Abstract
Flavobacterium psychrophilum is the etiological agent of bacterial coldwater disease (BCWD) and rainbow trout fry syndrome (RTFS). It causes disease primarily in fresh water-reared salmonids, but other fish species can also be affected. A diverse array of clinical conditions is associated with BCWD, including tail rot (peduncle disease), necrotic myositis, and cephalic osteochondritis. Degradation of connective and muscular tissues by extracellular proteases is common to all of these presentations. There are no effective vaccines to prevent BCWD or RTFS, and antibiotics are often used to prevent and control disease. To identify virulence factors that might permit development of an efficacious vaccine, cDNA suppression subtractive hybridization (SSH) was used to identify cold-regulated genes in a virulent strain of F. psychrophilum. Genes predicted to encode a two-component system sensor histidine kinase (LytS), an ATP-dependent RNA helicase, a multidrug ABC transporter permease/ATPase, an outer membrane protein/protective antigen OMA87, an M43 cytophagalysin zinc-dependent metalloprotease, a hypothetical protein, and four housekeeping genes were upregulated at 8°C versus the level of expression at 20°C. Because no F. psychrophilum gene was known to be suitable as an internal standard in reverse transcription-quantitative real-time PCR (RT-qPCR) experiments, the expression stability of nine commonly used reference genes was evaluated at 8°C and 20°C. Expression of the 16S rRNA was equivalent at both temperatures, and this gene was used in RT-qPCR experiments to verify the SSH findings. With the exception of the ATCC 49513 strain, similar patterns of gene expression were obtained with 11 other representative strains of F. psychrophilum.
Bacterial cold water disease (BCWD), caused by Flavobacterium psychrophilum, occurs at low water temperatures and can cause economic losses in the aquaculture industry as a result of direct mortality or vertebral deformities that decrease the market value of fish that survive the infection (31, 35). A number of putative virulence factors of this bacterium have been identified, including extracellular proteases involved in degradation of extracellular matrix components such as elastin, fibrinogen, type IV collagen, actin, and myosin (9, 37, 47, 48). The best studied of these, psychrophilic metalloproteases Fpp1 and Fpp2, are thought to be involved in destruction of host tissues; however, their roles have not yet been demonstrated in vivo (47, 48). In a recent study, Sudheesh et al. (53) used two-dimensional acrylamide gel electrophoresis and Western blot analysis to compare a virulent strain and a nonvirulent strain of F. psychrophilum and found a thermolysin that was unique to the virulent strain. A role for the iron uptake-associated gene exbD2 has also been demonstrated. Strains lacking this gene have decreased virulence and confer a high level of protection in rainbow trout fry when they are used for vaccination (4). Other putative virulence factors of F. psychrophilum include a lipopolysaccharide with an unusual O-antigen structure (16, 33), glycocalyx (29), and the tlpB (thiol oxidoreductase-like protein gene) locus, which is involved in gliding motility, growth on iron-depleted media, and extracellular proteolytic activity (3).
Decreasing the water temperature below the optimum for a particular species of fish usually reduces or delays the immune response (11, 40). In rainbow trout, the immunologically nonpermissive temperature is approximately 4°C (10), while the physiologically optimum temperature is 15°C (40). BCWD outbreaks commonly occur at water temperatures of between 8 and 12°C and do not occur at temperatures in excess of 15°C, even though F. psychrophilum grows very well at >15°C in vitro (25). While decreased host immune function associated with low temperature likely plays a role in the pathogenesis of BCWD, the fact that outbreaks commonly occur at temperatures higher than those considered to be immunologically nonpermissive suggests that temperature-regulated factors in the agent may be important in the pathogenesis of BCWD. To identify such factors, the current study was undertaken using a virulent strain of F. psychrophilum.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The F. psychrophilum strains used in this study (Table 1) were grown at 8°C or at 20°C in cytophaga broth (5) containing 0.05% tryptone, 0.05% yeast extract, 0.02% beef extract, 0.02% sodium acetate, 0.05% anhydrous calcium chloride, 0.05% magnesium chloride, and 0.05% potassium chloride, pH 7.5.
TABLE 1.
Flavobacterium psychrophilum strains used in this study
| FPG no. | Strain no. | Yr of isolation | Tissue/diseasea | Fish species | Sequence typeb |
|---|---|---|---|---|---|
| FPG2 | ATCC 49511 | 1986 | Kidney | O. mykiss | a |
| FPG3 | ATCC 49418 | 1947 | Kidney | O. mykiss | b |
| FPG15 | B305-94-L3 | 1994 | Necrotic myositis | O. mykiss | a |
| FPG17 | B305 94-L5 m | 1994 | Necrotic myositis | Oncorhynchus mykiss | c |
| FPG25 | B216-93-4 | 1993 | Ulcerative dermatitis and necrotizing stomatitis | O. mykiss | a |
| FPG63 | B382-90-4 | 1990 | Ulcerative dermatitis | Salvelinus fontinalis | a |
| FPG64 | B82-97 | 1997 | Ulcerative dermatitis, osteochondritis | Salvelinus alpinus | a |
| FPG96 | CAF8-06 | 2006 | Ulcerative dermatitis | S. alpinus | e |
| FPG100 | W3 | 2008 | Kidney, systemic disease | O. mykiss | a |
| FPG101 | W6 | 2008 | Kidney, systemic disease | O. mykiss | a |
| FPG102 | W7 | 2009 | Kidney, systemic disease | O. mykiss | a |
| FPG103 | W9 | 2009 | Kidney, systemic disease | O. mykiss | a |
Isolates were obtained from skin or superficial lesion, unless otherwise indicated.
16S rRNA sequence type described by Hesami et al. (25).
mRNA isolation.
Total RNA was extracted from log-phase F. psychrophilum cells grown at 8°C (tester) and at 20°C (driver) to an optical density at 600 nm (OD600) of 0.4 using the TRIzol reagent (Invitrogen Life Technologies, Burlington, ON, Canada), following the manufacturer's instructions. Cells grown at 8°C reached an OD600 of 0.4 after 72 h, while cells grown at 20°C reached an OD600 of 0.4 after 36 h. Contaminating DNA was removed using DNA-free DNase treatment and removal reagents (Ambion, Austin, TX). The integrity of the extracted RNA was examined following electrophoresis of samples on a 1% denaturing agarose gel, and the RNA concentration was estimated by spectrophotometery at 260 nm using a NanoDrop ND1000 spectophotometer (Thermo Scientific, Wilmington, DE). For the subtractive hybridization experiments, mRNA was purified from F. psychrophilum FPG25 total RNA using a MICROBExpress bacterial mRNA kit (Ambion), following the manufacturer's instructions. Enriched mRNA supernatants from five total RNA preparations were pooled before ethanol precipitation.
