Abstract
The surfaces of 8 bacterial and 23 archaeal species, including many hyperthermophilic Archaea, could be stained using succinimidyl esters of fluorescent dyes. This allowed us for the first time to analyze the mode of cell wall growth in Archaea by subculturing stained cells. The data obtained show that incorporation of new cell wall material in Archaea follows the pattern observed for Bacteria: in the coccoid species Pyrococcus furiosus incorporation was in the region of septum formation while for the rod-shaped species Methanopyrus kandleri and Methanothermus sociabilis, a diffuse incorporation of cell wall material over the cell length was observed. Cell surface appendages like fimbriae/pili, fibers, or flagella were detectable by fluorescence staining only in a very few cases although their presence was proven by electron microscopy. Our data in addition prove that Alexa Fluor dyes can be used for in situ analyses at temperatures up to 100°C.
The possibilities for in situ analyses of bacterial cells have been dramatically improved in recent times by the development of new instruments and new analytical tools. These developments include light microscopic detection of details on a nanometer scale (11), which today is possible using new instruments/techniques. As one example for the development of new electron microscopic techniques, electron cryotomography may be mentioned here (19); this technique allows the three-dimensional (3D) reconstruction of parts of cells with a resolution down to 3 to 5 nm (14).
The introduction of green fluorescent protein into analytical biology and its consequent further development allowed analysis of proteins/substructures in living cells and culminated in the 2008 Nobel Prize (see, e.g., 17). The ongoing development of existing methods like fluorescent in situ hybridization (FISH) (for a recent review, see reference 1) resulted in many variations, named by at least 39 acronyms (from ACM-FISH to Zoo-FISH) (27). Fluorescent semiconductor nanocrystals, also known as quantum dots (with a size from 2.5 to 10 nm), allow the study of various intracellular processes (16). Fluorescent dyes (like 4′,6′-diamidino-2-phenylindole [DAPI], fluorescein, Texas Red, etc.) and probes derived from them, like labeled antibodies, allow analyses of various processes and cellular targets by fluorescence microscopy (see, e.g., reference 8). Specialized derivatives of such dyes can be covalently coupled to functional groups of proteins and other cellular components.
The possibility of in situ analyses of flagella of the Gram-negative model Bacteria species Escherichia coli and Salmonella enterica serovar Typhimurium was revolutionized by a report of Turner et al. in 2000 (26). It was demonstrated that succinimidyl esters of fluorescent dyes (e.g., Alexa Fluor 488) can be covalently linked to flagella, thereby offering a very convenient way for analysis via fluorescence microscopy. This technique was used by the above-mentioned authors to analyze in situ the swimming behavior of these two species and of Rhodobacter sphaeroides, while a motile Streptococcus strain could not be analyzed since “the staining procedure rendered the latter cells immotile” (26). In addition, the movement of type IV pili during twitching motility of Pseudomonas aeruginosa could be visualized using this method (23).
In the case of Archaea, fluorescent probes for FISH analyses have been of great help in defining the spatial distribution of the microorganisms in their habitat. There are no reports available, however, on the direct labeling of archaeal cells and their cell appendages by succinimidyl esters of fluorescent dyes. Using this method, we observed that cell appendages of Archaea can be stained and thereby analyzed in situ but only in very few cases. On the other hand, we observed that cell surfaces were stained very effectively, allowing us to analyze the distribution of the label after subculturing the (anaerobic and hyperthermophilic) cells. The data we obtained by this approach indicate that the general mode of incorporation of new material into the cell wall does not differ fundamentally between Bacteria and Archaea. To the best of our knowledge, we present here the first report on the mode of cell wall growth in an archaeal organism.
MATERIALS AND METHODS
Microorganisms and growth conditions.
Details for the strains of Bacteria and Archaea, the growth conditions and media used, and the staining conditions are provided in Table S1 in the supplemental material. A short summary of the organisms is given in the following paragraphs; in most cases the type strains of these species were used.
Bacteria.
The following Bacteria species were used (growth temperature and medium): Campylobacter jejuni (37°C in CAB medium), E. coli (37°C in M9 medium), Geobacillus stearothermophilus (60°C in LB medium), Helicobacter pylori (37°C in WCB medium), Klebsiella pneumoniae (37°C in LB medium), Proteus mirabilis (37°C in LB medium), Rhodospirillum rubrum (20°C in RÄH medium), and Thermotoga maritima (85°C in MSH medium).
