Abstract
Healthy pigs are an important reservoir for the emerging human pathogen Arcobacter which can result in contamination of porcine carcasses and pork and the spread of arcobacters into the environment. Up to now, the excretion of arcobacters by pigs has been studied, but information about the transmission routes in fattening pigs is lacking. The present study aimed to elucidate the Arcobacter population dynamics in pigs during the fattening period on four farrow-to-finish farms. On each farm, 30 clinically healthy, 12-week-old piglets were selected. Fecal samples were collected on 10 sampling occasions until a slaughter age of 30 weeks was reached. Arcobacter spp. were isolated by a selective method and identified by multiplex PCR. The genetic diversity was examined by amplified fragment length polymorphism and enterobacterial repetitive intergenic consensus PCR. The Arcobacter presence in the fecal samples on the four farms ranged from 11.3 to 50.0%, with excretion levels of up to 104 CFU/g feces. The ratio in which Arcobacter species were isolated varied between the farms and over time. Characterization revealed a high degree of genotypic diversity among the isolates. Arcobacter strains persisted and spread within the finishing unit during the fattening period. The occurrence of both unique and shared genotypes in pigs in adjacent and nonadjacent pens demonstrates that transmission routes other than fecal-oral transmission occur.
In the late 1970s, the first isolation of aerotolerant campylobacters from aborted porcine fetuses was reported (10, 29). These organisms, for which the genus Arcobacter was created in 1991, are closely related to campylobacters but are able to grow in air and at temperatures of below 30°C (40). At present, nine species are characterized, of which five are animal related (3, 21, 27). In humans, Arcobacter cryaerophilus and Arcobacter skirrowii have been isolated from diarrheal stool samples (26, 35, 52), but predominantly Arcobacter butzleri has been associated with enteritis and septicemia (19). Moreover, A. butzleri was the fourth most common Campylobacteraceae species isolated in both a Belgian survey and a French survey (32, 42).
Contaminated drinking water has been identified as a major source of infection in developing countries (39). In industrialized countries, human infection is assumed to be food borne and probably occurs through the manipulation, consumption, or cross-contamination of raw and undercooked meat products. Close contact with pets and person-to-person contact are other potential risk factors for human infection (11, 20, 41). Arcobacters are commonly present in food of animal origin, with the highest prevalence reported for poultry, pork, and beef (7, 25, 34, 37, 46, 47). The origin of the contamination in poultry products is still debated, but fecal material is regarded as the main source of pork and beef carcass contamination (47).
In the past decade, A. butzleri, A. cryaerophilus, and A. skirrowii have been isolated from the feces of healthy pigs at slaughter age (14, 15, 23, 24, 43). However, they have also been implicated in reproduction disorders as late-term abortions and a higher rate of stillbirths and have been isolated from internal organs of aborted pig fetuses and placental, uterine, and oviductal tissues (5, 10, 36). They are present in vaginal swabs of normal producing sows and were isolated from preputial fluid of boars on farms with reproductive problems, though they have not been detected in the semen (6, 24). Nevertheless, insemination with experimentally infected semen seemed to induce a decrease in conception rates in sows.
Hitherto, intrauterine and horizontal transmission between sows and their piglets up to 3 weeks old has been studied (15), but there is no information about the transmission in pigs during the fattening period. Elucidation of the infection sources and transmission of arcobacters in fattening pigs is essential to develop intervention strategies for reducing Arcobacter infection at slaughter age and eventually to prevent carcass and meat contamination during slaughter. Hence, a longitudinal study on four Belgian farrow-to-finish farms was undertaken to explore the Arcobacter epidemiology within fattening pigs from 12 to 30 weeks old.
MATERIALS AND METHODS
Study setup.
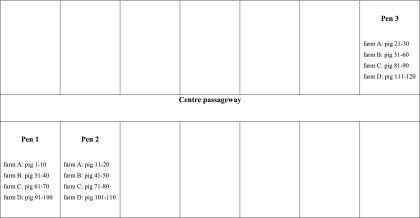
Four unrelated farrow-to-finish closed farms (farms A, B, C, and D) situated in the northern part of Belgium were randomly selected to participate in the present study. Details on farm management are shown in Table 1. Animals were accommodated with an average of 12 piglets per pen, and per farm 30 piglets of 12 weeks of age (both neutered male and female) were randomly selected. Ten piglets were randomly chosen in a first pen (pen 1), 10 in an adjacent second pen (pen 2), and another 10 in a nonadjacent third pen (pen 3) on the other side of the central passageway in the opposite corner of the pigsty (Fig. 1). Direct contact between animals was possible only in the adjacent pens 1 and 2, through the partially opened side wall. Fecal samples from the individually ear-tagged pigs (pigs 1 to 120) were collected rectally every 2 weeks using sterile gloves, starting at 12 weeks of age (around 20 kg) until the slaughter age of approximately 30 weeks (around 110 to 115 kg). Samples were transported under cooled conditions to the laboratory and were always processed within 4 h. Samplings took place from February until June (farms A and B), from April until August (farm C), and from May until September 2007 (farm D). Each animal and its fecal samples were represented by an unique code: farm (A to D)/sampling occasion (1 to 10)/pen (1 to 3)/pig number (1 to 120). In total, 1,100 fecal samples were collected on the four farms during the sampling period, with 282, 256, 266, and 296 samples taken on farms A, B, C, and D, respectively. The difference in the number of samples is due to occasional absence of fecal material in the rectum, death of certain animals, or selling of pigs. Doxycycline (doxycycline hyclate; 500 mg/g) was incorporated in the drinking water for 5 days, preventively between sampling points 2 and 3 on farm B and curatively (for cough) between sampling occasions 4 and 5 and sampling occasions 6 and 7 on farms A and C, respectively.