Suppression subtractive hybridization (SSH).
A PCR-Select cDNA subtraction kit (Clontech, Mountain View, CA) was used for subtractive hybridization of F. psychrophilum FPG25 cDNA with modifications described by De Long et al. (18). The first-strand cDNA was synthesized using 2 μg of mRNA-enriched RNA with 2 μl of random hexamer prokaryotic cDNA subtraction primers containing an RsaI site (5′-GTACN6-3′; 10 μM). The mixture was denatured at 70°C for 10 min and then cooled on ice for 2 min. Four microliters of 5× first-strand buffer, 2 μl of a deoxynucleoside triphosphate (dNTP) mixture (10 mM each), and 2 μl of SuperScript III reverse transcriptase (200 U/μl; Invitrogen Life Technologies, Burlington, ON, Canada) were added to each reaction mixture, which was incubated for 10 min at 25°C, followed by 1.5 h at 42°C. To improve cDNA yield, an additional 2 μl of SuperScript III reverse transcriptase was added, and the incubation was carried on for a further 1.5 h at 42°C (18). The second-strand cDNA was synthesized with DNA polymerase I, RNase H, Escherichia coli DNA ligase, DNA polymerase I, and 5× second-strand buffer, following the manufacturer's instructions (Invitrogen Life Technologies). cDNA products were extracted using a QIAquick PCR purification kit (Qiagen Inc., Mississauga, ON, Canada), according to the manufacturer's instructions. Tester and driver cDNAs were digested with RsaI and then checked for complete digestion on 2% agarose gels. Tester cDNA was subdivided into two portions; one portion was ligated to adaptor 1 and the second portion was ligated to adaptor 2R using T4 DNA ligase. In the first hybridization, an excess of RsaI-digested driver was added to RsaI-digested tester cDNA with adaptor 1 and with tester cDNA ligated to adaptor 2R. Samples were heat denatured at 98°C and allowed to anneal at 59°C. In the second hybridization, the products of the first hybridization were mixed without denaturation with fresh denatured RsaI-digested driver cDNA, and hybridization was allowed to take place overnight at 63°C. During this step, tester cDNA ligated to adaptor 1 and annealed to tester cDNA ligated to adaptor 2R. The entire population of molecules was then subjected to PCR amplification using PCR primer 1 (which targets a common sequence at the termini of adaptors 1 and 2R) to amplify the differentially expressed sequences. A secondary PCR amplification was performed using the products of the primary PCR amplification and 50 nM nested 1 and 2R primers to further reduce any nonspecific background PCR products (18). Nested primers 1 and 2R targeted sequences unique to adaptors 1 and 2R, respectively.
Cloning and sequencing.
PCR amplification products from the secondary nested PCR were cloned using a TOPO TA cloning kit for sequencing (Invitrogen Life Technologies), and recombinant plasmid DNA was purified using a QIAprep Spin miniprep kit (Qiagen Inc.), following the manufacturers' instructions. Primers M13F and M13R were used to amplify the insert cDNA in each recombinant plasmid. The 20-μl PCR mixtures consisted of 5.0 μl DNA template, 2.5 μl of 10× PCR buffer containing 3 mM MgCl2, 4.0 μl of dNTP, 1 μl each of primers M13 (forward [F] primer, 5′-GTAAAACGACGGCCAGTG-3′), and M13 (reverse [R] primer, 5′-CAGGAAACAGCTATGAC-3′), 1.0 μl Taq polymerase, and 5.5 μl of H2O. PCR products that differed in size were purified using a QIAquick PCR purification kit (Qiagen Inc.) and were sequenced at the Laboratory Services Division, University of Guelph, Guelph, ON, Canada. After the primer, vector, and adaptor sequences were removed, insert sequences were compared to publicly available sequences using the blastx program (www.ncbi.nlm.nih.gov/BLAST) (2).
Expression analysis of candidate reference genes.
Nine commonly used reference genes (i.e., DNA gyrase subunit A [gyrA], recombinase A [recA], DNA gyrase subunit B [gyrB], prolyl-tRNA synthesase [prolA], 50S ribosomal protein L9 [rplI], 50S ribosomal protein L17 [rplQ], transcription termination factor Rho [rho], 16S rRNA, and glutamine synthetase [glnA]) were evaluated as internal control genes for gene expression analysis by reverse transcription-quantitative real-time PCR (RT-qPCR) (13). Gene-specific primers for the genes used in RT-qPCR experiments were designed using Primer Express software (version 3.0) from Applied Biosystems, Carlsbad, CA (Table 2). The blastn program was used to compare all primer sequences with the F. psychrophilum JIP02/86 genome sequence available in GenBank to ensure amplification specificity (20). Primers were purchased from Sigma-Genosys, Oakville, ON, Canada. Synthesis of the cDNA was carried out using a high-capacity cDNA reverse transcription kit (Applied Biosystems) with 500 ng of DNase-treated total RNA (isolated as described above). Real-time PCR amplification was performed in 96-well plates using a StepOnePlus real-time system (Applied Biosystems). The 20 μl-PCR mixtures contained 10 μl of 2× Power SYBR green PCR master mix, 100 nM each forward and reverse primers, and 5 μl of template cDNA. To ensure that the same amount of input RNA was used, three independent RNA concentration determinations were made spectrophotometrically. The following thermocycler program was used: 95°C for 10 min for activation of the AmpliTAq Gold polymerase and 40 cycles at 95°C for 15 s for denaturation and 60°C for 1 min for annealing and extension.
TABLE 2.
