Abstract
In the current study, we adapted and optimized the lambda Red recombineering strategy to genetically manipulate the fastidious insect endosymbiont Sodalis glossinidius. This work greatly facilitates the application of genetics to the study of insect symbionts and should also prove useful in the context of long-awaited paratransgenic insect control strategies.
The lifestyle switch from facultative to obligate host association is often accompanied by a process of bacterial genome degeneration and size reduction. Whereas this process has a streamlining effect, eliminating genes that are no longer required for the symbiotic lifestyle, it also drives a reduction in metabolic plasticity, yielding fastidious bacteria that have proven to be difficult to manipulate in the laboratory due to their low growth rate, high level of susceptibility to contamination, and complex nutritional requirements (12). To date, only a few studies have utilized genetic techniques to explore the nature of host-symbiont interactions (4, 5, 14), and the implementation of these techniques has proved arduous and unreliable over the long term. In the current study, we report on the adaptation and optimization of the lambda Red recombineering strategy (6) for the genetic manipulation of the tsetse fly endosymbiont Sodalis glossinidius (Fig. 1).
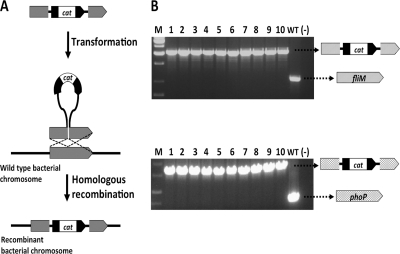
FIG. 1.
(A) Schematic representation of lambda Red-mediated chromosomal insertion. A linear construct containing a drug-resistant marker (e.g., the chloramphenicol resistance gene, cat) is transformed into hyper-recombinogenic cells. The plasmid-encoded lambda Red functions mediate the recombination of the construct into the homologous region of the bacterial chromosome, generating cells with recombinant chromosomes. (B) PCR analysis of recombinant S. glossinidius clones using primers that flank the putative insertion point of the constructs. PCR analysis carried out with chloramphenicol-resistant clones (1 to 10) and a wild-type (WT) strain of S. glossinidius using primers for the flagellar structural gene fliM (locus tag SG0046) (top) and the response regulator phoP (locus tag SG1082) (bottom). While the WT strain PCR product has a low molecular weight, the recombinant clones have high-molecular-weight bands resulting from the insertion of the cat cassette. Molecular weight markers (M) and a negative control (−) are also shown.
A plasmid harboring lambda Red functions under the control of the PBAD arabinose-inducible promoter (pKD46) (6) was transformed into S. glossinidius using heat shock (7). Sodalis glossinidius is a fastidious bacterium that grows optimally in rich medium formulations containing glucose or N-acetyl-d-glucosamine (NAG) as a carbon source. Since S. glossinidius cannot use arabinose as a carbon source (3) and glucose and NAG can potentially interfere with the expression of genes under the regulation of the PBAD promoter through catabolite repression (8), we examined the expression of the bet lambda Red gene by quantitative PCR (4) in the presence of different sugars and 3′-5′-cyclic AMP (cAMP). As expected, we found that the expression of the bet gene is catabolite repressed by glucose and NAG but that catabolite repression can be overcome by the addition of cAMP (Fig. 2). Indeed, we were unable to obtain any recombinants following lambda Red recombineering without the addition of cAMP.
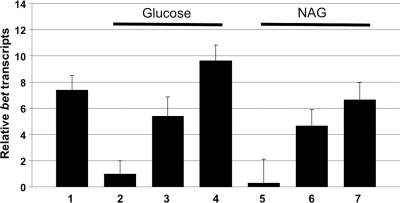
FIG. 2.
Regulation of the arabinose-inducible promoter PBAD in S. glossinidius. Relative bet transcripts were measured after S. glossinidius cells were incubated for 8 h in MM medium with 0.25% arabinose and no glucose (bar 1), MM medium plus 0.25% glucose (bar 2), MM medium plus 0.25% glucose plus 0.25% arabinose (bar 3), MM medium plus 0.25% glucose plus 0.25% arabinose plus 5 mM cAMP (bar 4), MM medium plus 0.25% N-acetyl-d-glucosamine (NAG) (bar 5), MM medium plus 0.25% NAG plus 0.25% arabinose (bar 6), and MM medium plus 0.25% NAG plus 0.25% arabinose plus 5 mM cAMP (bar 7). The observed changes in relative levels of bet transcripts in the presence of catabolites (glucose and NAG) and cAMP are consistent with catabolite repression of the PBAD promoter. Quantitative PCR measurements were performed in triplicate; error bars represent standard deviations.
