Abstract
Baker's yeast (Saccharomyces cerevisiae) whole-cell bioconversions of naringenin 7-O-β-glucoside revealed considerable β-glucosidase activity, which impairs any strategy to generate or modify flavonoid glucosides in yeast transformants. Up to 10 putative glycoside hydrolases annotated in the S. cerevisiae genome database were overexpressed with His tags in yeast cells. Examination of these recombinant, partially purified polypeptides for hydrolytic activity with synthetic chromogenic α- or β-glucosides identified three efficient β-glucosidases (EXG1, SPR1, and YIR007W), which were further assayed with natural flavonoid β-glucoside substrates and product verification by thin-layer chromatography (TLC) or high-performance liquid chromatography (HPLC). Preferential hydrolysis of 7- or 4′-O-glucosides of isoflavones, flavonols, flavones, and flavanones was observed in vitro with all three glucosidases, while anthocyanins were also accepted as substrates. The glucosidase activities of EXG1 and SPR1 were completely abolished by Val168Tyr mutation, which confirmed the relevance of this residue, as reported for other glucosidases. Most importantly, biotransformation experiments with knockout yeast strains revealed that only EXG1 knockout strains lost the capability to hydrolyze flavonoid glucosides.
Glycoside hydrolases, in particular glucosidases (EC 3.2.1.-), are widespread in pro- and eukaryotic organisms and play a pivotal role in many biological processes, such as the metabolism of oligosaccharides or the degradation of endogenous and exogenous glycosides. Beta-glucosidases (β-GHs) are among the oldest classes of enzymes, and microbial β-GHs have been identified often as molecular factors indispensable for growth; for example, they enable phytopathogenic fungi to colonize host plant tissues by hydrolyzing plant fungitoxic glucosides to less toxic or less soluble aglyca (5). They are also important for cleaving cellulose and fulfill essential functions in the sporulation of yeast cells (27, 38). In plants, β-GHs are involved in crucial growth processes, such as the degradation of endosperm cell walls during germination or the formation of intermediates in cell wall lignification, as well as in the activation of defense compounds and the formation of phytohormones (references 49 and 58 and references therein). Moreover, plant β-GHs are essential for turnover of flavonoid glucosides, which are exclusively found in β configuration, as had been reported for malonylglucosides of the isoflavones genistein and daidzein in soybean (21) or isoflavone 7-O-β-glucosides in chickpea (20). Even the endophytic bacterium Pseudomonas strain ZD-8 (61), as well as cell-associated β-GHs (bglH and yckE) from Bacillus subtilis natto, used for fermentation of soy products (28), were shown to metabolize apigenin 7-O-β-glucoside or glucosides and malonylglucosides of genistein and daidzein. The capacity of multiple Bifidobacterium strains of human origin to digest isoflavonoid glucosides (32, 41) is particularly noteworthy because of their relevance for the uptake of dietary isoflavones.
β-GHs utilize β-linked oligosaccharides or β-O-glucosides of alkyl and aryl compounds as substrates (37). Most of these enzymes show fairly high stability and are widely used by the food and beverage industries for biotechnological production processes, including the hydrolysis of bitter compounds (naringin) in citrus juice and the liberation of flavor from grape juice (monoterpenols) (3). Glucosidases hydrolyze their substrates in aqueous solution but are also capable of catalyzing the reverse reaction under appropriate reaction conditions. Accordingly, β-GHs are also used in the pharmaceutical, cosmetics, and detergent industries to synthesize alkyl- and arylglucosides from natural polysaccharides or their derivatives (11).
In recent years, flavonoids have come into focus as valuable nutritional factors due to their antioxidant potential, the ability to induce or inhibit enzymes of xenobiotic metabolism, and other health-promoting features (33, 56). Flavonoids are found ubiquitously in spermatophytic plants, mostly in the form of their β-glucosides, and play a pivotal role in plant UV protection, as well as flower coloration and defense. More than 10,000 flavonoid structures have been reported from natural sources (53). Flavonoid glycosylation enhances solubility and increases overall stability in planta compared to the corresponding aglyca. Both the scientific and commercial interests call for an efficient source of flavonoids, including their glucosides. However, the low flavonoid contents of plants, particularly rare flavonoids, limit the yield from tissue extraction (26). An alternative biotechnological approach using plant cell cultures is feasible (9, 15, 17, 19, 26) but has been shown to be inefficient and/or too expensive. The fact that all genes of flavonoid biosynthesis have been cloned, however, paved the way for the production of flavonoids and flavonoid glucosides by white biotechnology (57). Yan et al. (60), for example, described Escherichia coli transformants able to synthesize various anthocyanidin 3-O-β-glucosides from intermediates of the natural pathway. Nevertheless, several steps of flavonoid biosynthesis are catalyzed by membrane-bound cytochrome P450-dependent monooxygenases (Cyt P450), which are insufficiently expressed in bacteria. On the other hand eukaryotic systems, such as baker's yeast (Saccharomyces cerevisiae), likely hold more promise (42). Yeast cells were recently transformed with flavone synthase I (FNSI) from parsley (Petroselinum crispum, Apiaceae) and used in suspension for the biotransformation of several flavanones to the respective flavones (13, 14, 35). However, in a parallel study, we found that natural flavanone glucosides, which do not serve as substrates for FNSI (7), were converted under these conditions to their aglyca and the corresponding flavones (S. Martens, unpublished data). This indicates that suspension-cultured yeast cells express flavonoid O-β-glucoside hydrolase (GH) activity, excluding wild-type S. cerevisiae as a host from the production of flavonoid β-glucosides.
The present work aimed at the development of a yeast-based biotechnological platform for the synthesis of glucosylated flavonoids. The project was based on the hypothesis that knockout of the glycosidase(s) responsible for the hydrolysis of flavonoid glucosides in yeast should enable the recombinant generation of a eukaryotic host suitable for the efficient production of glycosylated flavonoids. As a first step, we identified and characterized in vitro the yeast glucohydrolases responsible for this activity and similarly tested knockout yeasts in whole-cell bioconversion experiments. The proposed system allows the catabolic activity of yeast β-GHs to be overcome and holds promise for the synthesis of glucosylated flavonoids.