Archaea.
The following Archaea species were used (growth temperature and medium): Archaeoglobus fulgidus (85°C in MGG medium), Archaeoglobus veneficus (75°C in MGG medium), Haloferax volcanii (42°C in DSMZ 97 medium), Ignicoccus hospitalis (90°C in 0.5× SME medium), Ignicoccus islandicus (90°C in 0.5×SME medium), Ignicoccus pacificus (90°C in 0.5× SME medium), Methanobacterium bryantii (37°C in MS medium), Methanobacterium formicicum (37°C in MS medium), Methanocaldococcus jannaschii (85°C in MGG medium), Methanocaldococcus villosus (80°C in MGG medium), Methanococcus vanniellii (37°C in MGG medium), Methanococcus voltae (37°C in MGG medium), Methanospirillum hungatei (37°C in MS medium), Methanopyrus kandleri (98°C in SME medium), Methanothermobacter marburgensis (65°C in MS medium), Methanothermobacter thermoautotrophicus (65°C in MS medium), Methanothermus fervidus (85°C in MS medium), Methanothermus sociabilis (85°C in MS medium), Pyrobaculum aerophilum (100°C in A medium), Pyrobaculum islandicum (100°C in A medium), Pyrobaculum organotrophum (100°C in A medium), Pyrococcus furiosus (95°C in 0.5× SME medium), Sulfolobus solfataricus (80°C in MM medium), and Thermoplasma volcanium (57°C in TA medium). In addition, eight not yet validly described archaeal isolates and one enrichment culture, containing three different morphotypes of archaea, were analyzed for staining of their cell surface appendages.
Staining of cells with succinimidyl esters of fluorescent dyes.
Staining of cells and cell appendages was performed as described previously by Turner et al. (26) with modifications and recommendations of the suppliers of the dyes (8; see also “Staining procedure” in the supplemental material). The succinimidyl esters of Alexa Fluor dyes were obtained from Invitrogen (Karlsruhe, Germany), Attotec dyes were from Attotec (Siegen, Germany), DyLight dyes were by Thermo Scientific (Bonn, Germany), and Fluo Probes dyes were from Interchim (Montlucon, France). The dyes were delivered in solid form, and aliquots were prepared as described in “Staining procedure” in the supplemental material.
Light and electron microscopic analyses: subculturing experiments.
Stained cells were analyzed by pipetting (using cut pipette tips to avoid shearing) 5-μl cell suspension aliquots onto thoroughly cleaned glass slides that had been covered with 1% agarose. Agarose was solubilized in the same buffered (organic-free) medium in which the stained cells were stored to minimize background fluorescence. Cells were observed using an Olympus BX50 fluorescence microscope using filter set F41-054 for Alexa Fluor 488 dye and filter set F41-003 for Alexa Fluor 532 and 555 dyes. Fluorescence and phase-contrast micrographs were recorded with a 14-bit digital camera (pco1600c; PCO Company, Kelheim, Germany).
For subculturing experiments, all centrifugation steps, including the staining procedure, were performed at room temperature in an anaerobic chamber (Coy Laboratory Products, Inc., Grass Lake, MI). After the final washing steps to remove unbound dye, cells were reinoculated into fresh, organic-containing (complete) medium; aliquots were sampled after various time intervals, and cells were analyzed via fluorescence microscopy and confocal laser scanning microscopy (CLSM). CLSM with an inverse LSM510-Meta instrument (Zeiss, Göttingen, Germany) was used to analyze confocal sections of cells that had been stained after regrowth (for Alexa Fluor 488-stained specimens; excitation wavelength, 488 nm; emission wavelength, 505 nm; long-pass filter.).
Transmission electron microscopy (TEM) and scanning electron microscopy (SEM) were done with samples prepared for fluorescence dye staining at the stage before washed cell samples were transferred into vials containing the fluorescent dye. TEM and SEM analyses were performed as described previously (20, 22).
