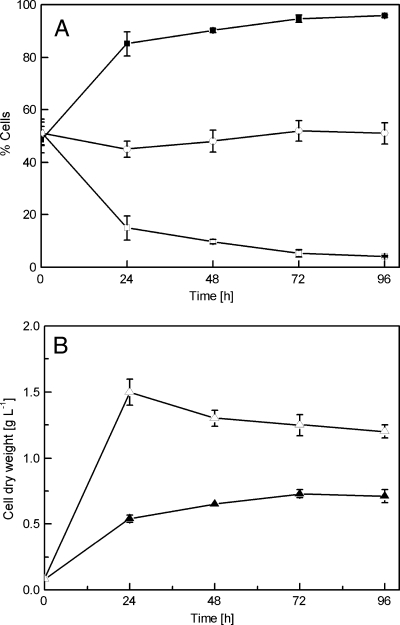
To examine the toxicity of styrene on planktonic cells, overnight cultures of Pseudomonas sp. strain VLB120ΔC-BT-gfp1 were grown as described in Materials and Methods and used for the inoculation of shake flasks containing styrene-saturated atmosphere (see the figure in the supplemental material). These cultures showed a 50% reduction of the growth rate and the final total cell dry weight compared to those of control cultures grown without styrene. Additionally, no further growth was observed after 48 h. The amount of damaged cells increased to approximately 90% after 24 h (Fig. 2). In contrast, the amount of dead/permeabilized cells in the control cultures stayed almost constant during the whole experimental period. In conclusion, the addition of the solvent styrene to the planktonic cell cultures resulted in a significantly decreased cell yield (total biomass) as well as an increased percentage of dead (permeabilized) cells.
FIG. 2.
Planktonic cell response to styrene. The graph shows the planktonic live and dead/permeabilized cells (A), as well as respective cell growth (B), from 0 to 96 h. □, percent live cells of styrene culture; ▪, percent dead cells of styrene culture; ○, percent dead cells as a control (without styrene); ▵, cell dry weight of control cultures; ▴, cell dry weight of cultures grown in the styrene environment. Data are mean values from three independent growth experiments.
Biofilm-grown cells showed quick adaptation to a styrene environment.
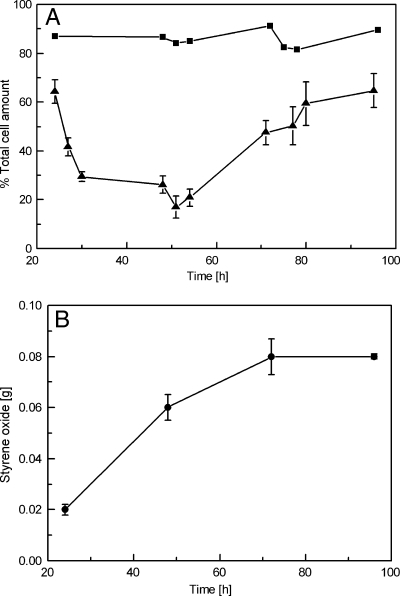
To investigate the influence of the toxic solvent styrene on biofilm growth, the real-time image analysis of biofilm cells was performed in styrene-saturated flow-cell reactors. The cell pillars described above also developed in the presence of styrene, which is similar to the case for standard conditions with regard to structure and thickness (Fig. 1D). However, more than 75% of the biomass stained red within the first 48 h of styrene exposure, indicating the presence of either dead or permeabilized cells with a damaged cell membrane (Fig. 1E), while in untreated biofilms only a few dead (permeabilized) cells were detected (<10%). Interestingly, the amount of live cells began to increase again in the course of the experiment. After 72 h, 65% of the cells were found to be intact again, as indicated by their green color in the image (Fig. 1F). It thus can be concluded that the biofilm-grown cells adapted to the toxic conditions and were able to recover.