MATERIALS AND METHODS
Materials and strains.
The yeast strain INV Sc1 (Invitrogen, Groningen, Netherlands) was used for RNA extraction and expression. Cells were grown in YPD medium (1% yeast extract, 1% peptone, 2% glucose) at 30°C, and cell growth was monitored by measuring the absorbance at 600 nm. The knockout strains EXG1, SPR1, and YIR007W and the respective wild-type strain BY4741 were purchased from Euroscarf (Frankfurt, Germany) (see Table S1 in the supplemental material).
Chemicals.
Chemicals were purchased from Roth (Karlsruhe, Germany) or Sigma-Aldrich (Deisenhofen, Germany); the biochemicals used were from MBI Fermentas (St. Leon-Rot, Germany).
All natural compounds and substrates were purchased from TransMIT Flavonoidforschung (Giessen/Marburg, Germany) or Extrasynthese (Genay, France) or were from our laboratory collection.
Cloning of glucoside hydrolase genes.
Putative GH genes annotated, e.g., with “unknown function” or as “involved in drug resistance” were identified by mining of the S. cerevisiae genome. Genes were amplified by reverse transcription (RT)-PCR according to the procedure generally used in our laboratory. RNA extraction was carried out with the GenElute Yeast Total RNA Purification Kit (Sigma-Aldrich) according to the manufacturer's instructions. cDNA synthesis was done with RevertAid H Minus M-MuLV-Reverse Transcriptase (MBI Fermentas) according to the supplier's instructions. First-strand cDNA products were PCR amplified in a reaction mixture containing 2.5 U Taq DNA polymerase (MBI Fermentas), 25 pmol each of the primers (see Table S2 in the supplemental material) (synthesized by Eurofins MWG GmbH, Martinsried, Germany). Forty cycles of PCR amplification were carried out, each cycle consisting of denaturation at 95°C for 1 min, appropriate annealing (see Table S2 in the supplemental material) for 1 min, and 72°C extension for 2 min using a Robocycler Gradient 96 (Stratagene, Amsterdam, Netherlands). The PCR amplification was completed with a final extension at 72°C for 10 min. PCR products were separated by electrophoresis on 1.5% agarose-Tris-acetate-EDTA (TAE) gels at 120 V for 20 min, stained with ethidium bromide, and visualized under UV irradiation. The amplified genes were finally cloned into the T/A yeast expression vector pYES 2.1 (Invitrogen).
Mutagenesis.
Mutagenesis was accomplished with the QuikChange Site-Directed Mutagenesis Kit (Stratagene, Heidelberg, Germany) used according to the manufacturer's instructions. The following primer sets were used to introduce single-amino-acid changes (underlined): mEXG1f, AACAGCTTGAAATATTGGGTTGATTTGCATGG, and mEXG1r, CCATGCAAATCAACCCAATATTTCAAGCTGTT for EXG1; mSPR1f, TATGGTTTGAAATATTGGATTGATCTTCATGG, and mSPR1r, CCATGAAGATCAATCCAATATTTCAAACCATA, for SPR1.
Yeast transformation and purification of expressed protein.
Introduction of plasmids harboring GH genes into different competent S. cerevisiae strains was achieved with the ScEasyComp Transformation Kit (Invitrogen), following the instructions in the manual. Transformants showing complementation of uracil auxotrophy were selected. Expression, cell disruption, and protein isolation were done as described previously (35). Recombinant His-tagged proteins were purified by immobilized metal affinity chromatography (IMAC column; Bio-Rad, Munich, Germany). Protein purification was done according to the manufacturer's instructions, and the proteins were used for activity assays and SDS-PAGE. Expression of the fusion proteins (fused to the C-terminal V5 epitope) was examined by Western blotting using alkaline phosphatase (AP)-conjugated anti-V5/AP monoclonal antibody from Invitrogen.
Protein concentrations were determined by the method of Bradford (6) using bovine serum albumin as a standard.
Glucosidase assays.
For measurement of GH activity, p-nitrophenyl-α- or -β-d-glucopyranoside (pNPG) was used. Assays of α-GH activity were conducted for 20 min at 37°C in 100 mM potassium phosphate buffer, pH 6.8 (450 μl), with the addition of 20 μl of 3 mM glutathione (reduced), 50 μl 10 mM α-pNPG, and 20 μl of enzyme solution (approximately 50 μg total protein) in a total volume of 200 μl. The solution was diluted with 100 mM Na2CO3 (800 μl), and the amount of p-nitrophenol released was determined by photometry at 400 nm. The β-GH activity was measured for 20 min at 45°C in 100 mM potassium phosphate buffer, pH 5.8 (450 μl), with the addition of 50 μl 10 mM β-pNPG and 100 μl of enzyme solution (approximately 250 μg total protein). The incubation mixture was diluted with 100 mM potassium phosphate buffer, pH 5.8 (500 μl), and the p-nitrophenol released was quantified by measuring the absorbance at 420 nm.
Analytical methods.
Incubations of β-GH assay mixtures with crude or purified enzyme were carried out for 30 min at 30°C in 100 mM potassium phosphate buffer at pH 5.8, and the products were subsequently extracted with ethyl acetate or isoamyl alcohol. The identities of products from β-GH activity assays with various natural glucoside substrates were verified by thin-layer chromatography (TLC) and high-performance liquid chromatography (HPLC). Flavonoid products were routinely analyzed by TLC on cellulose or silica plates (Merck, Darmstadt, Germany). All solvent systems are summarized in Table S3 in the supplemental material. HPLC analysis (Merck-Hitachi, Darmstadt, Germany) was carried out with a Nucleosil 100-10 C18 column (Macherey Nagel, Düren, Germany) according to the method of Isayenkova et al. (23) and using various UV detector and diode array detector (DAD) equipment. Analyses of biotransformation experiments with knockout strains were done according to the method of Romani et al. (45).