RESULTS AND DISCUSSION
Cell surface appendages of Archaea possess unique structures and functions (18, 20, 25), which is why we tested whether various species and unpublished new isolates of Archaea can be analyzed by staining with succinimidyl esters of fluorescent dyes. The initial question was whether cell surface appendages could be visualized using, e.g., Alexa Fluor 488. The outcome from such experiments was that the cell surface of nearly any species tested could readily be stained. This proved that the staining procedure itself worked in all experiments, meaning, on the other hand, that a lack of detection of archaeal flagella, pili, or fibers was not due to staining problems. Subculturing of cells after staining and subsequent microscopic analyses enabled us to investigate the mode of cell wall growth in Archaea. In the following, we first present data on the staining procedure and then use the optimized protocol to analyze the mode of cell wall growth in archaea.
Staining of cell surfaces and cell surface appendages.
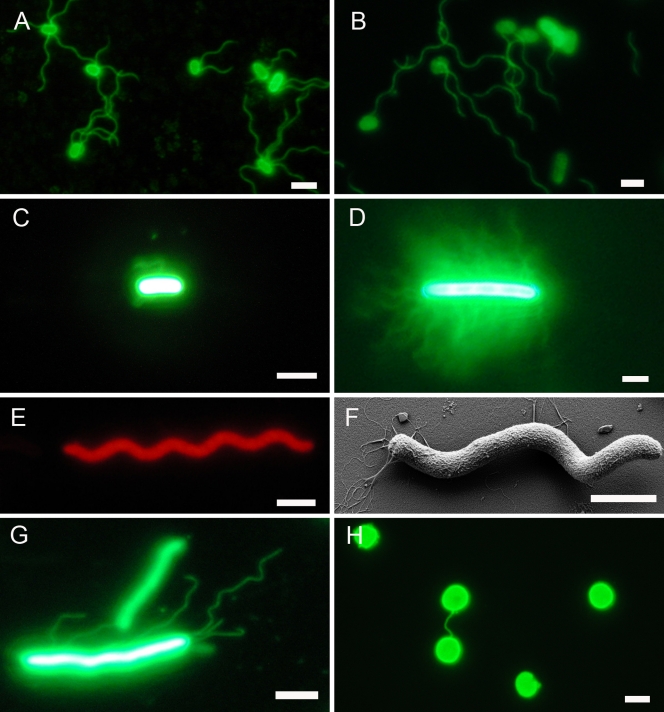
A short summary of the organisms we tested is given in Materials and Methods; details are found in Table S1 in the supplemental material. The staining of cell surfaces was successful in nearly all cases, yielding >40 strains/species with at least one of the fluorescent dyes. Only two out of three Pyrobaculum species tested could not be stained (data not shown). Staining intensity differed for various species but was strong enough in all cases to be detected readily without electronic enhancement. Details for optimization of the staining procedure and results obtained with different fluorescent dyes are given in “Staining results” in the supplemental material. Stained cells could be stored for prolonged times without significant loss of intensity; even after 4 months of (light-protected) storage at −20°C, E. coli cells and their flagella were nicely visible (Fig. 1 A and B).
FIG. 1.
Use of succinimidyl esters of fluorescent dyes to stain cell surfaces and cell surface appendages of Bacteria and Archaea. (A) E. coli stained with Alexa Fluor 488. The micrograph was taken directly after staining. (B) E. coli stained with Alexa Fluor 488. The micrograph was taken from cells stored for 4 months at −20°C after staining. (C) G. stearothermophilus stained with Alexa Fluor 488. (D) P. mirabilis cell from swarming plate stained with Alexa Fluor 488. (E) R. rubrum stained with Alexa Fluor 555. (F) R. rubrum scanning electron micrograph to demonstrate presence of flagella. (G) Methanothermobacter thermoautotrophicus stained with Alexa Fluor 488. (H) Pyrococcus furiosus stained with Alexa Fluor 488. Bar, 1 μm. It has to be emphasized that staining of cell bodies was of more or less equal intensity (to the human eye) in all cases. This means that cell appendages in panels C, D, and G were just detectable to the human eye; these micrographs, therefore, were enhanced electronically to result in good visibility of the cell appendages for reproduction, leading to “overexposure” of cell bodies.