TABLE 1.
Farm data
| Parameter | Farm A | Farm B | Farm C | Farm D |
|---|---|---|---|---|
| Breed | Hypor sow × Piétrain boar | French hybrid sow × Piétrain boar | Topics 20 sow × Piétrain boar | Topics 20 sow × Piétrain boar |
| No. of sows | 160 | 180 | 700 | 700 |
| No. of piglets (0-12 wk old) | 680 | 470 | 1,890 | 3,000 |
| No. of fattening pigs (12 wk old to slaughter) | 900 | 1,200 | 6,500 | 5,000 |
| Visible presence of rodents in pigsty | No | Yes | No | No |
| Possibility of presence of dogs or cats in pigsty | Yes | No | No | No |
| Presence of wet slatted floors in pigsty | No | Yes | No | No |
| Practice of tail biting | No | Yes | No | No |
| Application of drinking water medication | Yes | Yes | Yes | No |
| Ventilation system in finishing unit | Ceiling ventilation | Natural ventilation | Mechanical flap ventilation | Channel ventilation |
| Type of feed in finishing unit | Meal | Meal | Meal | Meal |
FIG. 1.
Floor plan of the finishing unit on the farms.
The statistical analysis of the differences in Arcobacter excretion within the sampling occasions on each farm and between the farms was performed with the chi-square test.
Arcobacter isolation, identification, and characterization.
For the selective isolation of arcobacters, 5 g feces was homogenized in 45 ml Arcobacter selective isolation broth (containing 24 g liter−1 Arcobacter broth [CM 965; Oxoid, Basingstoke, United Kingdom], 100 mg liter−1 5-fluorouracil [F6627; Sigma, St. Louis, MO], 100 mg liter−1 cycloheximide [C7698; Sigma], 10 mg liter−1 amphotericin B [A4888; Sigma], 16 mg liter−1 cefoperazone [C4292; Sigma], 32 mg liter−1 novobiocin [N1628; Sigma], 64 mg liter−1 trimethoprim [T0667; Sigma], and 50 ml liter−1 lysed defibrinated horse blood [E&O Laboratories Ltd., Bonnybridge, Scotland]) (43) using a stomacher blender (IUL Instruments, Barcelona, Spain) for 1 min at normal speed. For the quantitative analysis, 100 μl of each homogenate was inoculated onto an Arcobacter selective isolation agar plate (containing 24 g liter−1 Arcobacter broth, 12 g liter−1 Agar Technical No. 3 [LP0013; Oxoid], and the selective supplements described above) (43) using the spiral plating technique (Eddy Jet; IUL Instruments). Both plates and homogenates were incubated for 48 h at 28°C under microaerobic conditions by evacuating 80% of the normal atmosphere and introducing a gas mixture of 8% CO2, 8% H2, and 84% N2 into a jar. Following incubation, the plates were checked for typical bluish colonies using Henry transillumination and counted. In addition, 100 μl of each incubated homogenate was streaked onto an Arcobacter selective agar plate and incubated under the same conditions as described above. A maximum of 10 colonies were randomly picked from the plates used for counting, and 1 colony was picked from the plates after selective enrichment. The isolates were subcultured once on blood agar plates (containing 24 g liter−1 Arcobacter broth, 12 g liter−1 Agar Technical No. 3, and 50 ml liter−1 defibrinated horse blood) and stored in 500 μl defibrinated horse blood at −80°C until further identification and characterization. Template DNA was extracted by suspending the bacterial cells in 0.5 ml RS buffer (1,753 g NaCl and 744 mg EDTA adjusted with sterile water to a final volume of 200 ml [pH 8.0]). The suspensions were centrifuged for 2 min at 17,400 × g (Eppendorf 5417-R centrifuge; Eppendorf, Hamburg, Germany) to pellet the cells, and the supernatant was discarded. The pellets were then resuspended in 100 μl Tris-EDTA buffer, and genomic DNA was extracted by the guanidium thiocyanate method (31). The concentration of each DNA template was determined spectrophotometrically (Bio Photometer; Eppendorf) at 260 nm and adjusted to about 50 ng μl−1. Two microliters was used in the Arcobacter species-specific multiplex PCR (m-PCR) assay described by Douidah et al. (9). Isolates for which no species-specific band in the multiplex PCR was generated were further examined using the Arcobacter genus-specific PCR described by Harmon and Wesley (13).