Whole-cell bioconversion.
Suspension cultures of yeast transformants (250 ml) expressing the cDNA of either GH or parsley FNSI and flavanone 3-β-hydroxylase (FHT) (34) were used for bioconversion studies. The cultures were incubated for 17 h at 30°C in the presence of the substrate and subsequently extracted with 1 volume of ethyl acetate. Product formation was monitored by cellulose TLC and HPLC as described above.
RESULTS
Cloning and functional characterization of putative yeast GHs.
In silico mining of the yeast genome database (http://www.yeastgenome.org/) revealed a number of likely GH genes. Initially, 10 putative candidates and/or annotations, designated BGL2, CWH41, DSE2, EXG1, SPR1, SUC2, YGR287C, YIL172C, YIR007W, and YJL216C (Table 1), were selected. The coding regions of these genes were amplified by RT-PCR, using yeast poly(A)+ RNA as a template; ligated into the expression vector pYES 2.1; and overexpressed in INV Sc1 yeast cells. The expression of recombinant proteins in these transformants was monitored by Western blotting (data not shown). Initial screenings for α- or β-GH activity were carried out with crude cell extracts and using the chromogenic substrates α- and β-pNPG, respectively. Three proteins were unambiguously determined to possess α-GH activity (YGR287C, YIL172C, and YJL216C) and another three were determined to exhibit β-GH activity (EXG1, SPR1, and YIR007W). For simplicity, these proteins are referred to as α-GH and β-GH, respectively. The four remaining putative GHs (BGL2, CWH41, DSE2, and SUC2) could not be clearly assigned to one or the other category of enzymes (Table 2).
TABLE 1.
Putative glucoside hydrolases retrieved by in silico mining of a yeast genome database
| Gene | Chromosome location | Putative function | Reference | ORFa (bp) | Molecular mass (kDa) |
|---|---|---|---|---|---|
| BGL2 | VII: 1058731 to 1057790 | Endo-β-1,3-glucanase; abundant in the yeast cell wall | 25 | 942 | 33 |
| EXG1 | XII: 728957 to 730303 | Exo-1,3-β-glucanase of the cell wall; involved in cell wall β-glucan assembly | 29 | 1,347 | 51 |
| SPR1 | XV: 690696 to 692033 | Sporulation-specific exo-1,3-β-glucanase; contributes to ascospore thermoresistance | 38 | 1,338 | 52 |
| YIR007W | IX: 370701 to 372995 | Putative protein with unknown function | 22 | 2,295 | 87 |
| SUC2 | IX: 37385 to 38983 | Invertase; hydrolysis of sucrose and raffinose | 12 | 1,599 | 61 |
| YGR287C | VII: 1068998 to 1067229 | Unknown function; similarity to α-d-glucosidase | 50 | 1,770 | 69 |
| YJL216C | X: 26086 to 24341 | Unknown function; similarity to α-d-glucosidase | 55 | 1,746 | 68 |
| YIL172C | IX: 18553 to 16784 | Unknown function, similarity to glucosidases | 22 | 1,770 | 69 |
| DSE2 | VIII: 385513 to 386490 | Daughter cell-specific secreted protein; similarity to glucanases | 8 | 978 | 33 |
| CWH41 | VII: 446148 to 443647 | Integral membrane encoding protein of the ERb, removing the terminal glucose from core oligosaccharides | 51 | 2,502 | 97 |
ORF, open reading frame.
ER, endoplasmic reticulum.
TABLE 2.
Glucosidase activities of recombinant GHs in comparison to INV Sc1 cells using α/β-pNPG as a substrate
| GH | Activitya |
|
|---|---|---|
| α-Glucosidase | β-Glucosidase | |
| ScINVb | 100 | 100 |
| YJL216C | 670 | 160 |
| EXG1 | 180 | 720 |
| SPR1 | 110 | 580 |
| YIR007W | 160 | 630 |
| DSE 2 | 180 | 210 |
| CWH41 | 160 | 230 |
| SUC2 | 200 | 160 |
| YGR287C | 530 | 170 |
| YIL172C | 410 | 90 |
| BGL2 | 90 | 120 |
Activity is indicated as units/ml enzyme.
Nontransformed INV Sc1 cells served as a control; activity was set as 100.
The β-GHs EXG1, SPR1, and YIR007W are grouped in GH family 5 based on a unique sequence signature recognized by the Internet portal http://www.expasy.ch/prosite. This sequence element contains a conserved glutamic acid residue that is potentially involved in the catalytic mechanism (18). Both EXG1 and SPR1 carry a putative N-terminal secretory signal peptide of 20 amino acids, identified with SignalP (http://www.cbs.dtu.dk/services/SignalP/) and suggesting an extracellular location for these proteins, whereas YIR007W lacks such a signal peptide and is likely localized in the cytosol.
Catalytic properties of yeast GHs.