For comparison, the following bacterial species were analyzed for flagellum staining: E. coli was chosen as a positive control, and K. pneumonia was chosen as a nonmotile negative control; C. jejuni and H. pylori represented freshly isolated, clinically important species; P. mirabilis was chosen because of its ability to express hundreds of flagella during swarming on plates; R. rubrum was included because of its ability to swim very rapidly; G. stearothermophilus and T. maritima were chosen to represent thermophilic and hyperthermophilic species, respectively. It turned out that staining intensity of flagella was species dependent, with E. coli yielding by far the strongest signals. Flagella of G. stearothermophilus stained weakly and were just visible to the human eye (as in the “wave” above the cell in Fig. 1C). The multiple flagella of P. mirabilis cells swarming on agar plates produced weaker staining than E. coli flagella, but they were so numerous that single filaments could barely be resolved (Fig. 1D). As one example of cell surface appendages that could not be stained, data for R. rubrum are shown in Fig. 1E and F. Alexa Fluor 555 (and Alexa Fluor 488 [data not shown]) stained the cell body very strongly but not its flagella (Fig. 1E). On the other hand, flagella were clearly present on those cells, as demonstrated by SEM analysis (Fig. 1F) and by our observation of motility via phase-contrast light microscopy (data not shown). Our results that flagella of only three of seven tested bacterial species could be stained by fluorescent dyes are corroborated by data from other groups (26).
We also want to mention here that stained E. coli cells are an illustrative teaching tool to easily demonstrate the rotation of bacterial flagella for swimming. For instance, addition of rich medium to cells stored for a few days at 4°C resulted in restoration of motility, and rotating flagella were visible within a few minutes.
To study whether succinimidyl ester dye staining is applicable for analyzing cell surface appendages and cell walls of Archaea, we tested this method with 25 archaeal species, eight unpublished new archaeal isolates, and one archaeal enrichment culture containing three different morphotypes. In only two cases could single cell surface appendages be stained by the fluorescent dyes. The fimbriae of Methanothermobacter thermoautotrophicus stained with an intensity which was just visible to the human eye; within a single second, intensity faded to an extent that electronic enhancement was necessary to obtain micrographs as given in Fig. 1G. Fimbriae of Methanothermobacter marburgensis, a species very closely related to Methanothermobacter thermoautotrophicus, behaved similarly (data not shown). In the case of Pyrococcus furiosus and Methanocaldococcus villosus, although up to 50 flagella were present on one cell, they were not stained as single structures (Fig. 2); very interestingly, however, cell-cell connections formed by cable-like aggregated flagella (see reference 20 for details) could readily be visualized (Fig. 1H). We want to stress the fact that our data proved the applicability of this type of staining for partially characterized pure cultures and also enrichment cultures of Archaea (see Table S1 in the supplemental material); accordingly, this technique can and will be used by us for the first characterization of such strains and cultures. Many variations of the general staining procedure were tested, of which none, however, resulted in successful staining of individual flagella of the archaeal model organism Pyrococcus furiosus. These variations included use of up to 500 μg of fluorescent dye per staining reaction, staining at the growth temperature of 95°C, staining in the presence of subinhibitory concentrations of detergent (to potentially expose lysine residues not accessible to the dye in untreated flagella), and staining with detergent at 95°C.
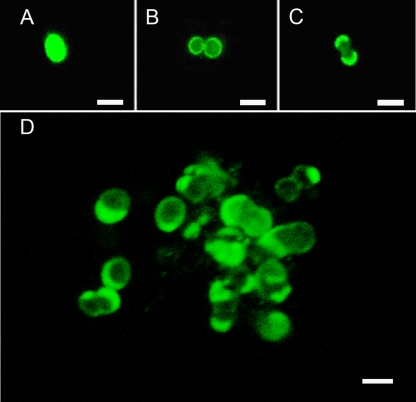
FIG. 2.
Use of succinimidyl esters of fluorescent dyes to study the mode of cell wall growth of the archaea Pyrococcus furiosus. Cells were stained using Alexa Fluor 488 and photographed as follows: directly after retransfer into fresh medium (A), 90 min after subculturing (B), and 180 min after subculturing (C). (D) Small cell aggregate 270 min after subculturing. The image is an overlay of 17 0.2-μm optical sections (LSM510-Meta; Zeiss, Germany). The same series of stacks was used to analyze the aggregate in three dimensions (see Video S1 in the supplemental material). Bar, 1 μm.