Primers used in RT-qPCR studies of cold-induced and reference genes of F. psychrophilum
| Gene description (designation or locus tag) | Primer sequence |
|---|---|
| Two-component system sensor histidine kinase (FP1516) | F: ATTCGGCGGCCAAAACTAAT |
| R: AAAACGCCGCAGTTCATTGT | |
| ATP-dependent RNA helicase (FP0666) | F: TTTTCCAGCGCCTTTTGG |
| R: CCAATGAGTTGCAAAAAGAAACAT | |
| Outer membrane protein (FP2096) | F: GGCTTTTTTGATCCCGAATCT |
| R: TTTGACTGGCTCCTTTTTCTACTAAAT | |
| Hypothetical protein (FP0029) | F: CATCGAAACCAATAAACTCAATCCT |
| R: GCTCCAGACCAATTCGGTAACT | |
| ATP-binding cassette transporters (FP0834) | F: CAGAAATGGTTAATGTAACGCCATA |
| R: AAAAGGCGGGCAATATCGA | |
| M43 cytophagalysin metalloprotease (FP1619) | F: TCACGAAATAGGTCACTGGATGAA |
| R: TGAATCATGTAACGGAGTATCTGAAAC | |
| DNA gyrase subunit A (gyrA) | F: GAAACCGGTGCACAGAAGG |
| R: CCTGTGGCTCCGTTTATTAA | |
| Recombinase A (recA) | F: CGGACCAGAATCATCAGGAAA |
| R: GGCGTGTTCTGCATCTATGAAA | |
| Prolyl-tRNA synthesase (prolA) | F: GTTGTCATCGGAATGTGTCATAACT |
| R: TTGCCAATGCCGAAGGAA | |
| DNA gyrase subunit B (gyrB) | F: CTAAGAAAGCCCGTGAAATGGT |
| R: CATTTTGCTGGATCTTGTTCAGAA | |
| 50S ribosomal protein L9 (rplI) | F: TGTTCAGCAACAATTTCATAAGCTAA |
| R: GGTATCGTGAAAAGAACAGGAAAATAT | |
| 50S ribosomal protein L17 (rplQ) | F: GCTTCGGTAGTTGGTTCAGCTT |
| R: AACGGTGGTAAAAAAGAAGAAGTGA | |
| Transcription termination factor Rho (rho) | F: TCGTATTATCGATTTGTTTTCTCCAA |
| R: GCTGCAATAGCATTAGCAATCTCTT | |
| 16S rRNA (FP0896) | F: AGACTCCTACGGGAGGCAGC |
| R: ATTACCGCGGCTGCTGG | |
| Glutamine synthetase (glnA) | F: CTAAGAAAGCCCGTGAAATGGT |
| R: CATTTTGCTGGATCTTGTTCAGAA |
RT-qPCR analysis of subtracted clones.
The expression of 10 differentially expressed genes identified in the SSH study whose sequences had significant similarities to the sequences of known genes in GenBank (≤10−5) was analyzed in FPG25 and 11 other F. psychrophilum strains by qRT-PCR. For relative quantification of target genes, cDNA was synthesized using a high-capacity cDNA reverse transcription kit (Applied Biosystems) containing random hexamers and 500 ng of DNase-treated total RNA isolated from F. psychrophilum cells grown at 8°C or 20°C in cytophaga broth. qRT-PCR experiments were performed in 96-well plates employing a StepOnePlus thermocycler (Applied Biosystems). Primers for qRT-PCR experiments (Table 2) were based on the F. psychrophilum JIP02/86 genome sequence (20) and were designed using Primer Express software (version 2.0; Applied Biosystems). Each 20-μl PCR mixture contained 2× Power SYBR green PCR master mix, 100 nM each forward and reverse primers, and 5 μl of template cDNA.
The following thermocycler program was used: heat activation of AmpliTaq Gold DNA polymerase at 95°C for 10 min and 40 cycles at 95°C for 15 s for denaturation and primer annealing and extension at 60°C for 1 min.
A melt curve was produced to confirm a single gene-specific peak and to detect primer-dimer formation by heating the samples from 60 to 95°C in 0.3°C increments. Gene amplification was confirmed by detection of a single peak in the melt curve analysis. No primer-dimer formation was detected. The PCR efficiencies were calculated using a relative standard curve derived from a cDNA mixture. For FPG25, PCR gene amplification was carried out with four independent biological replicates in duplicate under each condition (8°C or 20°C). Two biological replicates performed in duplicate were used for the 11 additional F. psychrophilum strains. The relative expression ratio of each target gene was determined according to the method described by Pfaffl (38). Expression of each gene was normalized to that of the internal control grown under the same culture conditions using the following equation: 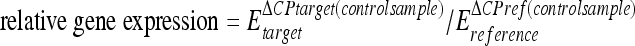 , where CP is the crossing point, Etarget is the real-time PCR efficiency of target gene transcript, Ereference is the real-time PCR efficiency of reference gene transcript, ΔCPtarget is the CP deviation of control − CP deviation of a sample of the target gene transcript, and ΔCPref is the CP deviation of control − CP deviation of a sample of the reference gene transcript.
, where CP is the crossing point, Etarget is the real-time PCR efficiency of target gene transcript, Ereference is the real-time PCR efficiency of reference gene transcript, ΔCPtarget is the CP deviation of control − CP deviation of a sample of the target gene transcript, and ΔCPref is the CP deviation of control − CP deviation of a sample of the reference gene transcript.
One-sample t test was performed to test the significance of the fold change between 8 and 20°C.
RESULTS
SSH.
Analysis of 100 random SSH recombinant plasmids revealed 10 genes predicted to encode the following proteins: a two-component system sensor histidine kinase, an ATP-dependent RNA helicase, an outer membrane (OM) protein (OMP), a hypothetical protein, an ATP-binding cassette transporter, an M43 cytophagalysin family metalloprotease, and four common housekeeping proteins (DNA gyrase subunit A and subunit B, recombinase A, and prolyl-tRNA synthetase) (Table 3).
TABLE 3.