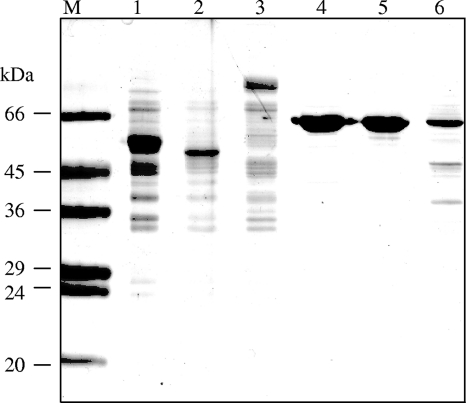
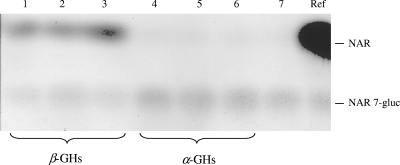
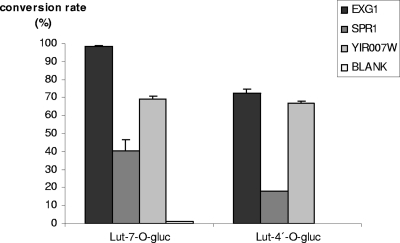
A more detailed characterization of the provisionally annotated α- and β-GHs (Table 1) was conducted with His-tagged affinity-purified proteins. The sizes of the protein bands on SDS-PAGE separation (Fig. 1) were fully compatible with the molecular masses (in kDa) calculated for the translated polypeptides (Table 1). d-Glucono-δ-lactone, which is produced by wine-degrading fungi (i.e., Botrytis cinerea), and glucose at high concentration are known as natural competitive inhibitors of β-GHs (4, 54), although glucose-tolerant β-GHs, which are of prime interest for wine and fruit processing, have also been described, for example, from Aspergillus oryzae (44). Individual incubations of the yeast GHs with pNPG substrate in the presence of increasing concentrations of the inhibitor d-glucono-δ-lactone (1.25, 2.5, and 3.75 mM) revealed 50% inhibitory concentrations (IC50s) for β-GHs of 1.7 mM (EXG1), 1.5 mM (SPR1), and 0.6 mM (YIR007W), while the yeast α-GHs were not affected (data not shown). Glucose at up to 100 mM inhibited neither α- nor β-GH activity. Naringenin 7-O-β-glucopyranoside (NAR 7-gluc) was used as a substrate in standard assays of β-GH activity. All three β-GHs (EXG1, SPR1, and YIR007W) clearly released the flavanone naringenin (NAR) from NAR 7-gluc and were investigated further. Several O-β-glucosides of other flavanones, as well as of flavones, flavonols, isoflavones, and anthocyanidins (see Table S4 in the supplemental material), were tested as potential substrates, and the product identities were confirmed by TLC on cellulose (see Table S3 in the supplemental material) and HPLC (see Materials and Methods). These β-GH assays documented a clear preference for 7-O-β-glucosides and 4′-O-β-glucosides (see Table S4 in the supplemental material). The assays were complemented by control incubations employing the annotated affinity-purified yeast α-GHs (YGR287C, YIL172C, and YJL216C) or boiled β-GH solutions. Neither the α-GHs (Fig. 2) nor the boiled β-GH samples showed flavonoid β-GH activity. The regiospecificities of yeast β-GHs were further substantiated by kinetic comparison of incubations with luteolin 7-O-β-glucoside (Lu 7-gluc) or luteolin 4′-O-β-glucoside (Lu 4′-gluc) as a substrate. All three β-GHs preferred Lu 7-gluc but differed in their relative turnover rates (Fig. 3). Additionally, various 3-O-β-glucosides and 3, 5-O-di-β-glucosides of anthocyanidins were tested. Significant hydrolysis of anthocyanidin 3-O-glucosides was observed only with EXG1 and SPR1, whereas YIR007W appeared inactive under the conditions of the assays (see Table S4 in the supplemental material). Flavonoid glycosides composed of sugars other than d-glucose, e.g., kaempferol 7-O-β-neohesperoside and delphinidin 3-O-β- rhamnoside, or flavonoid glycosyls (C ligated), such as vitexin, isovitexin, and homoorientin, were not hydrolyzed by any of the yeast GHs (see Table S4 in the supplemental material).
FIG. 1.
SDS-PAGE of recombinant proteins after His tag purification. Lanes: 1, EXG1; 2, SPR1; 3, YIR007W; 4, YIL172C; 5, YJL216C; 6, YGR287C; M, marker.
FIG. 2.
Enzyme assays with NAR 7-gluc as a substrate. Lanes: 1, EXG1; 2, SPR1; 3, YIR007W; 4, YIL172C; 5, YJL216C; 6, YGR287C; 7, negative control with boiled protein; Ref, references (NAR 7-gluc and NAR).
FIG. 3.
Comparison of conversion rates of luteolin 7-O-glucoside and luteolin 4′-O-glucoside as substrates. The error bars indicate standard deviations.
Identification of active-site valine.
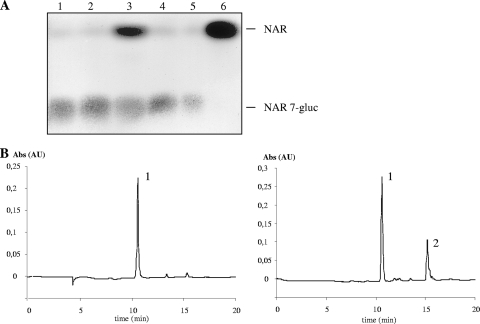
A cytosolic β-GH (human cytosolic β-glucosidase [hCBG]) from human liver assigned to family 1 of GHs (accession no. AF317840) has been reported to hydrolyze a number of flavonoid glucosides. Site-specific Val168Tyr mutation drastically decreased the catalytic activity of this enzyme (1, 2). Alignments of the yeast β-GH polypeptides with hCBG revealed that valine was conserved in equivalent positions in both EXG1 and SPR1 (Fig. 4). Point mutations analogous to those described by Berrin et al. (1, 2) were therefore introduced into EXG1 (Val170Tyr) and SPR1 (Val171Tyr), and the recombinant His-tagged mutant proteins were successfully expressed in yeast as monitored by Western blotting (data not shown). Subsequent activity assays with NAR 7-gluc as a substrate documented the complete loss of function of both mutant proteins (Fig. 5 A and B), underlining the relevance of the conserved valine residue for EXG1 and SPR1, as well.
FIG. 4.
Alignment of EXG1 and SPR1 with hCBG. The conserved Val is in boldface. Colon indicates similar amino acids; asterisks indicate identical amino acids.
FIG. 5.