Subsequently, the isolates were characterized at the strain level by two typing methods. For amplified fragment length polymorphism (AFLP) analysis, the protocol described by Debruyne et al. (4) was used. DNA integrity was controlled by 1.5% agarose-Tris-borate-EDTA (TBE) gel electrophoresis for 30 min at 70 V. One microgram of chromosomal DNA was simultaneously digested with the restriction enzymes HindIII and HhaI (New England BioLabs, Ipswich, MA). Restriction site-specific HindIII and HhaI adapters were ligated to the restriction fragments, after which a selective PCR with a fluorescence-labeled (6-carboxyfluorescein [FAM]) HindIII primer (5′-GACTGCGTACCAGCTT-3′) and a HhaI primer (with an additional 3′A nucleotide, 5′-GATGAGTCCTGATCGCA-3′) was carried out. One microliter of the final product was mixed with 8.6 μl of deionized formamide and 0.4 μl of the internal lane standard (Gene Scan-600 LIZ size standard; Applied Biosystems) and analyzed by means of capillary electrophoresis using an ABI 3130xl Genetic Analyzer (Applied Biosystems). AFLP profiles were collected with the data collection software 3.0 (Applied Biosystems). Computer-based normalization and interpolation of the DNA profiles and numerical analysis using the Pearson product moment correlation coefficient (1% position tolerance) were performed using the BioNumerics v. 4.61 software package (Applied Maths, Sint-Martens-Latem, Belgium). The correlation level was expressed as a percent similarity. Dendrograms were constructed using the unweighted-pair group method using average linkages (UPGMA).
For enterobacterial repetitive intergenic consensus PCR (ERIC-PCR) (17), 2 μl DNA (about 50 ng μl−1) was added to a 48-μl PCR mixture containing the ERIC primers 1R (5′-ATGTAAGCTCCTGGGGATTCAC-3′) and R2 (5′-AAGTAAGTGACTGGGGTGAGCG-3′) (48) at a concentration of 25 pmol each. The PCR products were size separated by electrophoresis of 10 μl of the reaction volume in 2% agarose-TBE gels for 2 h at 100 V. For interpretation of the fingerprints, the GelCompare 4.2 software package (Applied Maths) was used. Computer-based normalization and interpolation of the DNA profiles and numerical analysis using the Pearson product moment correlation coefficient, with 1% position tolerance, were performed. For convenience, the correlation level was expressed as a percent similarity. Dendrograms were constructed using UPGMA. DNA patterns that differed by one or more DNA fragments were considered different genotypes (2, 18, 44, 46).
RESULTS
Farm level.
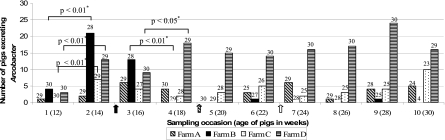
Arcobacters were present on all four farms. In total, 92 of the 120 pigs excreted arcobacters at least once during their lifetimes (19, 23, 21, and 29 animals on farms A, B, C, and D, respectively). None of the pigs excreted arcobacters throughout the whole study period of 18 weeks. Fifty-one pigs shed arcobacters only once during the finishing period. Of the 41 pigs shedding arcobacters more than once, 24 excreted arcobacters during consecutive sampling occasions, while another 17 shed arcobacters during nonconsecutive samplings in the study period. Significant variations in the number of the excreting pigs were observed between the farms (P < 0.01) and on certain sampling occasions (P < 0.05) on farms B, C, and D (Fig. 2).
FIG. 2.
Number of pigs shedding Arcobacter in the feces as a function of the age of the animals on the four farms. Arrows indicate the application of doxycyclin on farms A, B, and C. Numbers above bars indicate the number of fecal samples collected per sampling occasion. *, significant variation in Arcobacter excretion between consecutive sampling occasions.
In total, 52 pigs excreted arcobacters at levels of up to 104 CFU/g feces and 40 pigs shed Arcobacter at levels lower than 102 CFU/g feces. The Arcobacter counts in subsequent samples collected per animal showed a fluctuating pattern.
During the sampling period, arcobacters were not isolated from the gastrointestinal contents of eight rats present in the finishing unit of farm B (data not shown).
At three farms (A, B, and C), doxycycline hyclate was incorporated in the drinking water for 5 days during the course of the finishing period (Fig. 2). On the sampling occasion following the use of the antimicrobial agent, a nonsignificant (P > 0.05) decrease in the number of pigs excreting arcobacters was observed.
Arcobacter butzleri, A. cryaerophilus, A. skirrowii, and A. thereius were isolated on farms A and C, while A. butzleri, A. skirrowii, and A. thereius were recovered on farm B. On farm D, A. butzleri, A. cryaerophilus, and A. thereius were present (Table 2). In addition, 16 isolates were not identified using the m-PCR assay but generated a genus-specific fragment. AFLP analysis revealed 10 profiles among these isolates which clustered distinctly from the established Arcobacter species (Table 2 and data not shown). A taxonomic study published elsewhere demonstrated that these 16 isolates represent a novel Arcobacter species for which the name A. trophiarum sp. nov. has been proposed (8).
TABLE 2.
Arcobacter isolation load and species level identification results
| Farm | % Isolation (total no. of fecal samples collected) by: |
No. of isolates identified (no. of isolates obtained after direct plating/no. of isolates obtained after enrichment) |
|||||
|---|---|---|---|---|---|---|---|
| Direct plating | Enrichment | A. butzleri | A. cryaerophilus | A. skirrowii | A. thereius | A. trophiarum sp. nov. | |
| A | 4.6 (282) | 11.3 (282) | 10 (1/9) | 3 (0/3) | 1 (0/1) | 50 (47/3) | 1 (0/1) |
| B | 8.6 (256) | 15.6 (256) | 10 (0/10) | 0 (0/0) | 37 (36/1) | 78 (68/10) | 15 (4/11) |
| C | 4.1 (266) | 15.0 (266) | 44 (10/34) | 2 (1/1) | 1 (1/0) | 1 (0/1) | 0 (0/0) |
| D | 11.1 (296) | 50.0 (296) | 215 (78/137) | 7 (5/2) | 0 (0/0) | 10 (7/3) | 0 (0/0) |
Surprisingly, A. thereius was the species most commonly isolated on farms A and B (excreted by 10 and 18 animals, respectively) (see Fig. S1 in the supplemental material). On the other hand, the majority of the isolates from farms C and D was identified as A. butzleri. Moreover, A. butzleri was isolated from all pigs on farms C and D that excreted arcobacters at least once during the sampling period. One pig each on farms A and C and 11 pigs on farm B excreted A. skirrowii. Arcobacter cryaerophilus was isolated from three, one, and five pigs on farms A, C, and D, respectively.