Figure 3 shows the quantification of intact and damaged cells in a biofilm grown in a styrene environment. The strong toxic impact of the solvent is mirrored by a rapid decrease of living (non-PI-stained) biofilm cells within the first 48 h of contact with styrene. Seventy-five to 80% of the cells stained red, indicating a damaged cell membrane. However, in the following days, the biofilm obviously adapted to the harsh solvent conditions and recovered, which can be deduced from the increase in gfp-expressing cells to a final level of 65% (Fig. 3A). In contrast, Pseudomonas organisms growing in planktonic cultures responded much more dramatically to the presence of styrene. The amount of living cells accounted for less than 7%, and the culture did not recover in the course of the experiments (Fig. 2). In conclusion, biofilms showed quick adaptation and an enhanced tolerance to styrene without affecting their structural integrity, as observed through image analysis and quantification studies. As an additional readout for the physiological condition of the cells, the amount of styrene oxide produced by the biofilm was determined (Fig. 3B). The biofilm synthesized styrene oxide continuously throughout the experimental period, and therefore it can be concluded that although biofilm-grown cells were permeabilized rapidly in the styrene environment, the catalytic activity remained largely unaffected.
FIG. 3.
Graphical representation of fractional dynamics of live and dead (permeabilized) cells (A) and the (S)-styrene oxide production of biofilms growing in a styrene environment (B). Biofilm-forming cells were inoculated into the styrene-saturated flow-cell environment as described in Materials and Methods. ▴, percent intact gfp-expressing live cells; ▪, percent live cells as controls (without styrene); •, total styrene oxide produced in aqueous and organic phase. Live and dead (permeabilized) cells were determined by surface area covered compared to the total area. The aqueous- and organic-phase samples were collected every 24 h, and the concentration of styrene oxide was determined by GC. The organic-phase volume was 70 μl and was exchanged every 24 h. Data presented here are mean values from three independent growth experiments.
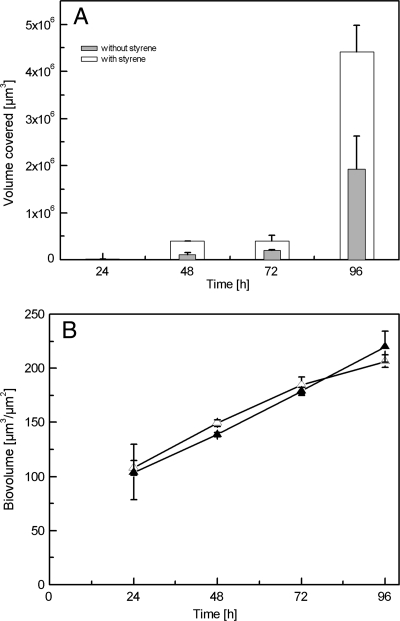
As mentioned above, EPS plays a major role in the structural integrity of biofilms. In addition, it was shown that toxic environmental conditions stimulate EPS production. In the present system, polysaccharide production was monitored and subsequently quantified in a nonsolvent environment, as well as in the presence of styrene. In the styrene-containing environment, polysaccharide production doubled compared to that in standard conditions (Fig. 4A), suggesting a protective function of these compounds for biofilm viability. The increase in polysaccharides was proportional to biomass growth over time both under standard and styrene-containing conditions.
FIG. 4.
Polysaccharide amount in biofilms grown in the presence of styrene and under standard/nonsolvent conditions. (A) Graph shows microbial polysaccharide production (given in volume covered) as analyzed during a period of 96 h. Biofilms were allowed to grow for 24 h under standard conditions before styrene was added. Data presented here are mean values from three independent growth experiments. (B) Increase in biofilm biovolume over time in standard and in styrene environments. ▵, biovolume of the biofilm grown in styrene environment; ▴, biovolume of the biofilm grown in a nonsolvent environment.
Styrene does not affect the biofilm growth velocity and structural integrity.
The progression of biofilm maturation can be described as an increase in biomass defined as biovolume given in μm3/μm2, as calculated by COMSTAT. The biovolume of the biofilm increased almost linearly over time, both in the presence of styrene and under standard growth conditions (Fig. 4B). A 24-h-old biofilm had a biovolume of 100 μm3/μm2 and reached 220 μm3/μm2 after 4 days. No significant difference in the biovolume was observed between biofilms grown in contact with styrene and under normal conditions, allowing the conclusion that styrene as a toxic solvent has no significant effect on the growth rate and the overall structural integrity of biofilms.
Cell distribution in solvent-stressed biofilms.
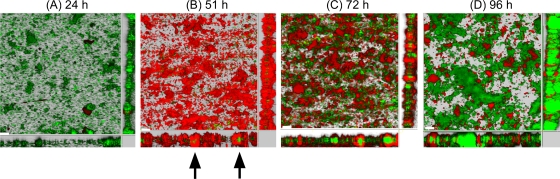
To investigate the distribution of dead/permeabilized cells within the biofilm, images were recorded at an early point of cultivation time (24 h, initial biofilm), after 51 h (intermediate biofilm), and at a later stage (72 and 96 h, mature biofilm). Selected images from at least three independent experiments and their sectional layers, as well as the respective side views, have been evaluated for an estimation of the cell distribution (Fig. 5).