(A) TLC analysis of mutated EXG1 and SPR1 proteins in comparison to SPR1 with NAR 7-gluc as a substrate. Lanes: 1, mEXG1; 2, mSPR1; 3, SPR1 (positive control); 4, without protein; 5, reference NAR 7-gluc; 6, reference NAR. (B) HPLC chromatograms from an enzyme assay with mutated EXG1. Shown are NAR 7-gluc as a substrate with mutated EXG1 (left) and native EXG1 (right) proteins. Peaks: 1, NAR 7-gluc; 2, NAR. Abs, absorption; AU, arbitrary units.
Bioconversion in β-GH knockout yeast strains.
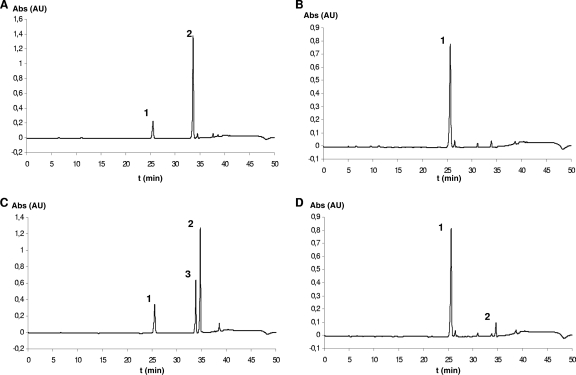
Knockout of the single β-GH EXG1, SPR1, or YIR007W in yeast has already been reported, and the respective strains, Y05210, Y02446, and Y02343 (see Table S1 in the supplemental material), are available. The suitability of these strains for whole-cell bioconversions of flavonoid glucosides was therefore examined by incubation of each single strain for 17 h in yeast-peptone-glucose-ethanol (YPGE) broth supplemented with up to 20 mg liter NAR 7-gluc−1. All three strains grew vigorously in the presence of the flavanone glucoside, reaching cell densities similar to those of the nonsupplemented control (data not shown). The proportions of NAR 7-gluc and NAR were subsequently examined in extracts of the yeast suspensions by TLC or HPLC and compared to those of controls employing wild-type strains Y00000 and INV Sc1 (see Table S1 in the supplemental material). Considerable hydrolysis of NAR 7-gluc was observed in incubations employing wild-type yeast cells and knockout strains that lacked either SPR1 or YIR007W (Fig. 6 A). However, no hydrolysis was observed in cultures of EXG1 knockout strains (Fig. 6B). Additional expression of parsley flavone synthase I, which oxidizes (2S)-NAR, but not its glucoside, to apigenin in the knockout strains corroborated these results. In fact, both NAR and apigenin were recovered from the bioconversions of NAR 7-gluc in the Y02446 and Y02342 transformants (Fig. 6C), whereas the knockout of EXG1 in the transformed strain Y05210 almost completely suppressed the formation of the products (Fig. 6D). Analogous transformation of the β-GH knockout strains with parsley flavanone 3-β-hydroxylase, which hydroxylates (2S)-NAR to dihydrokaempferol, confirmed the significance of EXG1 for the turnover of flavonoid glucosides in yeast. Also, in this case, SPR1 and YIR007W knockout strains metabolized NAR 7-gluc to NAR and further to dihydrokaempferol, whereas the EXG1 knockout strain lacked such activity (data not shown).
FIG. 6.
Bioconversion with yeast knockout strains. (A) representative chromatograms after incubation of 20 mg NAR 7-gluc with knockout strains 2343 and 2446 and wild-type strain Y0000. (B) Chromatogram of strain 5210 after incubation. (C) Coexpression of parsley FNSI in the knockout strains 2343 and 2446 and wild-type strain Y0000 and formation of NAR and Ap. (D) Coexpression of parsley FNSI in the knockout strain 5210. Peaks: 1, NAR 7-gluc; 2, NAR; 3, apigenin (Ap).
DISCUSSION
The use of yeasts as hosts for the biotechnological production of flavonoids is generally hampered by the metabolic activity of the yeasts' β-glucosidases, which hydrolyze the flavonoid β-glucosides. With few specific exceptions, the majority of the studies using yeasts in this field have highlighted this problem. Some of our bioconversion experiments have revealed clear-cut evidence for the rapid hydrolysis of flavonoid β-glucosides in suspension-cultured yeast cells (Martens, unpublished); several of our attempts at overexpressing flavonoid glycosyltransferase (FGT) from various plant species in wild-type yeasts have failed, probably for this reason (S. Schmidt and S. Witte, unpublished data). A recent work describing the expression in S. cerevisiae of an FGT from Dianthus that in vitro glucosylated NAR to NAR 7-glc (59) reported the effect of endogenous yeast glucosidase activity during whole-cell biocatalysis, which was responsible for greatly diminished product yield. Surprisingly, however, in this instance, the residual products (7- and 4′-O-glucosides of NAR) were exclusively recovered from the culture broth, and constitutive expression of the transferase was superior to the galactose-inducible expression. In this case the turnover rates of glucosyltransferase versus flavonoid β-GH and the time of bioconversion determined the amounts of products. Other plant glucosyltransferases were more successfully overexpressed in S. cerevisiae, thus providing the recombinant enzyme or the yeast transformant as a biocatalyst for glucosylation of natural products. For example, a single solanidine glucosyltransferase cDNA was isolated from a potato cDNA expression library in yeast cells (36), taking advantage of the toxicity of the alkaloid solanidine. A similar approach was followed by Poppenberger et al. (40) to isolate a zearalenone (ZON) 4-O-glucosyltransferase from Arabidopsis thaliana. The mycotoxin ZON is a metabolite of Fusarium spp. thriving on grain and causes some health concern because of its estrogenic effect. Out of six Arabidopsis glucosyltransferases overexpressed in yeast, one recombinant enzyme was capable of ligating ZON to ZON-4-Glu. This yeast transformant was propagated in suspensions containing ZON and converted up to 90% of the toxin to ZON-4-Glu. In both of these studies the glucosides were not deglucosylated, probably due to the limited substrate preferences of yeast endogenous GHs.