Arcobacter thereius and A. skirrowii were mostly isolated after direct plating and were consistently shed at levels higher than 102 CFU g−1 feces (Table 2). The majority of the A. butzleri and A. trophiarum sp. nov. isolates were recovered after enrichment and were shed at levels lower than 102 CFU g−1 feces. Except for one occasion, A. cryaerophilus was isolated at levels lower than 102 CFU g−1 feces.
At a similarity level of 85% used to discriminate genotypes, 110 AFLP genotypes were distinguished among all picked isolates (4). Isolates within these AFLP genotypes were often distinguishable by ERIC-PCR, and a total of 219 ERIC-PCR genotypes were obtained (Table 3). Two A. butzleri AFLP genotypes and one A. cryaerophilus AFLP genotype were found on two farms (C and D); in addition, one A. thereius AFLP genotype was present on farm A as well as on farm B. The majority (78/110) of the AFLP genotypes on the four farms was detected only once on a sampling occasion; the remaining 32 AFLP genotypes were isolated on multiple sampling occasions. Seventeen AFLP genotypes (farm A, 7/8; farm B, 1/8; farm C, 3/4; and farm D, 6/12) occurring more than once were excreted intermittently. Eleven AFLP genotypes (farm A, 1/8; farm B, 7/8; farm C, 1/4; and farm D, 2/12) were excreted on a number of consecutive samplings and then disappeared, and four AFLP genotypes on farm D were present predominantly during the finishing period and were shed on 9 (A. butzleri AFLP genotype B1 [18 pigs]), 8 (A. butzleri AFLP genotype B4 [15 pigs] and A. butzleri AFLP genotype B8 [17 pigs]), and 6 (A. butzleri AFLP genotype B11 [12 pigs]) sampling occasions.
TABLE 3.
Number of Arcobacter genotypes as determined by AFLP and ERIC-PCR
| Farm | No. of genotypes determined by: |
|||||||
|---|---|---|---|---|---|---|---|---|
| AFLP |
ERIC-PCR |
|||||||
| A. butzleri | A. cryaerophilus | A. skirrowii | A. thereius | A. butzleri | A. cryaerophilus | A. skirrowii | A. thereius | |
| A | 5 | 3 | 1 | 16 | 7 | 3 | 1 | 36 |
| B | 3 | —a | 15 | 27 | 5 | — | 28 | 57 |
| C | 9 | 2 | 1 | 1 | 13 | 2 | 1 | 1 |
| D | 20 | 4 | — | 3 | 55 | 4 | — | 6 |
—, not isolated.
Sixty-eight percent (75/110) of the AFLP genotypes occurring on the farms were excreted by the pigs at levels of between 102 and 104 CFU g−1 feces, while the other 35 Arcobacter AFLP genotypes were shed at levels below 102 CFU g−1 feces.
Pen level.
Arcobacters were recovered from fecal samples in all pens. The Arcobacter species distribution at the pen level on the four farms is shown in Fig. S1 in the supplemental material. On farms A and B, A. thereius and A. butzleri were excreted in all three pens. Arcobacter butzleri and A. thereius were isolated from the feces of 8 and 10 pigs, respectively, on farm A. On farm B, 7 and 18 pigs shed A. butzleri and A. thereius, respectively. Arcobacter skirrowii was isolated from the feces of 11 pigs housed in pens 1 and 2 on farm B, but on farm A this species was found in only one pig (A/3/2/17) accommodated in pen 2. In pen 1 (A/1/1/7), pen 2 (A/4/2/17), and pen 3 (A/3/3/26) of farm A, A. cryaerophilus was excreted by one animal. On the other hand, A. cryaerophilus was not isolated on farm B. On farm A, A. trophiarum sp. nov. was excreted by one pig (A/1/1/24) in the first pen, and on farm B this species was isolated from 12 pigs housed in pens 1 and 2. Arcobacter butzleri was isolated from 21 pigs housed in either pen 1, 2, or 3 on farm C. Conversely, A. cryaerophilus (C/2/1/64 and C/3/1/64), A. thereius (C/10/3/88), and A. skirrowii (C/7/3/87) were excreted by only one pig in either pen 1 or pen 3 on farm C. On farm D, A. butzleri and A. cryaerophilus were present in all pens and were shed by 29 and 5 pigs, respectively. Arcobacter thereius was excreted by three pigs (D/2/1/91, D/4/1/91, D/2/1/100, and D/1/2/109) in the first and second pens. Arcobacter skirrowii was not isolated on farm D.