In summary, we have found that Alexa Fluor dyes resulted in the best staining, especially at the very high temperatures we used in many cases. Flagella could be stained for three bacterial species but not for four other bacterial species. The same result was obtained regardless of whether staining was performed under aerobic or anaerobic conditions; in addition, we did not observe a difference in staining intensities if laboratory strains of E. coli or a fresh fecal isolate was used (see Table S1 in the supplemental material). In the case of Archaea, single cell surface appendages could be stained only in two cases, namely, Methanothermobacter thermoautotrophicus and Methanothermobacter marburgensis (Fig. 1; see also Table S1) although we had tested a total of 36 species/isolates (the presence of cell appendages had been proven via electron microscopy and/or observation of motility in 32 of these archaeal species/isolates).
This result raises the question of why the ability to stain cell surface appendages is the exception and not the rule. Obviously, this is not due to inadequate staining protocols because—with the exception of two of the three species of the genus Pyrobaculum—cell bodies were nicely stained in all cases.
Analyzing the cell surface growth of Archaea.
Our observation that cell surfaces could easily be stained allowed us to investigate the mode of incorporation of cell wall material in Archaea. This was done by an approach which in principle followed one used earlier for the study of cell wall growth in streptococci (4). In contrast to the original method of reacting cell walls with stained antibodies, we stained cell walls directly with fluorescent dyes and examined the distribution of the fluorescent signal after subculturing of the cells.
Pyrococcus furiosus is an archaeal model organism (20); it was chosen here as a model for archaeal cocci, and cells from mid-log phase were stained under anaerobic conditions. After transfer into fresh medium to a cell density slightly lower than before, stained samples were taken after various time intervals and analyzed by cell counts, fluorescence microscopy, and CLSM. It turned out that growth (as an increase in optical density [OD]) resumed ca. 90 to 120 min after the new incubation started, i.e., after approximately three “generation times” (optimal growth resulted in a doubling time of 37 min). Such a long lag phase is not too surprising if one takes into account that the total time needed for washing steps and labeling was more than 120 min and that cells were kept at room temperature during that time (in the case of E. coli the lag phase was between one and two generation times). The data we obtained clearly show that new cell wall material is incorporated in the archaeal coccus in the region of the septum. This was particularly obvious from the observation of dividing cells, in which the fluorescently labeled “old” cell wall was confined to the outer cell poles; this is shown by the time course from Fig. 2A (freshly labeled cell) to Fig. 2B (labeled cell starting to divide after 90 min of subculturing) to Fig. 2C (dividing cell at 180 min after subculturing). OD measurements indicated that roughly one doubling in cell mass took place for cells sampled for Fig. 2B and C. New cell wall material (synthesized after the subculture start) would not be labeled in this experiment and is clearly present in the region of division (Fig. 2). Since the small cell aggregate shown in Fig. 2D was analyzed by optical sectioning via CLSM, we used the AMIRA software package for a 3D visualization of the aggregate. These data convincingly demonstrate the dye distribution in the region of the old cell wall (see Video S1 in the supplemental material). There, some cells are labeled such that one half is fully stained but not the other; this staining pattern is explained if a cell pair, as stained in Fig. 2C, breaks apart after complete duplication.
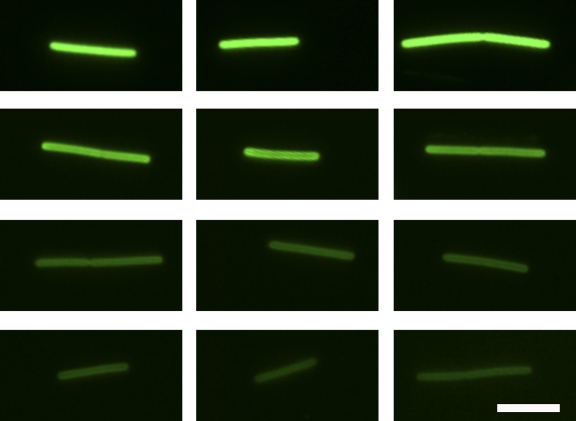
Methanopyrus kandleri was chosen as a model for rod-shaped Archaea because cells were stained brightly; the experimental setup (concerning growth, labeling, and subculturing) was as outlined for Pyrococcus furiosus. The doubling time under our growth conditions was around 50 min, and growth (as an increase in optical density) resumed after ca. 120 min. The cells of Methanopyrus kandleri were labeled over their entire length at equal intensities, and subculturing resulted, in contrast to Pyrococcus furiosus, in “fading” of the label over time (Fig. 3). This fading is not the result of a bleaching effect because cells taken after different incubation times were treated equally, and staining intensity does not diminish over 12 h if nongrowing cells are kept at 100°C (see also the supplemental material). Similar results were obtained for another rod-shaped archaeal organism, namely, Methanothermus sociabilis (data not shown). The main difference from growth of the coccoid Pyrococcus furiosus cells is that new cell wall material is incorporated equally over the whole cell length of these two rod-shaped Archaea. This mode of “diffuse” cell wall growth in the rod-shaped Archaea analyzed here, therefore, resembles the situation in most rod-shaped bacteria (5). We want to emphasize that very similar labeling patterns as those presented in Fig. 2 and 3 have been observed in not only one but at least three different experiments each.