In this work, we have identified three efficient yeast β-GHs: EXG1, SPR1, and YIR007W, that play relevant roles in flavonoid production in yeasts. Both EXG1 and SPR1 were annotated as exo-1,3-β-glucanases, while the function of YIR007W has not been classified yet. All three GHs efficiently hydrolyzed 7-O-β-glucosides or 4′-O-β-glucosides of flavanones, such as NAR 7-gluc, flavones, flavonols, and isoflavones, whereas 3-O-β-glucosides of flavonols or anthocyanidins were not accepted (see Table S4 in the supplemental material). This regioselectivity is reminiscent of hCBG, which split NAR 7-gluc, eriodictyol 7-O-β-glucoside, Lu 4′-gluc, genistin, and daidzin (see Table S4 in the supplemental material) but failed on kaempferol 3-O-β-glucoside and isorhamnetin 3-O-β-glucoside (1, 2).
Yeast β-GHs and hCBG were assigned to different GH families (family 5 versus family 1) but group together in clan GH-A, and the active site of hCBG had been identified by crystallization, homology modeling, and point mutations of Val168Tyr, Phe225Ser, and Tyr308Ala or Tyr308Phe (2). Each single mutation significantly reduced the turnover of flavonoid glucosides or pNPG. The residues Val168, Phe225, and Tyr308 form a hydrophobic cluster assumed to line the aglycon binding site, with Val168 being most important for affinity (2). Our results on EXG1 and SPR1 support this assumption, because replacement of the conserved Val residue (Val170 and Val171, respectively) completely abolished the glucosidase activity of NAR 7-gluc. The in vitro studies suggested that the activities of EXG1, SPR1, and/or YIR007W may interfere with the generation or modification of flavonoid glucosides in transformed yeast cells. Fortunately, the respective knockout strains are commercially available and were examined for whole-cell bioconversion of NAR 7-gluc. EXG1 was identified in situ as the β-GH relevant for flavonoid glucoside degradation. In yeast, EXG1 and SPR1 catalyze reactions in remodeling of the glucan network of the yeast cell wall, which consist of trimming and branching of the glucan molecule (30). Accordingly, overexpression of EXG1 caused a reduction of β-1,6-glucan in the cell wall (24). EXG1 encodes a constitutive exo-β-glucanase (Exg1p) that is initially secreted into the periplasmic space and then released into the growth medium (43, 48). Considering the extracellular accumulation of the enzyme, Exg1p conceivably metabolizes flavonoid glucosides in the culture broth.
Overall, the data demonstrated that the activity of EXG1 interferes with the biotechnological use of yeast cells to produce or modify flavonoid glucosides. Thus, EXG1 knockout strains are proposed as an option to develop a platform for expression of the pathway to flavonoid glucosides in yeast transformants.
Supplementary Material
Acknowledgments
This work was financially supported by the Deutsche Forschungsgemeinschaft (DFG).
Footnotes
Published ahead of print on 7 January 2011.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Berrin, J.-G., et al. 2002. Functional expression of human liver cytosolic β-glucosidase in Pichia pastoris. Eur. J. Biochem. 269:249-258. [DOI] [PubMed] [Google Scholar]
- 2.Berrin, J.-G., et al. 2003. Substrate (aglycone) specificity of human cytosolic beta-glucosidase. Biochem. J. 373:41-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhat, M. K. 2000. Cellulases and related enzymes in biotechnology. Biotechnol. Adv. 18:355-383. [DOI] [PubMed] [Google Scholar]
- 4.Bhiri, F., S. E. Chaabouni, F. Liman, R. Ghrir, and N. Marzouki. 2008. Purification and biochemical characterization of extracellular β-glucosidases from the hypercellulolytic Pol6 mutant of Penicillium occitanis. Appl. Biochem. Biotechnol. 149:169-182. [DOI] [PubMed] [Google Scholar]
- 5.Bouarab, K., R. Melton, J. Peart, D. Baulcombe, and A. Osbourn. 2002. A saponin-detoxifying enzyme mediates suppression of plant defences. Nature 418:889-892. [DOI] [PubMed] [Google Scholar]
- 6.Bradford, M. M. 1976. A rapid and sensitive method for the quantification of microgram protein quantities utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 7.Britsch, L. 1990. Purification and characterisation of flavone synthase I, a 2-oxoglutarate-dependent desaturase. Arch. Biochem. Biophys. 282:152-160. [DOI] [PubMed] [Google Scholar]
- 8.Colman-Lerner, A., T. E. Chin, and R. Brent. 2001. Yeast Cbk1 and Mob2 activate daughter-specific genetic programs to induce asymmetric cell fates. Cell 107:739-750. [DOI] [PubMed] [Google Scholar]
- 9.Ebel, J., and K. Hahlbrock. 1977. Enzymes of flavone and flavonol-glycoside biosynthesis. Coordinated and selective induction in cell-suspension cultures of Petroselinum hortense. Eur. J. Biochem. 75:201-209. [DOI] [PubMed] [Google Scholar]
- 10.Fowler, Z. L., and M. A. G. Koffas. 2009. Biosynthesis and biotechnological production of flavanones: current state and perspectives. Appl. Microbiol. Biotechnol. 83:799-808. [DOI] [PubMed] [Google Scholar]
- 11.Gargouri, M., I. Smaali, T. Maugard, M. D. Legoy, and N. Marzouki. 2004. Fungus β-glucosidases: immobilization and use in alkyl-β-glycoside synthesis. J. Mol. Catal. B: Enz. 29:89-94. [Google Scholar]
- 12.Gascón, S., N. P. Neumann, and J. O. Lampen. 1968. Comparative study of the properties of the purified internal and external invertases from yeast. J. Biol. Chem. 243:1573-1577. [PubMed] [Google Scholar]
- 13.Gebhardt, Y., et al. 2005. Molecular evolution of flavonoid dioxygenases in the family Apiaceae. Phytochemistry 66:1273-1284. [DOI] [PubMed] [Google Scholar]
- 14.Gebhardt, Y. H., S. Witte, H. Steuber, U. Matern, and S. Martens. 2007. Evolution of flavone synthase I from parsley flavanone 3β-hydroxylase by site-directed mutagenesis. Plant Physiol. 144:1442-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahlbrock, K., K.-H. Knobloch, F. Kreuzaler, J. R. M. Potts, and E. Wellmann. 1976. Coordinated induction and subsequent activity changes of two groups of metabolically interrelated enzymes. Light-induced synthesis of flavonoid glycosides in cell suspension cultures of Petroselinum hortense. Eur. J. Biochem. 61:199-206. [DOI] [PubMed] [Google Scholar]
- 16.Hänsel, R., and O. Sticher. 2007. Pharmakognosie-Phytopharmazie. Springer- Verlag, Berlin, Germany.