With the exception of farm B, no clear difference in the number of shared genotypes in pigs of adjacent pens (pens 1 and 2) in comparison with animals housed in nonadjacent pens (either pen 1 versus 3 or pen 2 versus 3) was found. On all farms, the majority (farm A, 21/25; farm B, 33/45; farm C, 10/13; and farm D, 16/27) of the AFLP genotypes were found in only one pen and were mostly excreted by only one animal (farm A, 17/21; farm B, 25/33; farm C, 9/10; and farm D, 14/16). Conversely, one (farms A and B), two (farm C), and seven (farm D) A. butzleri AFLP genotypes were excreted in two pens. On farms A, B, and D, three, seven, and one A. thereius AFLP genotype, respectively, were recovered from pigs in two pens. In addition, one A. cryaerophilus AFLP genotype (C1, farm D) and two A. skirrowii AFLP genotypes (S9 and S12, farm B) were shed by pigs housed in pens 1 and 2. Two genotypes present on farms B (A. butzleri AFLP genotype B2 and A. thereius AFLP genotype T2) and D (A. butzleri AFLP genotypes B1 and B4) and one A. butzleri AFLP genotype (B7) on farm C were excreted by pigs in all three pens examined.
Animal level.
The majority (57/92) of the animals excreted only one Arcobacter species at a time during the whole fattening period. The other pigs mostly shed two or three species in the course of the fattening period. Pig 36 excreted four Arcobacter species (A. thereius, A. skirrowii, A. butzleri, and A. trophiarum sp. nov.), and at 14 weeks of age, the species A. thereius, A. skirrowii, and A. butzleri were shed.
During the fattening period, 60 of the 92 pigs excreted more than one AFLP genotype per species. A large majority of these genotypes (78/110) were detected on one sampling occasion and were excreted mostly by one animal (65/110). On one sampling occasion, pigs maximally excreted five A. butzleri (D/4/2/107), eight A. thereius (B/2/1/36), and five A. skirrowii (B/2/2/45) genotypes or one A. cryaerophilus (for example, A/7/1/7, C/2/1/64, and D/8/2/110) genotype. However, the other 32 genotypes were excreted on several sampling occasions. For example, A. butzleri genotype B8 (farm D) was excreted by pig 99 on four successive samplings (D/6/1/99, D/7/1/99, D/8/1/99, and D/9/1/99). Both on farm A (A. thereius genotypes T9 and T11) and on farm B (A. thereius genotypes T2 and T19), two A. thereius genotypes were each recovered from the feces of one pig during two successive samplings. The A. cryaerophilus and A. skirrowii genotypes were shed only on one successive sampling occasion by one pig.
DISCUSSION
Arcobacters are commonly present in food of animal origin, with the highest prevalence reported for poultry, followed by pork (47). However, in contrast to the case for poultry, arcobacters are associated with pigs throughout the production chain, from piglet to ground meat. Information on the Arcobacter population dynamics during the fattening period, however, is lacking (15, 43, 44).
In the present study, 92 of the 120 pigs excreted arcobacters at least once during the finishing period without any clinical symptoms or notable influence on the rearing parameters. Arcobacters also do not seem to be part of the essential commensal intestinal flora, as the Arcobacter excretion was highly dynamic in time, numbers, and strains. This intermittent excretion and strain variability can be explained by temporal clearance followed by reinfection, by a resting phase of the bacteria through an accumulation at the bottom of the crypts in the intestinal mucosa, or by a tendency to accumulate in the rectal mucus, resulting in a heterogeneous distribution in the gut content (22, 28). Moreover, in contrast with previous findings (23, 44), the number of excreting animals and the number of arcobacters in the feces did not increase with the age of the animals.
Notwithstanding the variable species and genotype composition in and between animals during the fattening period in the present study, several Arcobacter transmission routes were identified. Although strains were mostly recovered from one animal only, transmission of the same strain between animals housed in the same pen and pigs in both adjacent and nonadjacent pens was demonstrated. On farm D, for example, Arcobacter butzleri genotype B4 was first excreted by pigs 109 (D/2/2/109) and 110 (D/2/2/110) at 14 weeks of age, after which it spread in pen 2 (pigs 102, 105, 106, 107, and 108) during the following samplings. From the fifth sampling occasion (20 weeks of age), this genotype was further recovered from seven pigs (111, 112, 115, 116, 117, 119, and 120) in pen 3 as well. In pen 1, only animal 95 (D/9/1/95) shed A. butzleri genotype B4 at 28 weeks of age (see Fig. S2 in the supplemental material). The presence of the same strain within the same pen and in adjacent pens can be explained by fecal-oral transmission during close contact through the partially opened side walls. Oral uptake of arcobacters followed by fecal excretion has previously been observed during an experimental in vivo study in 1-day-old piglets (51) and in piglets up to 3 weeks of age (15). The occurrence of unique Arcobacter strains in nonadjacent and adjacent pens demonstrates that other infection routes, such as contaminated water, rodents, the pig farmer, and iatrogenic transmission, are probably important (44, 45). In contrast to the case for Campylobacter (1), Arcobacter transmission through rodents could not be confirmed in the present study, as the gastrointestinal tracts of the rats examined did not harbor Arcobacter (data not shown).
As reported for Campylobacter coli in pigs (38, 49, 50), some Arcobacter strains spread over the pig house, resulting in dominant strains in several animals. For example strains B1, B4, B8, and B11 were predominantly excreted by several pigs throughout the finishing period on farm D. The dominance of such strains may suggest a greater capacity to survive the environmental stresses and to colonize the host gut, as previously described for Campylobacter jejuni (12, 33). Differences in colonization types can be due to genetic differences or differences in expression of colonization and invasion related genes (12).