FIG. 3.
Use of succinimidyl esters of fluorescent dyes to study the mode of cell wall growth of the archaea Methanothermus sociabilis. Three cells per time point, stained using Alexa Fluor 488, are shown, all of which were photographed using the identical camera settings (70 ms on a pco1600 14-bit digital camera) on an Olympus BX50 fluorescence microscope. Micrographs were taken directly after staining and retransfer into fresh medium (top row), 120 min after subculturing (second row), 180 min after subculturing (third row), and 240 min after subculturing (bottom row). As explained in the text, the different time points represent roughly one generation each. Bar, 2 μm.
To the best of our knowledge, no previous studies address the question of the spatial pattern of new cell wall material as it is incorporated into archaeal cell walls. Therefore, we show here for the first time on selected species that the general mode of cell wall growth is similar for bacterial and archaeal cocci, namely, incorporation in the septum region; bacterial and archaeal rod-shaped microorganisms, on the other hand, use another general mode of cell wall growth, namely, diffuse incorporation over the cell length. We also are aware of the fact that in other species of Archaea even other modes of cell wall material incorporation might occur; in the case of Bacteria exceptions to these general rules have already been identified (5).
Conclusions.
Cell surface appendages could be stained only in case of the three bacterial species, E. coli, P. mirabilis, and G. stearothermophilus, of eight potentially positive species. In the case of Archaea only the fimbriae of Methanothermobacter thermoautotrophicus and Methanothermobacter marburgensis could be visualized by fluorescence staining although electron microscopy clearly revealed that 23 from a total of the 25 validly described species possessed cell surface appendages, namely, flagella, fimbriae/pili, or fibers. Taken together, our data indicate that this type of fluorescent dye is of limited use for studying cell surface appendages; E. coli, in retrospect, turned out to be a lucky choice to visualize the rotation of flagella as the mechanism used for swimming by fluorescence microscopy. Staining occurs by covalent linkage to free, reactive −NH2 groups, particularly of lysines in proteins. It had been argued previously (26) that in the case of E. coli, only three lysines are present on the outer subdomain of flagellin; the other 11 lysines, therefore, are hidden inside the flagellar structure. The presence of various amounts of lysines might well explain why we observed different staining intensities for different bacterial flagella. In the case of hyperthermophilic Archaea, it even might be argued that their habitat might select against the presence of lysines in proteins (24) and therefore also on the outer surface of cell appendages. Indeed, most species tested by us grew best at very high temperatures; nevertheless, for the five archaeal species growing at 37°C, we could not observe any staining of the cell surface appendages although we had proven their presence by electron microscopy.
Our data demonstrate that fluorescent dyes, on the other hand, are a very powerful tool to stain bacterial and archaeal cell surfaces, allowing, e.g., analysis of the mode of cell wall growth. Only Alexa Fluor fluorescent dyes were found to be stable for at least 12 h at temperatures up to 100°C, allowing extended experimentation with hyperthermophilic organisms.