- 17.Hempel, J., et al. 1999. Flavonols and flavones of parsley cell suspension culture change the antioxidative capacity of plasma in rats. Nahrung 43:201-204. [DOI] [PubMed] [Google Scholar]
- 18.Henrissat, B., and G. Davies. 1997. Structural and sequence-based classification of glycoside hydrolases. Curr. Opin. Struct. Biol. 7:637-644. [DOI] [PubMed] [Google Scholar]
- 19.Henstrand, J. M., et al. 1992. Light and fungal elicitor induce 3-deoxy-d-arabino-heptulosonate 7-phosphate synthase mRNA in suspension cultured cells of parsley (Petroselinum crispum L.). Plant Physiol. 98:761-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hösel, W., and W. Barz. 1975. β-Glucosidases from Cicer arietinum L. Eur. J. Biochem. 57:607-616. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh, M. C., and T. L. Graham. 2001. Partial purification and characterization of a soybean β-glucosidase with high specific activity towards isoflavone conjugates. Phytochemistry 58:995-1005. [DOI] [PubMed] [Google Scholar]
- 22.Huh, W. K., et al. 2003. Global analysis of protein localization in budding yeast. Nature 425:686-691. [DOI] [PubMed] [Google Scholar]
- 23.Isayenkova, J., V. Wray, M. Nimtz, D. Strack, and T. Vogt. 2006. Cloning and functional characterisation of two regioselective flavonoid glucosyltransferases from Beta vulgaris. Phytochemistry 67:1598-1612. [DOI] [PubMed] [Google Scholar]
- 24.Jiang, B., A. F. Ram, J. Sheraton, F. M. Klis, and H. Bussey. 1995. Regulation of cell wall beta-glucan assembly: PTC1 negatively affects PBS2 action in a pathway that includes modulation of EXG1 transcription. Mol. Gen. Genet. 248:260-269. [DOI] [PubMed] [Google Scholar]
- 25.Klebl, F., and W. Tanner. 1989. Molecular cloning of a cell wall exo-beta-1,3-glucanase from Saccharomyces cerevisiae. J. Bacteriol. 171:6259-6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolewe, M. E., V. Gaurav, and S. C. Roberts. 2008. Pharmaceutically active natural product synthesis and supply via plant cell culture technology. Mol. Pharm. 5:243-256. [DOI] [PubMed] [Google Scholar]
- 27.Kotaka, A., et al. 2008. Direct ethanol production from barley β-glucan by sake yeast displaying Aspergillus oryzae β-glucosidase and endoglucanase. J. Biosci. Bioeng. 105:622-627. [DOI] [PubMed] [Google Scholar]
- 28.Kuo, L.-C., and K.-T. Lee. 2008. Cloning, expression and characterization of two β-glucosidases from isoflavone glycoside-hydrolizing Bacillus subtilis natto J. Agric. Food Chem. 56:119-125. [DOI] [PubMed] [Google Scholar]
- 29.Larriba, G., R. D. Basco, E. Andaluz, and J. P. Luna-Arias. 1993. Yeast exoglucanases. Where redundancy implies necessity. Arch. Med. Res. 24:293-299. [PubMed] [Google Scholar]
- 30.Lesage, G., and H. Bussey. 2006. Cell wall assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 70:317-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu, H., et al. 2009. Absorption and disposition of ginsenosides after oral administration of Panax notoginseng extract to rats. Drug Metab. Dispos. 37:2290-2298. [DOI] [PubMed] [Google Scholar]
- 32.Marotti, I., A. Bonetti, B. Biavati, P. Catizone, and G. Dinelli. 2007. Biotransformation of common bean (Phaseolus vulgaris L.) flavonoid glycosides by Bifidobacterium species from human intestinal origin. J. Agric. Food Chem. 55:3913-3919. [DOI] [PubMed] [Google Scholar]
- 33.Martens, S., and A. Mithöfer. 2005. Flavones and flavone synthases, Phytochemistry 66:2399-2407. [DOI] [PubMed] [Google Scholar]
- 34.Martens, S., G. Forkmann, U. Matern, and R. Lukacin. 2001. Cloning of parsley flavone synthase I. Phytochemistry 58:43-46. [DOI] [PubMed] [Google Scholar]
- 35.Martens, S., et al. 2003. Divergent evolution of flavonoid 2-oxoglutarate-dependent dioxygenases in parsley. FEBS Lett. 544:93-98. [DOI] [PubMed] [Google Scholar]
- 36.Moehs, C. P., P.V. Allen, M. Friedman, and W. R. Belknap. 1997. Cloning and expression of solanidine UDP-glucose glucosyltransferase from potato. Plant J. 11:227-236. [DOI] [PubMed] [Google Scholar]
- 37.Morant, A. V., et al. 2008. β-Glucosidases as detonators of plant chemical defence. Phytochemistry 69:1795-1813. [DOI] [PubMed] [Google Scholar]
- 38.Muthukumar, G., S. H. Suhng, P. T. Magee, R. D. Jewell, and D. A. Primerano. 1993. The Saccharomyces cerevisiae SPR1 gene encodes a sporulation-specific exo-1,3-beta-glucanase which contributes to ascospore thermoresistance. J. Bacteriol. 175:386-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neculai, A. M., D. Ivanov, and M. A. Bernards. 2009. Partial purification and characterization of three ginsenoside-metabolizing beta-glucosidases from Pythium irregulare. Phytochemistry 70:1948-1957. [DOI] [PubMed] [Google Scholar]