The large heterogeneity of Arcobacter populations, in both species and strain composition, has been discussed almost since the first description of the genus in 1992. Like for Campylobacter (33, 49), multiple parent genotypes from different infection sources and genomic rearrangements and DNA uptake from the environment have been suggested as the driving force for this diversity, but none of these hypotheses have been firmly confirmed. Apart from the biological variation, the isolation and typing method also can bias the outcome of diversity studies. The strains recovered may reflect a superior adaptation to the isolation procedure and medium components rather than their dominant occurrence, especially when an enrichment step is applied. In the present study, a previously validated Arcobacter isolation method with both direct isolation and isolation after enrichment was applied (43). In contrast to other Arcobacter isolation methods, the composition of the selective supplement in the medium allows growth of all currently known mammal-associated species with a maximum suppression of the accompanying flora (16). The lower rates of isolation of A. cryaerophilus and A. skirrowii can be explained either by a lower occurrence in the intestinal tract or by the more fastidious growth requirements of those species (16, 43). As in previous studies, direct plating revealed a larger diversity of Arcobacter strains than in enrichment, where mostly one genotype was recovered (44, 45). In addition to the isolation method, the typing method also biases the results. Although no gold standard typing method has presently been established for the genus Arcobacter, the application of different typing methods confirms that this heterogeneity truly exists (23, 30, 44).
In the present study, a large number of A. thereius strains were isolated for the first time from pig feces. On farms A and B, A. thereius was the species most commonly excreted during the fattening period. ERIC-PCR, rather than AFLP, was most suited for A. thereius characterization. As reported by Debruyne et al. (4), the number of peaks observed in the AFLP profiles was significantly lower than for other Arcobacter species.
In conclusion, in the present study, arcobacters were isolated from the majority of animals on all farms. Although differences in farm management were observed, Arcobacter occurrence did not seem to be related to hygiene measurements or farm management. Moreover, within each farm, the Arcobacter excretion in animals is not static but is highly dynamic. The overwhelming number of strains present on each farm seriously complicates the study of Arcobacter epidemiology and hampers the identification of possible infection and transmission routes.
Supplementary Material
Acknowledgments
We thank the swine farmers who contributed to the study. We greatly appreciate the technical support of Karen Taerwe during AFLP analysis. We kindly thank Johan Van Hende for help in the performance of the statistical analysis.
Footnotes
Published ahead of print on 7 January 2011.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Alter, T., et al. 2005. Prevalences and transmission routes of Campylobacter spp. strains within multiple pig farms. Vet. Microbiol. 108:251-261. [DOI] [PubMed] [Google Scholar]
- 2.Aydin, F., K. S. Gümüçssoy, H. I. Atabay, T. Ica, and S. Abay. 2007. Prevalence and distribution of Arcobacter species in various sources in Turkey and molecular analysis of isolated strains by ERIC-PCR. J. Appl. Microbiol. 103:27-35. [DOI] [PubMed] [Google Scholar]
- 3.Collado, L., I. Cleenwerck, S. Van Trappen, P. De Vos, and M. J. Figueras. 2009. Arcobacter mytili sp. nov., an indoxyl acetate-hydrolysis-negative bacterium isolated from mussels. Int. J. Syst. Evol. Microbiol. 59:1391-1396. [DOI] [PubMed] [Google Scholar]
- 4.Debruyne, L., K. Houf, L. Douidah, S. De Smet, and P. Vandamme. 2010. Reassessment of the taxonomy of Arcobacter cryaerophilus. Syst. Appl. Microbiol. 33:7-14. [DOI] [PubMed] [Google Scholar]
- 5.de Oliveira, S. J., A. L. Baetz, I. V. Wesley, and K. M. Harmon. 1997. Classification of Arcobacter species isolated from aborted pig fetuses and sows with reproductive problems in Brazil. Vet. Microbiol. 57:347-354. [DOI] [PubMed] [Google Scholar]
- 6.de Oliveira, S. J., et al. 1999. Arcobacter cryaerophilus and Arcobacter butzleri isolated from preputial fluid of boars and fattening pigs in Brazil. J. Vet. Diagn. Invest. 11:462-464. [DOI] [PubMed] [Google Scholar]
- 7.De Smet, S., L. De Zutter, J. Van Hende, and K. Houf. 2010. Arcobacter contamination on pre- and post-chilled bovine carcasses and in minced beef at retail. J. Appl. Microbiol. 108:299-305. [DOI] [PubMed] [Google Scholar]