FIG. 5.
Confocal micrographs (sectional views) showing the distribution of live and dead (permeabilized) cells in the layers of biofilm at different time frames. (A) Early stage (24 h); (B) intermediate stage (51 h); (C and D) matured biofilms (72 and 96 h, respectively). Arrows indicate the intact cells that are located nearly exclusively inside the red (permeabilized) cell layers. Scale bar, 30 μm.
As can be deduced from the previous experiments (Fig. 1), no significant cell permeabilization is visible at the early stage of biofilm development. During biofilm development in a styrene-saturated environment, strong cell permeabilization occurs after approximately 50 h (Fig. 1 and 3). The side views of the respective figure (Fig. 5B) show that permeabilized cells dominate the biofilm, and only a minor portion of still-intact cells are located nearly exclusively inside the red (permeabilized) cell layers, as indicated by the arrows in Fig. 5B. In the course of the experiment, the fraction of nonpermeabilized cells significantly increased and seems to displace the permeabilized cell population (Fig. 5C and D). However, at this stage it is not possible to state if the permeabilized cells recover or detach and leave the biofilm.
DISCUSSION
The tolerance of bacterial biofilms to physical stress, chemical agents, and toxic solvents depends on several intrinsic parameters and their interactions (4). The underlying mechanisms of this tolerance behavior are poorly understood and have not been explored yet. This work intended to study in real time biofilm maturation and its response to solvent stress, and it addresses some of the key parameters, like the three-dimensional structure development, the increased polysaccharide (ConA-stainable fraction) production, and the adaptation and recovery of microbial cells in a biofilm. The biofilms were grown in modified flow-cell reactors. Simultaneously, the response of planktonic, nonadapted cells to the contact with the toxic solvent styrene was monitored in a comparable setup.
Compared to control experiments with planktonic cells, Pseudomonas biofilms adapted much better to the styrene environment. Approximately 65% of the biofilm cells were intact, compared to 7% in the planktonic cultures (Fig. 2 and 3). Some Pseudomonas species are solvent tolerant, i.e., are able to thrive in high concentrations of organic solvents (for example, in styrene, toluene, benzene, and xylene) even in their planktonic or freely swimming state. Numerous studies have been conducted in this direction, and important findings have been reported regarding their solvent tolerance mechanisms, such as changes in the membrane phospholipid composition and membrane fluidity or active efflux pumps to excrete toxic compounds (32, 37). Previous studies showed that Pseudomonas putida S12 cells were able to adapt and acquire resistance to the solvent styrene after a long lag phase. However, this adaptation phase was significantly reduced when preadapted cells were transferred to a fresh medium containing styrene. A rapid decline in the viable cell count was observed in the stationary phase. The counterbalance of the susceptibility and solvent adaptation of the cells could be due to reduced membrane fluidity (39) or the involvement of active solvent efflux pumps (20). Park and coworkers showed that Pseudomonas sp. strain VLB120ΔC is able to grow on LB or mineral medium agar plates covered with a layer of liquid styrene. These adapted cells then were able to grow in liquid medium containing styrene (10%, vol/vol). However, intrinsic enzymatic activities might still be inhibited in solvent-containing environments (31). A direct comparison between shake flask-grown planktonic cells and cells derived from biofilms is difficult because of differences in the growth models. In addition, bacteria growing in biofilms can differ greatly from their planktonic counterparts in many aspects, such as phenotypic characteristics, physiology, and adaptive responses to stress. These changes can provide survival advantages and protection to cells in a biofilm under a wide range of extreme environmental conditions (4, 27, 41, 42). Although planktonic cells were shown to be more susceptible to organic solvents than cells growing in an encased biofilm matrix, one has to keep in mind that our planktonic experiments were not based on preadapted cells, while a biofilm also could be described as a chemostat, with a continuous selection pressure for the most robust subpopulation.