In comparison to model organisms such as Escherichia coli, S. glossinidius divides very slowly under standard culture conditions. This is largely due to the fact that S. glossinidius cultures are maintained under anaerobic or microaerophilic conditions because the bacterium fails to grow on agar plates under atmospheric levels of oxygen (3). However, in a recent study, we found that S. glossinidius has a quorum-sensing system that regulates a large number of genes involved in the bacterial response to oxidative stress (11). Subsequently, we found that it was possible to significantly increase the growth rate of S. glossinidius by placing cultures in a shaking incubator once they had reached a cell density sufficient to ensure activation of a robust oxidative-stress response (optical density at 600 nm [OD600] ≈ 0.03). With shaking, cultures reached an OD600 of ∼0.9 in 2 days, whereas cultures maintained at rest reached an OD600 of only ∼0.14 in 6 days (Fig. 3). Since the efficiency of lambda Red-mediated recombination is expected to increase as a function of DNA replication rate (13), we elected to perform all subsequent experiments with cells derived from shaking cultures.
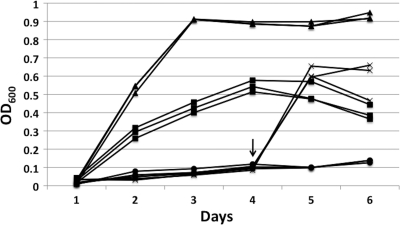
FIG. 3.
Growth dynamics of S. glossinidius. Cultures were maintained at 25°C at rest (filled circles), in an 80-rpm shaking incubator (filled squares), in a 200-rpm shaking incubator (filled triangles), or at rest followed by transfer into a 200-rpm shaking incubator at day 4 (indicated by the arrow) (multiplication signs). For each condition, the figure depicts growth curves obtained from three independent experiments.
Because overexpression of the lambda Red genes can be mutagenic (9), we first determined the minimal induction time needed to generate hyper-recombinogenic S. glossinidius cells. Cultures of S. glossinidius harboring pKD46 were grown with shaking to an OD600 of ∼0.5. These cultures were then induced for 0, 0.5, 1, 3, and 6 h in Mitsuhashi and Maramorosch medium (MM medium) (3) supplemented with 0.5% (wt/vol) arabinose and 5 mM cAMP. Following induction, cells were made chemically competent and transformed (7) with 2 μg of a fliM::cat allele containing 1 kbp of homology in the target sequence flanking the genetic marker. We found that 0.5 h of induction was sufficient to obtain hyper-recombinogenic S. glossinidius cells (as determined by the number of recombinants recovered at each time point [data not shown]). Because of the known mutagenic activity of lambda Red (9), we tested for the emergence of rifampin-resistant bacteria following lambda Red induction. We were unable to recover rifampin-resistant clones at 0.5 h of induction, after plating ∼1 × 109 CFU, indicating that the transient expression of the lambda Red genes did not significantly increase the mutation rate.
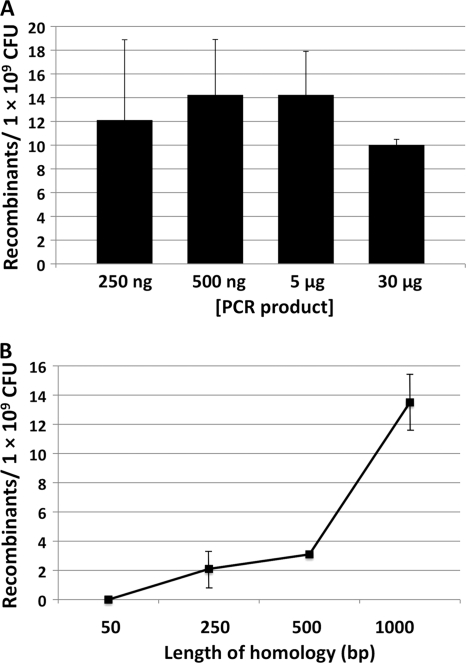
In E. coli, the rate of homologous recombination increases in accordance with DNA concentration and the length of target sequence homology (10). In S. glossinidius, equivalent numbers of recombinants were obtained when cells were transformed with 250 ng or 30 μg of a replacement allele, indicating that DNA concentration was not a limiting factor in recombination efficiency (Fig. 4 A). However, the length of target sequence homology affected the number of recombinants obtained following transformation (Fig. 4B). As expected, longer flanking sequences yielded more recombinants, but constructs with regions of target sequence homology of >1 kbp may prove difficult to amplify using standard PCR techniques.
FIG. 4.
Effects of DNA concentration (A) and length of target sequence homology (B) on the frequency of lambda Red-mediated recombination in S. glossinidius. (A) Cultures of S. glossinidius were transformed with different amounts of a phoP::cat replacement allele that maintains 1,000 bp of target sequence homology flanking the genetic marker. (B) Cultures of S. glossinidius were transformed with fliM::cat replacement alleles maintaining different lengths of target sequence homology flanking the genetic marker. The frequency of recombination was found to increase in proportion to the length of target sequence homology. Transformations were carried out using 2 μg of each replacement allele. All optimization experiments were performed in triplicate; error bars represent standard deviations.