Closest homologs of cold-induced genes of F. psychrophilum FPG25 identified by subtractive hybridization
| Clone (insert size [bp]) | Protein name (source) | Locus tag | GenBank accession no. | Function | E valuea | IDb (%) |
|---|---|---|---|---|---|---|
| A (770, 669, 530, 510, 335, 315, 221, 150, 145) | Two-component system sensor histidine kinase (Flavobacterium psychrophilum JIP02/86) | FP1516 | YP_00129639 | Signal transduction, some similarities with two-component sensor histidine kinase LytS | 3e−137 | 100 |
| Two-component system sensor histidine kinase (Gramella forsetii KT0803) | GFO_2625 | YP_862648 | Signal transduction | 5e−58 | 99 | |
| Possible sensor protein (Kordia algicida OT-1) | KAOT1_11837 | ZP_02159970 | Predicted periplasmic ligand-binding sensor domain | 2e−34 | 98 | |
| B (233, 230, 210, 185, 180, 153, 61) | ATP-dependent RNA helicase, DEAD box family (F. psychrophilum JIP02/86) | FP0666 | YP_001295586 | RNA modification | 6e−31 | 100 |
| Possible ATP-dependent RNA helicase (Flavobacterium bacterium BAL38) | FBBAL38_00395 | ZP_01732763 | RNA modification | 2e−21 | 69 | |
| UW101DEAD/DEAH box helicase domain protein (Flavobacterium johnsoniae UW101) | Fjoh_5046 | YP_001197364 | RNA modification | 9e−21 | 57 | |
| C (770,730,680, 456,159,100, 60) | Outer membrane protein (F. psychrophilum JIP02/86) | FP2096 | YP_001296960 | Similar to outer membrane protein/protective antigen OMA87 COG4775 | 3e−123 | 100 |
| Outer membrane protein (F. johnsoniae UW101) | Fjoh_1690 | YP_001194041 | Surface antigen (D15) | 6e−98 | 65 | |
| Outer membrane protein (Flavobacterium bacterium BAL38) | FBBAL38_07235 | ZP_01734125 | Putative outer membrane protein | 5e−97 | 77 | |
| D (120, 117, 112, 110, 100, 80) | Hypothetical protein (F. psychrophilum JIP02/86) | FP0029 | YP_001294969 | Protein of unknown function | 5e−20 | 100 |
| E (340, 308, 264, 268, 186, 128, 114) | Multidrug ABC transporter permease/ATPase (F. psychrophilum JIP02/86) | FP0834 | YP_001295750 | ABC transporter, permease, and ATP-binding protein (IM-ABC), DPL family, MDL subfamily, drug export | 6e−43 | 100 |
| ABC transporter related (Flavobacterium johnsoniae UW101) | Fjoh_2815 | YP_001195156 | ABC transporter, permease, and ATP-binding protein | 8e−34 | 78 | |
| ABC superfamily ATP-binding cassette transporter, membrane protein (Sphingobacterium spiritivorum ATCC 33861) | HMPREF0766_0778 | ZP_04778237 | ABC transporter, permease, and ATP-binding protein | 5e−25 | 55 | |
| F (466, 350, 334, 299, 202, 80) | M43 cytophagalysin family metalloprotease (F. psychrophilum JIP02/86) | FP1619 | YP_001296495 | Zinc-dependent metalloprotease | 9e−41 | 100 |
| Hypothetical protein Fjoh_4597 (F. johnsoniae UW101) | Fjoh_4597 | YP_001196915 | Probable zinc-dependent metalloprotease | 6e−19 | 60 | |
| Hypothetical protein (Gramella forsetii KT0803) | GFO_3129 | YP_863140 | Probable zinc-dependent metalloprotease | 3e−14 | 48 | |
| G (710, 690, 310, 284, 260, 255, 250, 240, 75, 60) | DNA gyrase subunit A (F. psychrophilum JIP02/86) | FP0748 | YP_001295667 | DNA replication and transcription | 2e−48 | 100 |
| H (228, 210, 196) | DNA gyrase subunit B (F. psychrophilum JIP02/86) | FP0527 | YP_001295451 | DNA replication and transcription | 2e−23 | 100 |
| I (524, 311, 155) | Recombinase A (F. psychrophilum JIP02/86) | FP2245 | YP_001297102 | DNA recombination and repair | 3e−81 | 100 |
| J (510, 188, 119) | Prolyl-tRNA synthesase (F. psychrophilum JIP02/86) | FP1400 | YP_001296285 | Catalyzes the formation of prolyl-tRNA | 3e−29 | 100 |
| K (212) | SprA protein (F. psychrophilum JIP02/86) | FP2121 | YP_001296985 | Similar to SprA protein involved in gliding motility and chitin utilization of F. johnsoniae | 0.055 | 60 |
| L (294) | Acyl (acyl carrier protein) desaturase (F. psychrophilum JIP02/86) | FP1155 | YP_001296053 | Metabolism of lipids | 3.3 | 31 |
| M (154) | Oxidoreductase (F. psychrophilum JIP02/86) | FP1029 | YP_001295934 | Dioxygenase, similar to isopenicillin N synthase and related dioxygenases COG3491 | 0.021 | 73 |
| N (360, 354, 210, 80) | Streptococcus mutans biomarker 0001 | EU918292 | YP_002997673 | Putative ABC transporter, ATP-binding protein | 6e−24 | 49 |
| O (388, 256, 122, 115) | HmwA (Haemophilus influenzae strain AAr105) | AY601283 | AAT27425 | Uncharacterized protein conserved in bacteria, hemagglutination activity | 1e−31 | 67 |
| P (460, 440, 328, 100) | Hypothetical protein BACCAP_03832 (Bacteroides capillosus ATCC 29799) | BACCAP_03836 | EDM98348 | Unknown function | 2e−07 | 52 |
| Q (770, 590, 580, 498, 195) | Hypothetical protein KP1_0491 (Klebsiella pneumoniae NTUH-K2044) | KP1_0491 | YP_00291743 | Unknown function | 3e−14 | 100 |
| R (585, 565, 510, 453) | IS1 ORFc (Shigella sonnei Ss046) | SSON_0977 | YP_309947 | Transposase_27, insertion sequence | 7e−88 | 100 |
Of the longest insert sequence.
ID, identity.
ORF, open reading frame.
Sequences that displayed low levels of similarity with SprA, an oxidoreductase similar to isopenicillin N synthase, and an acyl (acyl carrier protein) desaturase were also detected (Table 3). In addition, 21 inserts with sequences with no significant similarity with the F. psychrophilum JIP02/86 genome were obtained; however, they showed similarities to genes of other bacteria, including an insertion sequence (IS) homologous to IS1 of Shigella sonnei Ss046 (Table 3). The remaining sequences (15 inserts) did not show similarity to any genes in the database.
RT-qPCR analysis of differentially expressed genes.
Among the nine candidate reference genes, including four housekeeping genes that were previously detected by SSH, the 16S rRNA showed the least variation in expression at the two temperatures in RT-qPCR experiments (Table 4) and was therefore used as an internal control to normalize gene expression. Two genes for ribosomal proteins, rplI and rplQ, were markedly upregulated at 8°C versus the level of expression at 20°C, while glnA was downregulated ∼5-fold. The level of expression of cold-induced genes in strain FPG25 was evaluated in RT-qPCR experiments. All 10 cold-induced genes identified by SSH were upregulated from ∼3.5- to 28.36-fold at 8°C, as measured by RT-qPCR (Table 4). To determine if the same genes were cold induced in other strains, 11 additional representative isolates of F. psychrophilum were evaluated at 8°C and 20°C (Table 5). Seven of the 10 genes were downregulated in the FPG3 strain at 8°C; however, the fold changes in the levels of expression of the outer membrane protein, ATP-binding cassette transporter, and M43 cytophagalysin metalloprotease genes were 1.037, 1.37, and 1.16, respectively, when this bacterium was grown at 8°C versus the levels at 20°C. Other than the FPG3 strain, seven genes, i.e., two-component his kinase, ATP-dependent RNA helicase, outer membrane protein (with the exception of FPG63), hypothetical protein, M43 cytophagalysin metalloprotease, recombinase A, and prolyl-tRNA synthesase genes, appeared to be upregulated in the other 11 isolates of F. psychrophilum (Table 5).