Many studies of biofilm resistance and its aid in the survival of various adverse environmental stress conditions have been performed, and plausible mechanisms have been reported in recent years. A direct comparison of stress responses of planktonic cells and biofilm-grown cells was done for some of the microorganisms. Pseudomonas aeruginosa biofilms showed a reduced susceptibility when challenged with the antimicrobial agent colistin, and it was suggested that the enhanced resistance is due to the development of a tolerant subpopulation in the biofilm. The resistance mechanisms also were linked to the adaptation or activation of a surviving phenotype, targeting physiological mechanisms, and mutations (3, 9, 29). Kubota and coworkers reported the increased resistance of Lactobacillus plantarum JCM 1149 biofilms to organic acids and concluded that biofilms are protected by extracellular polymeric secretions, and the three-dimensional structure of the biofilm then protects the cells inside the biofilm matrix. It is suggested that not only the structure of biofilms but also individual cells in biofilms have an effect on the increased resistance toward the acidic environment. For L. plantarum JCM 1149, individual cells in biofilms alter gene expression profiles and regulation differently than planktonic bacteria (23, 24). The enhanced resistance of selected biofilms to heavy metals also was studied extensively. Here, biofilm tolerance results from diffusion and the binding of heavy metals to EPS, as well as the phenotypic diversification of bacterial cells, namely, persister cells and other mutations, within the biofilm (13, 14, 15, 38). Studies regarding the biofilm resistance to toxic solvents are sparse. Artificially immobilized (entrapped in calcium alginate beads) cells of E. coli, Staphylococcus aureus, and Pseudomonas putida tolerated higher phenol concentrations than free cells. The number of generations of the immobilized cells as well as the formation of microcolonies and cell aggregates were reported to be the main reasons for the observed increase in phenol tolerance (16, 19). Zymomonas mobilis biofilms have been reported to be tolerant against benzaldehyde. However, specific mechanisms for this enhanced tolerance were not described (27). The overall biofilm morphology remained unaffected after exposure to benzaldehyde, which is similar to observations made in this study. No significant difference in biofilm biovolume was observed between biofilms grown in the presence of styrene and those without solvent contact, respectively. Thus, it may be concluded that the solvent styrene did not affect the growth rate and overall biofilm structural integrity (Fig. 4B). However, the molecular basis of the solvent tolerance of biofilms remains elusive, and it is important to quantitatively describe this phenomenon in detail for later in-depth studies on the deactivation and stability of the cells growing in biofilms.
The detailed analysis of the live and dead (permeabilized) cells throughout the z-plane of the biofilm gave a strong indication that the recovery of the biofilm was due mainly to the growth of adapted unaffected cells rather than to the cell recovery of damaged cells. A widely employed staining method to assess cell viability is commonly referred to as the live/dead cell bacterial viability kit and is based on two DNA binding stains, SYTO9 and propidium iodide (PI). Here, we replaced SYTO9 with green fluorescent protein but still used PI for staining cells with compromised membranes. PI actually does not distinguish between different grades of membrane permeabilization. PI is a fluorogenic compound that binds to intracellular DNA. At this stage, we cannot exclude that this dye also binds to extracellular DNA (eDNA), which also would contribute to the strong signal we see after 48 h (Fig. 1). It is, by now, widely accepted that eDNA is secreted into the EPS matrix as part of the initial attachment of biofilm-forming organisms (8, 22). Whether or not eDNA is present in Pseudomonas VLB120ΔC biofilms and whether it is secreted or simply liberated by cell lysis upon solvent stress remains pure speculation at this point. Propidium iodide is membrane impermeant and generally excluded from viable cells, and it enters the cells only through damaged cell membranes. However, there is an ongoing discussion about when a cell is really defined as dead, which depends upon the methods applied for verification of death (30, 43). The most common technique, cultivability, reveals only a minor fraction of organisms, neglecting those that still show metabolic activity, although they are currently not able to divide. The grade of membrane permeabilization is certainly an important factor in this respect. The fact that obviously damaged cells remain a part of the biofilm population, rather than being washed out of the film, indicates that these organisms still are metabolically active. Earlier studies evaluating the application of Pseudomonas VLB120ΔC biofilms as biocatalysts reported that the addition of styrene did not hamper the catalytic activity (productivity) of the biofilm, which also strengthens this assumption (10). It was possible to detect catalytic activity through product (styrene oxide) formation, although the flow-cell format is not optimal (in terms of oxygen supply, biomass, and hydrodynamic conditions) for the biotransformation of styrene to styrene oxide. The productivity of flow-cell reactors is 0.5 g liter−1 day−1 compared to 24 g liter−1 day−1 in an optimized tubular biofilm reactor (10). Biofilm-grown cells displayed stable catalytic activity, producing (S)-styrene oxide continuously during the experimental period regardless of the cell permeabilization and adaptation in the styrene environment (Fig. 3B). These results were similar to those of earlier studies conducted in other biofilm reactor formats for styrene oxide synthesis, such as tubular, solid support membrane aerated reactors. These experiments also showed that the styrene oxide formation rate increased concomitantly with the biofilm biomass and was not hampered by either substrate or product (10, 11).