In the case of Bacteria, the mode of incorporation of cell wall material differs for various morphotypes. In coccoid Bacteria, incorporation is in the septum region (4, 9, 21), as we observed here for the coccoid archaeal organism Pyrococcus furiosus; we are not aware of an exception to this rule in coccoid Bacteria. In rod-shaped Bacteria, the actin homolog MreB forms spiral-shaped cytoskeletal filaments directing peptidoglycan incorporation and therefore is required to form rod-shaped cells. This spatial pattern of MreB results in an overall diffuse pattern of cell wall growth over the cell length (5, 10), as we observed here for the rod-shaped Archaea Methanopyrus kandleri and Methanothermus sociabilis. This mode of cell wall growth seems to be the general one in rod-shaped Bacteria. In the case of Streptomycetes and coryneform Bacteria, however, special modes of cell wall growth have been described (5); it remains open at the moment if such special modes of cell wall growth might occur also in special species of Archaea. Bacterial cell shape and the mode of cell wall growth are known to be interconnected (2, 7, 15) and depend on various cytoskeletal proteins like FtsZ, Mbl, MreBCD, RodZ, DivIVA, and others. Since homologs of the corresponding genes are also present in many archaeal genomes, it is not too surprising that the general spatial pattern of incorporation of cell wall material in Archaea seems not to differ from the general one observed in Bacteria.
On the other hand, cell walls of Bacteria and Archaea are made from completely different components; in Bacteria, cell walls consist of murein, while Archaea use pseudomurein, polysaccharides, glycoprotein S-layers, or other proteins for synthesizing cell walls. Our data allow us to state that new cell wall material (containing free −NH2 groups) in Archaea is incorporated in a general spatial pattern similar to that in Bacteria during growth; we cannot, however, define at the moment which specific component of the cell wall is incorporated in that manner. The outermost cell wall component of Pyrococcus furiosus is a “layer of stained material” in ultrathin sections, which was interpreted as an S-layer (6); its presence has not been directly proven, however, and in the genome no S-layer gene was annotated. The cell wall of Methanopyrus kandleri is composed of pseudomurein, covered by a protein, putatively an S-layer (12); for Methanothermus sociabilis no data of the composition of its outermost cell layer are available (13). Today, it is known that in Bacteria the spatial pattern of murein (composing the primary cell wall) incorporation and that of “accessory cell wall material” like teichoic acid or lipopolysaccharide are in a spatially congruent way dependent on the same cytoskeletal proteins as anchors for the assembly machineries. As mentioned above, the presence of homologs of the bacterial cytoskeletal proteins in Archaea is proven. An MreB homolog, e.g., is present in the rod-shaped M. kandleri but not in the coccoid P. furiosus.
Therefore, it is safe to state that the general mode of cell wall growth in Archaea is similar to the general one in Bacteria. Since “So far no genes of enzymes involved in the cell wall biosynthesis have been unambiguously assigned” in Archaea (3), any statement about which of the above-mentioned cytoskeletal proteins might be involved in the pattern of cell wall material synthesis we observed here would be pure speculation.
Supplementary Material
Acknowledgments
This work was supported by DFG-grant WI 731/10-1 to R.W. and R.R.
R.W. thanks the numerous dedicated students who contributed to this study, particularly N. Donner, M. Frank, L. Heine, G. Kunz, D. Näther, S. Schmid, and C. Thoma. The expert technical assistance of Elke Papst and Sylvia Dobler is gratefully acknowledged.
Footnotes
Published ahead of print on 17 December 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Amann, R., and B. M. Fuchs. 2008. Single-cell identification in microbial communities by improved fluorescence in situ hybridization techniques. Nat. Rev. Microbiol. 6:339-348. [DOI] [PubMed] [Google Scholar]
- 2.Carballido-Lopez, R., and J. Errington. 2003. The bacterial cytoskeleton: in vivo dynamics of the actin-like protein Mbl of Bacillus subtilis. Dev. Cell 4:19-28. [DOI] [PubMed] [Google Scholar]
- 3.Claus, H., and H. König. 2010. Cell envelopes of methanogens, p. 231-251. In H. König, H. Claus, and A. Varma (ed.), Prokaryotic cell wall compounds. Springer-Verlag, Berlin, Germany.