TABLE 4.
Fold change of Flavobacterium psychrophilum target and reference gene expression at 8°C versus that at 20°C in FPG25 measured by RT-qPCRb
| Gene description (designation or locus tag) | Fold changea ± SD | P value |
|---|---|---|
| Target genes | ||
| Two-component histidine sensor kinase (FP1516) | 18.15 ± 1.47 | 0.0004 |
| ATP-dependent RNA helicase (FP0666) | 11.31 ± 1.32 | 0.0041 |
| Outer membrane protein (FP2096) | 10.15 ± 1.77 | 0.0034 |
| Hypothetical protein (FP0029) | 7.16 ± 1.17 | 0.0017 |
| ATP-binding cassette transporter (FP0834) | 4.90 ± 0.71 | 0.0041 |
| M43 metalloprotease (FP1619) | 3.44 ± 0.33 | 0.0092 |
| Target and reference genes | ||
| DNA gyrase subunit A (gyrA) | 28.36 ± 9.08 | 0.1430 |
| DNA gyrase subunit B (gyrB) | 3.48 ± 0.25 | 0.0459 |
| Recombinase A (recA) | 4.33 ± 0.82 | 0.0037 |
| Prolyl-tRNA synthesase (pro1A) | 3.52 ± 0.83 | 0.0084 |
| Reference genes | ||
| 50S ribosomal protein L9 (rplI) | 67.40 ± 21.30 | 0.0079 |
| 50S ribosomal protein L17 (rplQ) | 31.39 ± 1.08 | 0.0000 |
| Transcription termination factor Rho (rho) | 2.64 ± 0.62 | 0.0043 |
| Glutamine synthetase (glnA) | 0.18 ± 0.04 | 0.0000 |
Fold change is the fold increase or decrease in the level of expression of a gene at 8°C relative to the level of expression of the gene at 20°C, measured by real-time PCR.
Duplicate measurements of four biological replicates were made for all genes except gryA and gryB, where two replicates were done.
TABLE 5.
Putative cold-induced gene expression change at 8°C versus that at 20°C in 11 F. psychrophilum strains measured by RT-qPCR
| FPG no. | Avg fold change |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| His kinase | RNA helicase | OMP | Hypothetical protein | ABC transporter | M43 metalloprotease | gyrA | recA | Prolyl-tRNA synthesase | gyrB | |
| FPG2 | 2.31 | 2.31 | 2.30 | 3.25 | 1.42 | 3.34 | NDa | ND | ND | ND |
| FPG3 | 0.46 | 0.33 | 1.037 | 0.0 | 1.37 | 1.16 | 0.54 | 0.53 | 0.5 | 0.78 |
| FPG15 | 3.45 | 6.22 | 5.20 | 5.15 | 3.21 | 2.07 | 2.17 | 2.34 | 3.43 | 3.64 |
| FPG17 | 10.69 | 6.07 | 4.10 | 3.8 | 43.9 | 1.18 | 2.28 | 1.95 | 3.12 | 0.51 |
| FPG63 | 79.94 | 104.92 | 0.42 | ND | 0.37 | 18.53 | 0.99 | 2.34 | 204.17 | 1.12 |
| FPG64 | 47.19 | 194.57 | 2.76 | 4.48 | 0.45 | 116.24 | 0.64 | 4.49 | 546.14 | 2.78 |
| FPG96 | 81.06 | 229.89 | 70.11 | 44.43 | 41.25 | 44.46 | 36.46 | 13.48 | 92.51 | 33.49 |
| FPG100 | 23.97 | 21.94 | 9.67 | 7.93 | 5.88 | 1.34 | 6.25 | 7.39 | 8.13 | 3.65 |
| FPG101 | 5.8 | 9.35 | 5.80 | 5.09 | 3.67 | 2.83 | 6.43 | 3.38 | 7.81 | 2.97 |
| FPG102 | 2.63 | 14.88 | 5.46 | 14.48 | 22.1 | 54.37 | 1.35 | 2.91 | 36.08 | 2.91 |
| FPG103 | 5.55 | 18.84 | 10.42 | 7.75 | 8.60 | 1.10 | 13.04 | 4.45 | 13.57 | 5.41 |
ND, not detected.
DISCUSSION
The psychrophilic bacterium F. psychrophilum is capable of growing at 4°C to 20°C, with optimum growth occurring at 12°C to 15°C (8). The ability of microorganisms to adapt to low-temperature conditions likely depends on their capacity to sense changes in temperature and respond by altering gene expression. Some (putative) virulence genes are known to be induced or upregulated at low temperatures and downregulated at higher temperatures. For example, more Fpp1 extracellular metalloprotease is produced by F. psychrophilum cells at 12°C than at 18°C (47). To better understand how F. psychrophilum causes BCWD at low temperatures, an SSH approach was utilized to identify genes that are differentially expressed at low temperature.
On the basis of significant similarity with homologs in the GenBank database and the frequency of recovery, 10 cold-induced genes were identified by SSH, and their upregulation in F. psychrophilum FPG25 was confirmed by RT-qPCR. One of these genes, FP1516, which encodes a two-component system sensor histidine kinase, was upregulated 18.15-fold at 8°C. Pathogenic bacteria often use two-component gene regulatory systems to control expression of genes encoding proteins for drug resistance, surface structures, restriction modification, toxins, adhesins, quorum sensing, biofilm formation, and other virulence-associated molecules that interact with the host and mediate survival in vivo (7, 41). The putative two-component regulatory system sensor kinase FP1516 identified in this study had the greatest similarity with the two-component sensor histidine kinase LytS, which is involved in regulation of cell autolysis in a variety of bacteria, including Escherichia coli SMS-3-5, Staphylococcus aureus, and Bacillus subtilis (12). A recent study of the LytSR two-component regulatory system indicates that the products of this system regulate biofilm development in S. aureus (49). FP1516 and a partner regulator (or a similar regulatory system) might control biofilm formation in F. psychrophilum, since four proteins similar to the alginate-O-acetyltransferases of Pseudomonas aeruginosa (thought to be involved in biofilm formation) are present in the genome of this bacterium (20).