EPS may play a significant role in tolerating a toxic environment. Fang and coworkers described that the presence of toxic metals (Cd, Cu, Pb, Zn, Al, and Cr) and chemicals (glutaraldehyde and phenol) stimulated the EPS production of the sulfate-reducing bacterial biofilms (7). These results are very similar and in agreement with results achieved in this study (Fig. 4A). Campanac and coworkers also have confirmed the involvement of EPS in particular causing an increased hydrophobicity of biofilm-grown cells under stress conditions by biocide cationic agents as quaternary ammonium compounds (4). Real-time confocal microscopic observations showed that dead (permeabilized) cells were located throughout the biofilm, and this indicates that the styrene diffusion to the biofilm inner layer was not limited. The enhanced survival and tolerance of Pseudomonas sp. strain VLB120ΔC biofilms in environments containing toxic solvents is supposedly a combination of effects, such as the three-dimensional structure, increased polysaccharide production, physiological changes due to adaptation, slow growth, and other, still unknown, resistance mechanisms.
Time-resolved, on-line, noninvasive analysis showed that biofilm-grown Pseudomonas sp. strain VLB120ΔC cells can adapt and grow in environments containing the toxic solvent styrene. The results also show that under solvent stress conditions, biofilm growth velocity was not decreased and structural biofilm integrity was not significantly changed. The catalytic activity remained unaffected, suggesting that Pseudomonas sp. strain VLB120ΔC biofilms are robust systems for performing the continuous biocatalysis of challenging substrates in organic syntheses. Investigations of biofilm architecture revealed an increase in polysaccharide production upon solvent addition. However, the regulation of polysaccharide production under solvent stress conditions remains unclear. Based on these findings, future studies should be directed toward elucidating the molecular mechanisms underlying stress responses in catalytic biofilms. The reported findings can be beneficial for further exploration in the direction of bacterial resistance and response mechanisms to other environmental stresses, such as pH, acids, heat, and toxic inorganic/organic chemicals, etc. Understanding these mechanisms, however, is important for exploiting the full potential of biofilms for biotechnological applications, especially in fine chemical syntheses or in new process concepts, like biorefineries or syngas conversions.
Supplementary Material
Acknowledgments
We gratefully acknowledge and sincerely thank Claus Sternberg of DTU for providing required plasmids and strains. We also thank Thomas Letzel, Rieke Bufe, and Jennifer Hesse for their excellent experimental support. We are very much indebted to Klaus Hirschfeld (Department of Biochemical and Chemical Engineering, TU Dortmund) for fabricating the flow cell reactors.