- 4.Cole, R. M., and J. J. Hahn. 1962. Cell wall replication in Streptococcus pyogenes. Science 135:722-724. [DOI] [PubMed] [Google Scholar]
- 5.Daniel, R. A., and J. Errington. 2003. Control of cell morphogenesis in bacteria: two distinct ways to make a rod-shaped cell. Cell 113:767-776. [DOI] [PubMed] [Google Scholar]
- 6.Fiala, G., and K. O. Stetter. 1986. Pyrococcus furiosus sp. nov. represents a novel genus of marine heterotrophic Archaebacteria growing optimally at 100°C. Arch. Microbiol. 146:56-61. [Google Scholar]
- 7.Goehring, N. W., and J. Beckwith. 2005. Diverse paths to midcell: assembly of the bacterial cell division machinery. Curr. Biol. 15:R514-R526. [DOI] [PubMed] [Google Scholar]
- 8.Haugland, R. P. 2005. The handbook: a guide to fluorescent probes and labeling techniques. Invitrogen Corp., Carlsbad, CA.
- 9.Higgins, M. L., and G. D. Shockman. 1970. Model for cell wall growth of Streptococcus faecalis. J. Bacteriol. 101:643-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones, L. J., R. Carballido-Lopez, and J. Errington. 2001. Control of cell shape in bacteria: helical, actin-like filaments in Bacillus subtilis. Cell 104:913-922. [DOI] [PubMed] [Google Scholar]
- 11.Keller, P., et al. 2009. Magnifying power. Nature 459:629. [DOI] [PubMed] [Google Scholar]
- 12.Kurr, M., et al. 1991. Methanopyrus kandleri, gen. and sp. nov. represents a novel group of hyperthermophilic methanogens, growing at 110° C. Arch. Microbiol. 156:239-247. [Google Scholar]
- 13.Lauerer, G., J. K. Kristjansson, T. A. Langworthy, H. König, and K. O. Stetter K. 1986. Methanothermus sociabilis sp. nov., a second species within the Methanothermaceae growing at 97°C. Syst. Appl. Microbiol. 8:100-105. [Google Scholar]
- 14.Liu, J., et al. 2009. Intact flagellar motor of Borrelia burgdorferi revealed by cryo-electron tomography: evidence for stator ring curvature and rotor/C-ring assembly flexion. J. Bacteriol. 191:5026-5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Margolin, W. 2009. Sculpting the bacterial cell. Curr. Biol. 19:R812-R822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michalet, X., et al. 2005. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 307:538-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyawaki, A. 2008. Green fluorescent protein glows gold. Cell 135:987-990. [DOI] [PubMed] [Google Scholar]
- 18.Müller, D., et al. 2009. The Iho670 fibers of Ignicoccus hospitalis: a new type of archaeal cell surface appendage. J. Bacteriol. 191:6465-6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy, G. E., and G. J. Jensen. 2007. Electron cryotomography. Biotechniques 43:413-423. [DOI] [PubMed] [Google Scholar]
- 20.Näther, D. J., R. Rachel, G. Wanner, and R. Wirth. 2006. Flagella of Pyrococcus furiosus: multifunctional organelles, made for swimming, adhesion to various surfaces, and cell-cell contacts. J. Bacteriol. 188:6915-6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinho, M. G., and J. Errington. 2003. Dispersed mode of Staphylococcus aureus cell wall synthesis in the absence of the division machinery. Mol. Microbiol. 50:871-881. [DOI] [PubMed] [Google Scholar]
- 22.Rachel, R., et al. 2010. Analysis of the ultrastructure of Archaea by electron microscopy. Methods Cell Biol. 96:47-69. [DOI] [PubMed] [Google Scholar]
- 23.Skerker, J. M., and H. C. Berg. 2001. Direct observation of extension and retraction of type IV pili. Proc. Natl. Acad. Sci. U. S. A. 98:6901-6904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szilagyi, A., and P. Zavodsky. 2000. Structural differences between mesophilic, moderately thermophilic and extremely thermophilic protein subunits: results of a comprehensive survey. Structure 8:493-504. [DOI] [PubMed] [Google Scholar]
- 25.Thoma, C., et al. 2008. Fimbriae of Methanothermobacter thermoautotrophicus are encoded by mth60: first characterization of an archaeal fimbrium. Environ. Microbiol. 10:2785-2795. [DOI] [PubMed] [Google Scholar]
- 26.Turner, L., W. S. Ryu, and H. C. Berg. 2000. Real-time imaging of fluorescent flagellar filaments. J. Bacteriol. 182:2793-2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volpi, E. V., and J. M. Bridger. 2008. FISH glossary: an overview of the fluorescence in situ hybridization technique. Biotechniques 45:385-409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.