Another gene identified in the SSH library was the ATP-dependent RNA helicase homolog FP0666, which was upregulated 11.31-fold at 8°C. This gene encodes a protein that is a member of the DEAD (Asp-Glu-Ala-Asp) box RNA helicase family. RNA helicase genes have been demonstrated to be cold induced in a number of different bacteria (44) and are thought to be overexpressed at low temperature in psychrophilic bacteria so that they can destabilize the mRNA secondary structures and facilitate initiation of translation of related cold-induced proteins (32, 45). Several studies have suggested a role for DEAD box proteins in bacterial virulence. For instance, they may be involved in regulating the important virulence factor urease in Helicobacter pylori (26), and in the anaerobic pathogen Clostridium perfringens, a DEAD box RNA helicase is thought to play a role in the adaptive response to oxidative stress (26). Secretion systems can be associated with virulence by transporting toxins and proteases to the bacterial surface. Seven SSH recombinant plasmids contained sequences related to the gene that encodes the multidrug ABC transporter permease/ATPase FP0834, which was upregulated 4.90-fold at 8°C. A number of bacterial ABC-type transporters have been associated with virulence (17, 27, 36, 43). For example, ABC transporters are involved in export of capsular polysaccharide across the cytoplasmic membrane in Gram-negative bacteria (24), and in Listeria monocytogenes, multidrug resistance transporters are reported to activate a cytosolic surveillance pathway of host innate immunity (14).
Another cDNA fragment obtained from the SSH library showed similarity to the outer membrane protein/protective antigen OMA87 COG4775. This OMP (FP2096) was upregulated 10.15-fold at 8°C. The D15/Oma87 protein family members are 87-kDa outer membrane antigens (Oma87) known to be encoded by some bacterial pathogens (42). Several immunogenic surface molecules of F. psychrophilum have been implicated in pathogenesis, and some of these may be good candidates for F. psychrophilum vaccines (15, 21-23, 34, 39). While immunization with whole-cell bacterins induces partial protection, antigens associated with the OM fractions of F. psychrophilum have been reported to elicit protective immunity in experimentally infected fish (28, 34, 53). The FP2096 OMP identified in this study belongs to the D15/Oma87 protein family, which is highly immunogenic and highly conserved among a wide range of bacterial species, although the exact function of D15/Oma87 is not known (1, 42).
The M43 cytophagalysin family zinc-dependent metalloprotease FP1619 identified in the SSH study was upregulated 3.44-fold at 8°C (Table 5). Because there is a correlation between proteolytic activity and the virulence of this organism, the M43 cytophagalysin may be involved in pathogenesis through destruction of host tissues. Interestingly, Ostland et al. (37) reported that an extracellular preparation of an Ontario strain of F. psychrophilum recovered from a case of necrotic myositis contained zinc-dependent and heat-stable metalloprotease activities that were able to induce severe muscle necrosis in rainbow trout.
Genes for DNA gyrase subunit A (gyrA) and subunit B (gyrB), recombinase A (recA), and prolyl-tRNA synthetase (pro1A) were also identified in the SSH experiments, and their induction at 8°C was confirmed by RT-qPCR. GyrA has previously been identified to be a cold-inducible protein in prokaryotes (44). The gyrA and gyrB genes were upregulated 28.36- and 3.48-fold at 8°C, respectively, in this study. The reason that gyrB was not induced at the same level as gyrA is unclear, but as suggested by other authors (44), the induction of gyrA seems to be sufficient to increase the DNA negative supercoiling after cold treatment.
An IS homologous to IS1 of Shigella sonnei Ss046 that was identified in the SSH study is not present in the published F. psychrophilum genome (20). Bacterial IS elements can cause disruption of gene function, gene activation, or DNA rearrangements, all of which may modify bacterial characteristics, particularly in relation to pathogenesis or antimicrobial resistance (46); further, the transposition activity of IS elements is known to be induced in response to various stress conditions in bacteria (19). The regions flanking an IS element insertion(s) need to be identified and sequenced before the significance of this cold-induced gene can be determined.
To assess the level of upregulation of these cold-induced genes, RT-qPCR was carried out. Because no reference gene was known to be suitable for use as an internal standard for F. psychrophilum, the stability of expression of nine reference genes, including the four housekeeping genes identified in the SSH experiments (gyrA, gyrB, recA, and pro1A), was evaluated under the two temperature conditions (Table 4). The nine genes selected for analysis encode proteins involved in different metabolic activities. rplI and rplQ (30), glnA, rho, recA, gyrA, gyrB, and pro1A (54), and 16S rRNA are housekeeping genes that have been used as reference genes in similar studies of various bacteria (51, 55). Of the nine genes tested, only the 16S rRNA gene was stably expressed at the two temperatures. Although housekeeping genes involved in basic cellular metabolism are usually constitutively expressed, some bacteria respond to low temperature by altering protein synthesis and cellular metabolism (6, 52). Hence, the upregulation of seven of the remaining candidate reference genes was not unexpected.
To determine if the upregulation of the 10 genes identified by SSH was strain specific, a further 11 strains were evaluated. With the exception of the FPG3 strain, the 10 cold-induced genes were, for the most part, upregulated at 8°C versus the level of expression at 20°C in the other F. psychrophilum strains. In addition, expression of the ATP-binding cassette transporter and DNA gyrase subunit A genes in two strains (FPG63 and FPG64), the outer membrane protein in one strain (FPG63), and DNA gyrase subunit B gene in one strain (FPG17) was downregulated at 8°C versus the level of expression at 20°C (Table 5). The difference in expression of the 10 cold-induced genes in the FPG3 strain versus that in the others may be due to the fact that it is avirulent (50). Although strain FPG3 was originally isolated from a systemic infection, it might have lost virulence upon repeated passage or it might have been an avirulent strain isolated from a polyclonal infection.
In conclusion, genes encoding a number of putative virulence factors, including a two-component system sensor histidine kinase, an ATP-dependent RNA helicase, an ATP-binding cassette transporter, an outer membrane protein, an M43 cytophagalysin metalloprotease, and a hypothetical protein (FP0029), plus seven housekeeping genes, were upregulated in F. psychrophilum cells after growth at 8°C compared with the level of expression after growth at 20°C. Further research, including mutation analysis, is needed to determine the molecular link between these cold-regulated genes and BCWD. Nevertheless, several of these genes (e.g., those for the metalloprotease and OMP) have potential for use in the development of a recombinant vaccine, whereas homologs of the cold-induced reference genes are possible candidates for the development of live attenuated vaccines.
Acknowledgments
We thank Jing Zhang from the Genomics Facility at the University of Guelph for her expert help with the RT-qPCR experiments.