Footnotes
Published ahead of print on 30 December 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Bao, Y., D. P. Lies, H. Fu, and G. P. Roberts. 1991. An improved Tn7-based system for the single-copy insertion of cloned genes into chromosomes of gram-negative bacteria. Gene 109:167-168. [DOI] [PubMed] [Google Scholar]
- 2.Branda, S. S., S. Vik, L. Friedman, and R. Kolter. 2005. Biofilms: the matrix revisited. Trends Microbiol. 13:20-26. [DOI] [PubMed] [Google Scholar]
- 3.Brown, M. R., D. G. Allison, and P. Gilbert. 1988. Resistance of bacterial biofilms to antibiotics: a growth-rate related effect? J. Antimicrob. Chemother. 22:777-780. [DOI] [PubMed] [Google Scholar]
- 4.Campanac, C., L. Pineau, A. Payard, G. Baziard-Mouysset, and C. Roques. 2002. Interactions between biocide cationic agents and bacterial biofilms. Antimicrob. Agents Chemother. 46:1469-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, M. Y., D. J. Lee, Z. Yang, X. F. Peng, and J. Y. Lai. 2006. Fluorecent staining for study of extracellular polymeric substances in membrane biofouling layers. Environ. Sci. Technol. 40:6642-6646. [DOI] [PubMed] [Google Scholar]
- 6.Emmerling, M., et al. 2002. Metabolic flux responses to pyruvate kinase knockout in Escherichia coli. J. Bacteriol. 184:152-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang, H. H., L. C. Xu, and K. Y. Chan. 2002. Effects of toxic metals and chemicals on biofilm and biocorrosion. Water Res. 36:4709-4716. [DOI] [PubMed] [Google Scholar]
- 8.Flemming, H. C., T. R. Neu, and D. J. Wozniak. 2007. The EPS matrix: the “house of biofilm cells.” J. Bacteriol. 189:7945-7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folkesson, A., J. A. Haagensen, C. Zampaloni, C. Sternberg, and S. Molin. 2008. Biofilm induced tolerance towards antimicrobial peptides. PLoS One 3:e1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gross, R., B. Hauer, K. Otto, and A. Schmid. 2007. Microbial biofilms: new catalysts for maximizing productivity of long-term biotransformations. Biotechnol. Bioeng. 98:1123-1134. [DOI] [PubMed] [Google Scholar]
- 11.Gross, R., K. Lang, K. Bühler, and A. Schmid. 2010. Characterization of a biofilm membrane reactor and its prospects for fine chemical synthesis. Biotechnol. Bioeng. 105:705-717. [DOI] [PubMed] [Google Scholar]
- 12.Halan, B., A. Schmid, and K. Buehler. 2010. Maximizing the productivity of catalytic biofilms on solid supports in membrane aerated reactors. Biotechnol. Bioeng. 106:516-527. [DOI] [PubMed] [Google Scholar]
- 13.Harrison, J. J., H. Ceri, C. A. Stremick, and R. J. Turner. 2004. Biofilm susceptibility to metal toxicity. Environ. Microbiol. 6:1220-1227. [DOI] [PubMed] [Google Scholar]
- 14.Harrison, J. J., H. Ceri, and R. J. Turner. 2007. Multimetal resistance and tolerance in microbial biofilms. Nat. Rev. Microbiol. 5:928-938. [DOI] [PubMed] [Google Scholar]
- 15.Harrison, J. J., et al. 2006. The use of microscopy and three-dimensional visualization to evaluate the structure of microbial biofilms cultivated in the Calgary biofilm device. Biol. Proc. Online 8:194-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heipieper, H. J., H. Keweloh, and H. J. Rehm. 1991. Influence of phenols on growth and membrane permeability of free and immobilized Escherichia coli. Appl. Environ. Microbiol. 57:1213-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heydorn, A., et al. 2000. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146(Pt 10):2395-2407. [DOI] [PubMed] [Google Scholar]
- 18.Kessler, B., V. de Lorenzo, and K. N. Timmis. 1992. A general system to integrate lacZ fusions into the chromosomes of gram-negative eubacteria: regulation of the Pm promoter of the TOL plasmid studied with all controlling elements in monocopy. Mol. Gen. Genet. 233:293-301. [DOI] [PubMed] [Google Scholar]
- 19.Keweloh, H., H. J. Heipieper, and H. J. Rehm. 1989. Protection of bacteria against toxicity of phenol by immobilization in calcium alginate. Appl. Microbiol. Biotechnol. 31:383-389. [Google Scholar]
- 20.Kieboom, J., J. J. Dennis, G. J. Zylstra, and J. A. de Bont. 1998. Active efflux of organic solvents by Pseudomonas putida S12 is induced by solvents. J. Bacteriol. 180:6769-6772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koch, B., L. E. Jensen, and O. Nybroe. 2001. A panel of Tn7-based vectors for insertion of the gfp marker gene or for delivery of cloned DNA into Gram-negative bacteria at a neutral chromosomal site. J. Microbiol. Methods 45:187-195. [DOI] [PubMed] [Google Scholar]