In E. coli and related enteric bacteria, the lambda Red functions are often maintained on plasmids that have a temperature-sensitive origin of replication (6, 15). After the desired genetic modifications have been engineered, the plasmid is cured by growing cells in the absence of plasmid selection at the nonpermissive temperature (i.e., 42°C). Because S. glossinidius replicates at a lower temperature (3), curing was achieved by simply growing cells at 25°C (with shaking) in the absence of plasmid selection. After only five passages, over 98% of cells were ampicillin sensitive, indicating the loss of pKD46 (data not shown).
The lambda Red genetic modification strategy mediates the integration of foreign DNA into the bacterial chromosome via homologous recombination, and it can be used to engineer a wide range of genetic modifications, including chromosomal insertions, duplications and inversions (15). This technique should prove useful in functional studies of cultured insect endosymbionts and in the development of a paratransgenic tsetse control strategy in which the symbiont S. glossinidius is used as a platform to express transgenes that reduce or eliminate the capability of the tsetse fly host to transmit parasitic trypanosomes (1, 2). The optimizations reported in this study may also prove useful in the application of the lambda Red recombineering strategy to other fastidious organisms.
Acknowledgments
We thank Serap Aksoy (Yale University) and Kelly Hughes (University of Utah) for the provision of bacterial strains.
This research was supported by National Science Foundation grant EF-0523818 (to C.D.). M.H.P. was supported by the Stringfellow Award at the University of Utah.
Footnotes
Published ahead of print on 7 January 2011.
REFERENCES
- 1.Aksoy, S., I. Maudlin, C. Dale, A. S. Robinson, and S. L. O'Neill. 2001. Prospects for control of African trypanosomiasis by tsetse vector manipulation. Trends Parasitol. 17:29-35. [DOI] [PubMed] [Google Scholar]
- 2.Aksoy, S., B. Weiss, and G. Attardo. 2008. Paratransgenesis applied for control of tsetse transmitted sleeping sickness. Adv. Exp. Med. Biol. 627:35-48. [DOI] [PubMed] [Google Scholar]
- 3.Dale, C., and I. Maudlin. 1999. Sodalis gen. nov. and Sodalis glossinidius sp. nov., a microaerophilic secondary endosymbiont of the tsetse fly Glossina morsitans morsitans. Int. J. Syst. Bacteriol. 49:267-275. [DOI] [PubMed] [Google Scholar]
- 4.Dale, C., T. Jones, and M. Pontes. 2005. Degenerative evolution and functional diversification of type-III secretion systems in the insect endosymbiont Sodalis glossinidius. Mol. Biol. Evol. 22:758-766. [DOI] [PubMed] [Google Scholar]
- 5.Dale, C., S. A. Young, D. T. Haydon, and S. C. Welburn. 2001. The insect endosymbiont Sodalis glossinidius utilizes a type III secretion system for cell invasion. Proc. Natl. Acad. Sci. U. S. A. 98:1883-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inoue, H., H. Nojima, and H. Okayama. 1990. High efficiency transformation of Escherichia coli with plasmids. Gene 96:23-28. [DOI] [PubMed] [Google Scholar]
- 8.Miyada, C. G., L. Stoltzfus, and G. Wilcox. 1984. Regulation of the araC gene of Escherichia coli: catabolite repression, autoregulation, and effect on araBAD expression. Proc. Natl. Acad. Sci. U. S. A. 81:4120-4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy, K. C., and K. G. Campellone. 2003. Lambda Red-mediated recombinogenic engineering of enterohemorrhagic and enteropathogenic E. coli. BMC Mol. Biol. 4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy, K. C., K. G. Campellone, and A. R. Poteete. 2000. PCR-mediated gene replacement in Escherichia coli. Gene 246:321-330. [DOI] [PubMed] [Google Scholar]
- 11.Pontes, M. H., et al. 2008. Quorum sensing primes the oxidative stress response in the insect endosymbiont, Sodalis glossinidius. PLoS One 3:e3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pontes, M. H., and C. Dale. 2006. Culture and manipulation of insect facultative symbionts. Trends Microbiol. 14:406-412. [DOI] [PubMed] [Google Scholar]
- 13.Poteete, A. R. 2008. Involvement of DNA replication in phage lambda Red-mediated homologous recombination. Mol. Microbiol. 68:66-74. [DOI] [PubMed] [Google Scholar]
- 14.Runyen-Janecky, L. J., A. N. Brown, B. Ott, H. G. Tujuba, and R. V. Rio. 2010. Regulation of high-affinity iron acquisition homologues in the tsetse fly symbiont Sodalis glossinidius. J. Bacteriol. 192:3780-3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sawitzke, J. A., et al. 2007. Recombineering: in vivo genetic engineering in E. coli, S. enterica, and beyond. Methods Enzymol. 421:171-199. [DOI] [PubMed] [Google Scholar]