This study was supported by the Ontario Ministry of Agriculture, Food, and Rural Affairs, Fisheries, and Oceans Canada and the Natural Sciences and Engineering Research Council.
Footnotes
Published ahead of print on 7 January 2011.
REFERENCES
- 1.Adler, B., et al. 1996. Candidate vaccine antigens and genes in Pasteurella multocida. J. Biotechnol. 73:83-90. [DOI] [PubMed] [Google Scholar]
- 2.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez, B., P. Secades, M. Prieto, M. J. McBride, and J. A. Guijarro. 2006. A mutation in Flavobacterium psychrophilum tlpB inhibits gliding motility and induces biofilm formation. Appl. Environ. Microbiol. 72:4044-4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez, B., J. Alvarez, A. Menéndez, and J. A. Guijarro. 2008. A mutant in one of two exbD loci of a TonB system in Flavobacterium psychrophilum shows attenuated virulence and confers protection against cold water disease. Microbiology 154:1144-1151. [DOI] [PubMed] [Google Scholar]
- 5.Anacker, R. L., and E. J. Ordal. 1959. Study on the myxobacterium Chondrococcus columnaris. I. Serological typing. J. Bacteriol. 78:25-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Awano, N., M. Inouye, and S. Phadtare. 2008. RNase activity of polynucleotide phosphorylase is critical at low temperature in Escherichia coli and is complemented by RNase II. J. Bacteriol. 190:5924-5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beier, D., and R. Gross. 2006. Regulation of bacterial virulence by two-component systems. Curr. Opin. Microbiol. 9:143-152. [DOI] [PubMed] [Google Scholar]
- 8.Bernardet, J. F., and J. P. Bowman. 2006. The genus Flavobacterium, p. 481-531. In M. Dworkin et al. (ed.), The prokaryotes, vol. 7. Springer-Verlag, New York, NY. [Google Scholar]
- 9.Bertolini, J. M., H. Wakabayashi, V. G. Watral, M. J. Whipple, and J. S. Rohovec. 1994. Electrophoretic detection of protease from selected strains of Flexibacter psychrophilus and assessment of their variability. J. Aquat. Anim. Health 6:224-233. [Google Scholar]
- 10.Bly, J. E., and L. W. Clem. 1992. Temperature and teleost immune functions. Fish Shellfish Immunol. 2:159-171. [Google Scholar]
- 11.Bowden, T. J. 2008. Modulation of the immune system of fish by their environment. Fish Shellfish Immunol. 25:373-383. [DOI] [PubMed] [Google Scholar]
- 12.Brunskill, E. W., and K. W. Bayles. 1996. Identification of LytSR-regulated genes from Staphylococcus aureus. J. Bacteriol. 178:5810-5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bustin, S. A., et al. 2009. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55:611-622. [DOI] [PubMed] [Google Scholar]
- 14.Crimmins, G. T., et al. 2008. Listeria monocytogenes multidrug resistance transporters activate a cytosolic surveillance pathway of innate immunity. Proc. Natl. Acad. Sci. U. S. A. 105:10191-10196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crump, E. M., J. Burian, P. D. Allen, and W. W. Kay. 2005. Identification and expression of a host recognized antigen, FspA, from Flavobacterium psychrophilum. Microbiology 151:3127-3135. [DOI] [PubMed] [Google Scholar]
- 16.Dalsgaard, I., and L. Madsen. 2000. Bacterial pathogens in rainbow trout, Oncorhynchus mykiss (Walbaum), reared at Danish freshwater farms. J. Fish Dis. 23:199-206. [Google Scholar]
- 17.Davidson, A. L., E. Dassa, C. Cedric Orelle, and J. Chen. 2008. Structure, function, and evolution of bacterial ATP-binding cassette systems. Microbiol. Mol. Biol. Rev. 72:317-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Long, S. K., K. A. Kinney, and M. J. Kirisits. 2008. Prokaryotic suppression subtractive hybridization PCR cDNA subtraction, a targeted method to identify differentially expressed genes. Appl. Environ. Microbiol. 74:225-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drevinek, P., et al. 2010. Oxidative stress of Burkholderia cenocepacia induces insertion sequence-mediated genomic rearrangements that interfere with macrorestriction-based genotyping. J. Clin. Microbiol. 48:34-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duchaud, E., et al. 2007. Complete genome sequence of the fish pathogen Flavobacterium psychrophilum. Nat. Biotechnol. 25:763-769. [DOI] [PubMed] [Google Scholar]
- 21.Dumetz, F., et al. 2006. A protective immune response is generated in rainbow trout by an OmpH-like surface antigen (P18) of Flavobacterium psychrophilum. Appl. Environ. Microbiol. 72:4845-4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dumetz, F., S. E. LaPatra, E. Duchaud, S. Claverol, and M. Le Henaff. 2007. The Flavobacterium psychrophilum OmpA, an outer membrane glycoprotein, induces a humoral response in rainbow trout. J. Appl. Microbiol. 103:1461-1470. [DOI] [PubMed] [Google Scholar]
- 23.Dumetz, F., et al. 2008. Analysis of the Flavobacterium psychrophilum outer-membrane subproteome and identification of new antigenic targets for vaccine by immunomics. Microbiology 154:1793-1801. [DOI] [PubMed] [Google Scholar]
- 24.Garmory, H. S., and R. W. Titball. 2004. ATP-binding cassette transporters are targets for the development of antibacterial vaccines and therapies. Infect. Immun. 72:6757-6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hesami, S., et al. 2008. Phenotypic and genotypic analysis of Flavobacterium psychrophilum isolates from Ontario salmonids with coldwater disease. Can. J. Microbiol. 54:619-629. [DOI] [PubMed] [Google Scholar]
- 26.Heung, L. J., and M. Del Poeta. 2005. Unlocking the DEAD-box: a key to cryptococcal virulence. J. Clin. Invest. 115:593-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janulczyk, R., S. Ricci, and L. Bjorck. 2003. MtsABC is important for manganese and iron transport, oxidative stress resistance, and virulence of Streptococcus pyogenes. Infect. Immun. 71:2656-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LaFrentz, B. R., S. E. LaPatra, G. R. Jones, and K. D. Cain. 2004. Protective immunity in rainbow trout Oncorhynchus mykiss following immunization with distinct molecular mass fractions isolated from Flavobacterium psychrophilum. Dis. Aquat. Organ. 59:17-26. [DOI] [PubMed] [Google Scholar]