- 40.Poppenberger, B., et al. 2006. Heterologous expression of Arabidopsis UDP-glucosyltransferases in Saccharomyces cerevisiae for production of zearalenone-4-O-glucoside. Appl. Environ. Microbiol. 72:4404-4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raimondi, S., et al. 2009. Bioconversion of soy isoflavones daidzin and daidzein by Bifidobacterium strains. Appl. Microbiol. Biotechnol. 81:943-950. [DOI] [PubMed] [Google Scholar]
- 42.Ralston, L., S. Subramanian, M. Matsuno, and O. Yu. 2005. Partial reconstruction of flavonoid and isoflavonoid biosynthesis in yeast using soybean type I and type II chalcone isomerases. Plant Physiol. 137:1375-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramírez, M., L. M. Hernández, and G. Larriba. 1989. A similar protein portion of two exoglucanases secreted by Saccharomyces cerevisiae. Arch. Microbiol. 151:391-398. [DOI] [PubMed] [Google Scholar]
- 44.Riou, C., J.-M. Salmom, M.-J. Vallier, Z. Guenta, and P. Barre. 1998. Purification, characterisation, and substrate specificity of a novel highly glucose-tolerant β-glucosidase from Aspergillus oryzae. Appl. Environ. Mircobiol. 64:3607-3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romani, A., A. Baldi, N. Mulinacci, F. F. Vincieri, and M. Tattini. 1996. Extraction and identification procedures of polyphenolic compounds and carbohydrates in Phillyrea (Phillyrea angustifolia L.) leaves. Chromatographia 42:571-577. [Google Scholar]
- 46.Romero-Pérez, A. I., M. Ibern-Gómez, R. M. Lamuela-Raventós, and M. C. de la Torre-Boronat. 1999. Piceid, the major resveratrol derivative in grape juices. J. Agric. Food Chem. 47:1533-1536. [DOI] [PubMed] [Google Scholar]
- 47.Ruan, C. C., et al. 2009. Biotransformation of ginsenoside Rf to Rh1 by recombinant beta-glucosidase. Molecules 14:2043-2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sánchez, A., J. R. Villanueva, and T. G. Villa. 1982. Saccharomyces cerevisiae secrets 2 exo-β-glucanases. FEBS Lett. 138:209-212. [DOI] [PubMed] [Google Scholar]
- 49.Sánchez-Pérez, R., K. Joergensen, M. S. Motawia, F. Dicenta, and B. L. Moeller. 2009. Tissue and cellular localization of individual β-glycosidases using a substrate-specific sugar reducing assay. Plant J. 60:894-906. [DOI] [PubMed] [Google Scholar]
- 50.Sickmann, A., et al. 2003. The proteome of Saccharomyces cerevisiae mitochondria. Proc. Natl. Acad. Sci. U. S. A. 100:13207-13212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simons, J. F., M. Ebershold, and A. Helenius. 1998. Cell wall 1,6-beta-glucan synthesis in Saccharomyces cerevisiae depends on ER glucosidases I and II, and the molecular chaperone BiP/Kar2p. EMBO J. 17:396-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suzuki, K., T. Yabe, Y. Maruyama, K. Abe, and T. Nakajima. 2001. Characterization of recombinant yeast exo-beta-1,3-glucanase (Exg 1p) expressed in Escherichia coli cells. Biosci. Biotechnol. Biochem. 65:1310-1314. [DOI] [PubMed] [Google Scholar]
- 53.Tahara, S. 2007. A journey of twenty-five years through the ecological biochemistry of flavonoids. Biosci. Biotechnol. Biochem. 71:1387-1404. [DOI] [PubMed] [Google Scholar]
- 54.Tanaka, A., et al. 1999. Steady-state inhibitory kinetic studies on the ligand binding modes of Aspergillus niger glucoamylase. Biosci. Biotechnol. Biochem. 63:1548-1552. [DOI] [PubMed] [Google Scholar]
- 55.Vandenbol, M., et al. 1994. Sequence analysis of a 40.2 kb DNA fragment located near the left telomere of yeast chromosome X. Yeast 10:1657-1662. [DOI] [PubMed] [Google Scholar]
- 56.Ververidis, F., et al. 2007. Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part I. Chemical diversity, impacts on plant biology and human health. Biotechnol. J. 2:1214-1234. [DOI] [PubMed] [Google Scholar]
- 57.Ververidis, F., et al. 2007. Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part II. Reconstruction of multienzyme pathways in plants and microbes. Biotechnol. J. 2:1235-1249. [DOI] [PubMed] [Google Scholar]
- 58.Vetter, J. 2000. Plant cyanogenic glycosides. Toxicon 38:11-36. [DOI] [PubMed] [Google Scholar]
- 59.Werner, S. R., and J. A. Morgan. 2009. Expression of a Dianthus flavonoid glucosyltransferase in Saccharomyces cerevisiae for whole-cell biocatalysis. J. Biotechnol. 142:233-241. [DOI] [PubMed] [Google Scholar]
- 60.Yan, Y., L. Zhen, and M. A. G. Koffas. 2008. High-yield anthocyanin biosynthesis in engineered Escherichia coli. Biotechnol. Bioeng. 100:126-140. [DOI] [PubMed] [Google Scholar]
- 61.Yang, L., Z. S. Ning, C. Z. Shi, Z. Y. Chang, and L. Y. Huan. 2004. Purification and characterization of an isoflavone-conjugates-hydrolyzing β-glucosidase from endophytic bacterium. J. Agric. Food Chem. 52:1940-1944. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.