- 22.Koerdt, A., J. Godeke, J. Berger, K. M. Thormann, and S. V. Albers. 2010. Crenarchaeal biofilm formation under extreme conditions. PLoS One 5:e14104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kubota, H., S. Senda, N. Nomura, H. Tokuda, and H. Uchiyama. 2008. Biofilm formation by lactic acid bacteria and resistance to environmental stress. J. Biosci. Bioeng. 106:381-386. [DOI] [PubMed] [Google Scholar]
- 24.Kubota, H., S. Senda, H. Tokuda, H. Uchiyama, and N. Nomura. 2009. Stress resistance of biofilm and planktonic Lactobacillus plantarum subsp. plantarum JCM 1149. Food Microbiol. 26:592-597. [DOI] [PubMed] [Google Scholar]
- 25.Laane, C., S. Boeren, K. Vos, and C. Veeger. 1987. Rules for optimization of biocatalysis in organic solvents. Biotechnol. Bioeng. 30:81-87. [DOI] [PubMed] [Google Scholar]
- 26.Lambertsen, L., C. Sternberg, and S. Molin. 2004. Mini-Tn7 transposons for site-specific tagging of bacteria with fluorescent proteins. Environ. Microbiol. 6:726-732. [DOI] [PubMed] [Google Scholar]
- 27.Li, X. Z., J. S. Webb, S. Kjelleberg, and B. Rosche. 2006. Enhanced benzaldehyde tolerance in Zymomonas mobilis biofilms and the potential of biofilm applications in fine-chemical production. Appl. Environ. Microbiol. 72:1639-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, H., and H. H. Fang. 2002. Extraction of extracellular polymeric substances (EPS) of sludges. J. Biotechnol. 95:249-256. [DOI] [PubMed] [Google Scholar]
- 29.Mah, T. F., and G. A. O'Toole. 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9:34-39. [DOI] [PubMed] [Google Scholar]
- 30.Oliver, J. D. 2005. The viable but nonculturable state in bacteria. J. Microbiol. 43:93-100. [PubMed] [Google Scholar]
- 31.Park, J. B., B. Buhler, S. Panke, B. Witholt, and A. Schmid. 2007. Carbon metabolism and product inhibition determine the epoxidation efficiency of solvent-tolerant Pseudomonas sp. strain VLB120ΔC. Biotechnol. Bioeng. 98:1219-1229. [DOI] [PubMed] [Google Scholar]
- 32.Ramos, J. L., et al. 2002. Mechanisms of solvent tolerance in gram-negative bacteria. Annu. Rev. Microbiol. 56:743-768. [DOI] [PubMed] [Google Scholar]
- 33.Ramos, J. L., E. Duque, M. J. Huertas, and A. Haidour. 1995. Isolation and expansion of the catabolic potential of a Pseudomonas putida strain able to grow in the presence of high concentrations of aromatic hydrocarbons. J. Bacteriol. 177:3911-3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sambrook, J., and D. W. Russell. 2001. Molecular cloning, a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 35.Sardessai, Y., and S. Bhosle. 2002. Tolerance of bacteria to organic solvents. Res. Microbiol. 153:263-268. [DOI] [PubMed] [Google Scholar]
- 36.Sardessai, Y. N., and S. Bhosle. 2004. Industrial potential of organic solvent tolerant bacteria. Biotechnol. Prog. 20:655-660. [DOI] [PubMed] [Google Scholar]
- 37.Sikkema, J., J. A. de Bont, and B. Poolman. 1995. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 59:201-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teitzel, G. M., and M. R. Parsek. 2003. Heavy metal resistance of biofilm and planktonic Pseudomonas aeruginosa. Appl. Environ. Microbiol. 69:2313-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weber, F. J., L. P. Ooijkaas, R. M. Schemen, S. Hartmans, and J. A. de Bont. 1993. Adaptation of Pseudomonas putida S12 to high concentrations of styrene and other organic solvents. Appl. Environ. Microbiol. 59:3502-3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Welin-Neilands, J., and G. Svensater. 2007. Acid tolerance of biofilm cells of Streptococcus mutans. Appl. Environ. Microbiol. 73:5633-5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Welin, J., J. C. Wilkins, D. Beighton, and G. Svensater. 2004. Protein expression by Streptococcus mutans during initial stage of biofilm formation. Appl. Environ. Microbiol. 70:3736-3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Welin, J., et al. 2003. Effect of acid shock on protein expression by biofilm cells of Streptococcus mutans. FEMS Microbiol. Lett. 227:287-293. [DOI] [PubMed] [Google Scholar]
- 43.Xu, H. S., et al. 1982. Survival and viability of nonculturable Escherichia coli and Vibrio cholerae in the estuarine and marine-environment. Microb. Ecol. 8:313-323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.