- 29.LaFrentz, B. R., N. M. Lindstrom, S. E. LaPatra, D. R. Call, and K. D. Cain. 2007. Electrophoretic and Western blot analyses of the lipopolysaccharide and glycocalyx of Flavobacterium psychrophilum. Fish Shellfish Immunol. 23:770-780. [DOI] [PubMed] [Google Scholar]
- 30.Laskowski, M. A., and B. I. Kazmierczak. 2006. Mutational analysis of RetS, an unusual sensor kinase-response regulator hybrid required for Pseudomonas aeruginosa virulence. Infect. Immun. 74:4462-4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leeds, T. D., et al. 2010. Response to selection for bacterial cold water disease resistance in rainbow trout. J. Anim. Sci. 88:1936-1946. [DOI] [PubMed] [Google Scholar]
- 32.Lim, J., T. Thomas, and R. Cavicchioli. 2000. Low temperature regulated DEAD-box RNA helicase from the Antarctic archaeon, Methanococcoides burtonii. J. Mol. Biol. 297:553-567. [DOI] [PubMed] [Google Scholar]
- 33.MacLean, L. L., E. Vinogradov, E. M. Crump, M. B. Perry, and W. W. Kay. 2001. The structure of the lipopolysaccharide O-antigen produced by Flavobacterium psychrophilum. Eur. J. Biochem. 268:2710-2716. [DOI] [PubMed] [Google Scholar]
- 34.Merle, C., D. Faure, M. C. Urdaci, and M. Le Henaff. 2003. Purification and characterization of a membrane glycoprotein from the fish pathogen Flavobacterium psychrophilum. J. Appl. Microbiol. 94:1120-1127. [DOI] [PubMed] [Google Scholar]
- 35.Nematollahi, A., A. Decostere, F. Pasmans, and F. Haesebrouck. 2003. Flavobacterium psychrophilum infections in salmonid fish. J. Fish Dis. 26:563-574. [DOI] [PubMed] [Google Scholar]
- 36.Nishi, J., et al. 2003. The export of coat protein from enteroaggregative Escherichia coli by a specific ATP binding cassette transporter system. J. Biol. Chem. 278:45680-45689. [DOI] [PubMed] [Google Scholar]
- 37.Ostland, V. E., P. J. Byrne, G. Hoover, and H. W. Ferguson. 2000. Necrotic myositis of rainbow trout, Oncorhynchus mykiss (Walbaum): proteolytic characteristics of a crude extracellular preparation from Flavobacterium psychrophilum. J. Fish Dis. 23:329-336. [Google Scholar]
- 38.Pfaffl, M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rahman, H., et al. 2002. The outer membrane fraction of Flavobacterium psychrophilum induces protective immunity in rainbow trout and ayu. Fish Shellfish Immunol. 12:169-179. [DOI] [PubMed] [Google Scholar]
- 40.Raida, M. K., and K. Buchmann. 2007. Temperature-dependent expression of immune-relevant genes in rainbow trout following Yersinia ruckeri vaccination. Dis. Aquat. Org. 77:41-52. [DOI] [PubMed] [Google Scholar]
- 41.Ribardo, D. A., T. J. Lambert, and K. S. McIver. 2004. Role of Streptococcus pyogenes two-component response regulators in the temporal control of Mga and the Mga-regulated virulence gene emm. Infect. Immun. 72:3668-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robb, C. W., C. J. Orihuelaa, M. B. Ekkelenkampa, and D. W. Niesel. 2001. Identification and characterization of an in vivo regulated D15/Oma87 homologue in Shigella flexneri using differential display polymerase chain reaction. Gene 262:169-177. [DOI] [PubMed] [Google Scholar]
- 43.Roset, M. S., A. E. Ciocchini, R. A. Ugalde, and N. Inon de Iannino. 2004. Molecular cloning and characterization of cgt, the Brucella abortus cyclic beta-1,2-glucan transporter gene, and its role in virulence. Infect. Immun. 72:2263-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scherer, S., and K. Neuhaus. 2006. Life at low temperatures, p. 210-262. In M. Dworkin et al. (ed.), The prokaryotes, vol. 2. Springer-Verlag, New York, NY. [Google Scholar]
- 45.Schmid, S. R., and P. Linder. 1992. D-E-A-D protein family of putative RNA helicases. Mol. Microbiol. 6:283-292. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt, H., and M. Hensel. 2004. Pathogenicity islands in bacterial pathogenesis. Clin. Microbiol. Rev. 17:14-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Secades, P., B. Alvarez, and J. A. Guijarro. 2001. Purification and characterization of a psychrophilic calcium induced, growth-phase-dependent metalloprotease from the fish pathogen Flavobacterium psychrophilum. Appl. Environ. Microbiol. 67:2436-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Secades, P., B. Alvarez, and J. A. Guijarro. 2003. Purification and properties of a new psychrophilic metalloprotease (Fpp2) in the fish pathogen Flavobacterium psychrophilum. FEMS Microbiol. Lett. 226:273-279. [DOI] [PubMed] [Google Scholar]
- 49.Sharma-Kuinkel, B. K., et al. 2009. The Staphylococcus aureus LytSR two-component regulatory system affects biofilm formation. J. Bacteriol. 191:4767-4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soule, M., K. Cain, S. LaFrentz, and D. R. Call. 2005. Combining suppression subtractive hybridization and microarrays to map the intraspecies phylogeny of Flavobacterium psychrophilum. Infect. Immun. 73:3799-3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stevenson, D. M., and P. J. Weimer. 2005. Expression of 17 genes in Clostridium thermocellum ATCC 27405 during fermentation of cellulose or cellobiose in continuous culture. Appl. Environ. Microbiol. 71:4672-4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stubs, D., T. M. Fuchs, B. Schneider, A. Bosserhoff, and R. Gross. 2005. Identification and regulation of cold-inducible factors of Bordetella bronchiseptica. Microbiology 151:1895-1909. [DOI] [PubMed] [Google Scholar]
- 53.Sudheesh, P. S., et al. 2007. Identification of potential vaccine target antigens by immunoproteomic analysis of a virulent and a non-virulent strain of the fish pathogen Flavobacterium psychrophilum. Dis. Aquat. Org. 74:37-47. [DOI] [PubMed] [Google Scholar]
- 54.Takle, G. W., I. K. Toth, and M. B. Brurberg. 2007. Evaluation of reference genes for real-time RT-PCR expression studies in the plant pathogen Pectobacterium atrosepticum. BMC Plant Biol. 7:50-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Venkatesh, B., et al. 2006. The Erwinia chrysanthemi 3937 PhoQ sensor kinase regulates several virulence determinants. J. Bacteriol. 188:3088-3098. [DOI] [PMC free article] [PubMed] [Google Scholar]