- 8.De Smet, S., et al. 19 March 2010, posting date. Arcobacter trophiarum sp. nov. isolated from fattening pigs. Int. J. Syst. Evol. Microbiol. doi: 10.1099/ijs.0.022665-0. [DOI] [PubMed]
- 9.Douidah, L., L. De Zutter, P. Vandamme, and K. Houf. 2010. Identification of five human and mammal associated Arcobacter species by a novel multiplex-PCR assay. J. Microbiol. Methods 80:281-286. [DOI] [PubMed] [Google Scholar]
- 10.Ellis, W. A., S. D. Neill, J. J. O'brien, and J. Hanna. 1978. Isolation of spirillum-like organisms from pig fetuses. Vet. Rec. 102:106. [DOI] [PubMed] [Google Scholar]
- 11.Fera, M. T., C. E. La, M. Carbone, D. Malara, and M. G. Pennisi. 2009. Pet cats as carriers of Arcobacter spp. in Southern Italy. J. Appl. Microbiol. 106:1661-1666. [DOI] [PubMed] [Google Scholar]
- 12.Hänel, I., et al. 2009. Genomic and phenotypic changes of Campylobacter jejuni strains after passage of the chicken gut. Vet. Microbiol. 136:121-129. [DOI] [PubMed] [Google Scholar]
- 13.Harmon, K. M., and I. V. Wesley. 1996. Identification of Arcobacter isolates by PCR. Lett. Appl. Microbiol. 23:241-244. [DOI] [PubMed] [Google Scholar]
- 14.Harvey, R. B., et al. 1999. Prevalence of Campylobacter, Salmonella and Arcobacter species at slaughter in market age pigs. Adv. Exp. Med. Biol. 473:237-239. [DOI] [PubMed] [Google Scholar]
- 15.Ho, T. K. H., L. J. A. Lipman, L. van der Graaf-van Bloois, M. van Bergen, and W. Gaastra. 2006. Potential routes of acquisition of Arcobacter species by piglets. Vet. Microbiol. 114:123-133. [DOI] [PubMed] [Google Scholar]
- 16.Houf, K., L. A. Devriese, L. De Zutter, J. Van Hoof, and P. Vandamme. 2001. Development of a new protocol for the isolation and quantification of Arcobacter species from poultry products. Int. J. Food Microbiol. 71:189-196. [DOI] [PubMed] [Google Scholar]
- 17.Houf, K., L. De Zutter, J. Van Hoof, and P. Vandamme. 2002. Assessment of the genetic diversity among arcobacters isolated from poultry products by using two PCR-based typing methods. Appl. Environ. Microbiol. 68:2172-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houf, K., L. De Zutter, B. Verbeke, J. Van Hoof, and P. Vandamme. 2003. Molecular characterization of Arcobacter isolates collected in a poultry slaughterhouse. J. Food Prot. 66:364-369. [DOI] [PubMed] [Google Scholar]
- 19.Houf, K., and R. Stephan. 2007. Isolation and characterization of the emerging foodborn pathogen Arcobacter from human stool. J. Microbiol. Methods 68:408-413. [DOI] [PubMed] [Google Scholar]
- 20.Houf, K., S. De Smet, J. Baré, and S. Daminet. 2008. Dogs as carriers of the emerging pathogen Arcobacter. Vet. Microbiol. 130:208-213. [DOI] [PubMed] [Google Scholar]
- 21.Houf, K., et al. 2009. Arcobacter thereius sp. nov., isolated from pigs and ducks. Int. J. Syst. Evol. Microbiol. 59:2599-2604. [DOI] [PubMed] [Google Scholar]
- 22.Hugdahl, M. B., and M. P. Doyle. 1985. Chemotactic behaviour of Campylobacter jejuni, p. 143. In A. D. Pearson, M. B. Skirrow, H. Lior, and B. Rowe (ed.), Campylobacter III. Public Health Laboratory Service, London, United Kingdom.
- 23.Hume, M. E., et al. 2001. Genotypic variation among Arcobacter isolates from a farrow-to-finish swine facility. J. Food Prot. 64:645-651. [DOI] [PubMed] [Google Scholar]
- 24.Kabeya, H., et al. 2003. Distribution of Arcobacter species among livestock in Japan. Vet. Microbiol. 93:153-158. [DOI] [PubMed] [Google Scholar]
- 25.Keller, S., S. Raber, T. Tasara, and R. Stephan. 2006. Prevalence of Arcobacter butzleri in fecal samples, on carcasses and in retail meat of cattle, pig and poultry in Switzerland. Arch. Lebensmittelhygiene 57:64-68. [Google Scholar]
- 26.Kiehlbauch, J. A., et al. 1991. Campylobacter butzleri sp. nov. isolated from humans and animals with diarrheal illness. J. Clin. Microbiol. 29:376-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim, H. M., C. Y. Hwang, and B. C. Cho. 2010. Arcobacter marinus sp. nov. Int. J. Syst. Evol. Microbiol. 60:531-536. [DOI] [PubMed] [Google Scholar]
- 28.Lee, A., J. L. O'Rourke, P. J. Barrington, and T. J. Trust. 1986. Mucus colonization as a determinant of pathogenicity in intestinal infection by Campylobacter jejuni: a mouse caecal model. Infect. Immun. 51:536-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neill, S. D., W. A. Ellis, and J. J. O'Brien. 1979. Designation of aerotolerant Campylobacter-like organisms from porcine and bovine abortions to the genus Campylobacter. Res. Vet. Sci. 27:180-186. [PubMed] [Google Scholar]
- 30.On, S. L. W., T. K. Jensen, V. Bille-Hansen, S. E. Jorsal, and P. Vandamme. 2002. Prevalence and diversity of Arcobacter spp. isolated from the internal organs of spontaneous porcine abortions in Denmark. Vet. Microbiol. 85:159-167. [DOI] [PubMed] [Google Scholar]
- 31.Pitcher, D. G., N. A. Saunders, and R. J. Owen. 1989. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett. Appl. Microbiol. 8:151-156. [Google Scholar]
- 32.Prouzet-Mauleon, V., L. Labadi, N. Bouges, A. Menard, and F. Megraud. 2006. Arcobacter butzleri: underestimated enteropathogen. Emerg. Infect. Dis. 12:307-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ridley, A. M., M. J. Toszeghy, S. A. Cawthraw, T. M. Wassenaar, and D. G. Newell. 2008. Genetic instability is associated with changes in the colonization potential of Campylobacter jejuni in the avian intestine. J. Appl. Microbiol. 105:95-104. [DOI] [PubMed] [Google Scholar]
- 34.Rivas, L., N. Fegan, and P. Vanderlinde. 2004. Isolation and characterisation of Arcobacter butzleri from meat. Int. J. Food Microbiol. 91:31-41. [DOI] [PubMed] [Google Scholar]
- 35.Samie, A., C. L. Obi, L. J. Barrett, S. M. Powell, and R. L. Guerrant. 2007. Prevalence of Campylobacter species, Helicobacter pylori and Arcobacter species in stool samples from the Venda region, Limpopo, South Africa: studies using molecular diagnostic methods. J. Infect. 54:558-566. [DOI] [PubMed] [Google Scholar]
- 36.Schroeder-Tucker, L., et al. 1996. Phenotypic and ribosomal RNA characterization of Arcobacter species isolated from porcine aborted fetuses. J. Vet. Diagn. Invest. 8:186-195. [DOI] [PubMed] [Google Scholar]
- 37.Scullion, R., C. S. Harrington, and R. H. Madden. 2006. Prevalence of Arcobacter spp. in raw milk and retail raw meats in northern Ireland. J. Food Prot. 69:1986-1990. [DOI] [PubMed] [Google Scholar]
- 38.Soultos, N., and R. H. Madden. 2007. A genotyping investigation of the colonization of piglets by Campylobacter coli in the first 10 weeks of life. J. Appl. Microbiol. 102:916-920. [DOI] [PubMed] [Google Scholar]
- 39.Taylor, D. N., J. A. Kiehlbauch, W. Tee, C. Pitarangsi, and P. Echeverria. 1991. Isolation of group 2 aerotolerant Campylobacter species from Thai children with diarrhea. J. Infect. Dis. 163:1062-1067. [DOI] [PubMed] [Google Scholar]
- 40.Vandamme, P., et al. 1991. Revision of Campylobacter, Helicobacter, and Wolinella taxonomy: emendation of generic descriptions and proposal of Arcobacter gen. nov. Int. J. Syst. Bacteriol. 41:88-103. [DOI] [PubMed] [Google Scholar]
- 41.Vandamme, P., et al. 1992. Outbreak of recurrent abdominal cramps associated with Arcobacter butzleri in an Italian school. J. Clin. Microbiol. 30:2335-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vandenberg, O., et al. 2004. Arcobacter species in humans. Emerg. Infect. Dis. 10:1863-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Driessche, E., K. Houf, J. Van Hoof, L. De Zutter, and P. Vandamme. 2003. Isolation of Arcobacter species from animal feces. FEMS Microbiol. Lett. 229:243-248. [DOI] [PubMed] [Google Scholar]
- 44.Van Driessche, E., et al. 2004. Occurrence and strain diversity of Arcobacter species isolated from healthy Belgian pigs. Res. Microbiol. 155:662-666. [DOI] [PubMed] [Google Scholar]
- 45.Van Driessche, E., K. Houf, F. Vangroenweghe, L. De Zutter, and J. Van Hoof. 2005. Prevalence, enumeration and strain variation of Arcobacter species in the faeces of healthy cattle in Belgium. Vet. Microbiol. 105:149-154. [DOI] [PubMed] [Google Scholar]
- 46.Van Driessche, E., and K. Houf. 2007. Discrepancy between the occurrence of Arcobacter in chickens and broiler carcass contamination. Poult. Sci. 86:744-751. [DOI] [PubMed] [Google Scholar]
- 47.Van Driessche, E., and K. Houf. 2007. Characterization of the Arcobacter contamination on Belgian pork carcasses and raw retail pork. Int. J. Food Microbiol. 118:20-26. [DOI] [PubMed] [Google Scholar]
- 48.Versalovic, J., T. Koeuth, and R. Lupski. 1991. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 19:6823-6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weijtens, M. J., R. D. Reinders, H. A. Urlings, and J. Van der Plas. 1999. Campylobacter infections in fattening pigs; excretion pattern and genetic diversity. J. Appl. Microbiol. 86:63-70. [DOI] [PubMed] [Google Scholar]
- 50.Weijtens, M. J., H. A. Urlings, and J. Van der Plas. 2000. Establishing a Campylobacter-free pig population through a top-down approach. Lett. Appl. Microbiol. 30:479-484. [DOI] [PubMed] [Google Scholar]
- 51.Wesley, I. V., A. L. Baetz, and D. J. Larson. 1996. Infection of cesarean-derived colostrum-deprived 1-day-old piglets with Arcobacter butzleri, Arcobacter cryaerophilus, and Arcobacter skirrowii. Infect. Immun. 64:2295-2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wybo, I., J. Breynaert, S. Lauwers, F. Lindenburg, and K. Houf. 2004. Isolation of Arcobacter skirrowii from a patient with chronic diarrhea. J. Clin. Microbiol. 42:1851-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.