Abstract
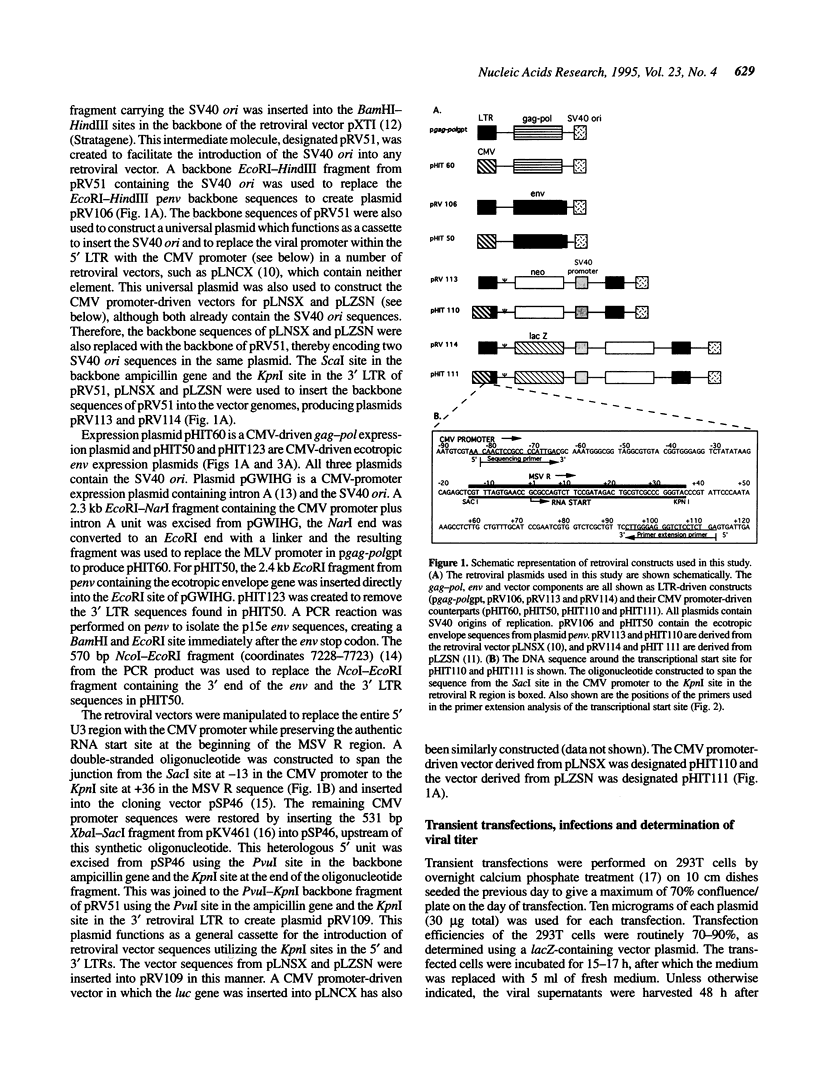
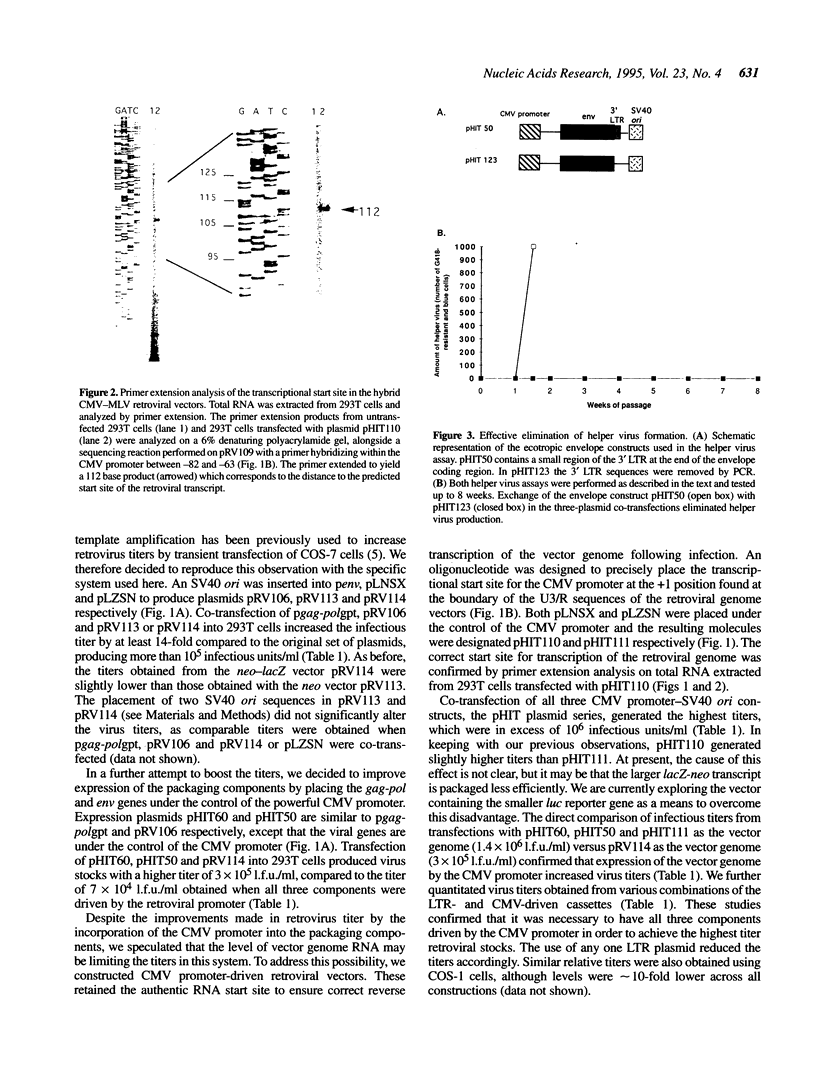
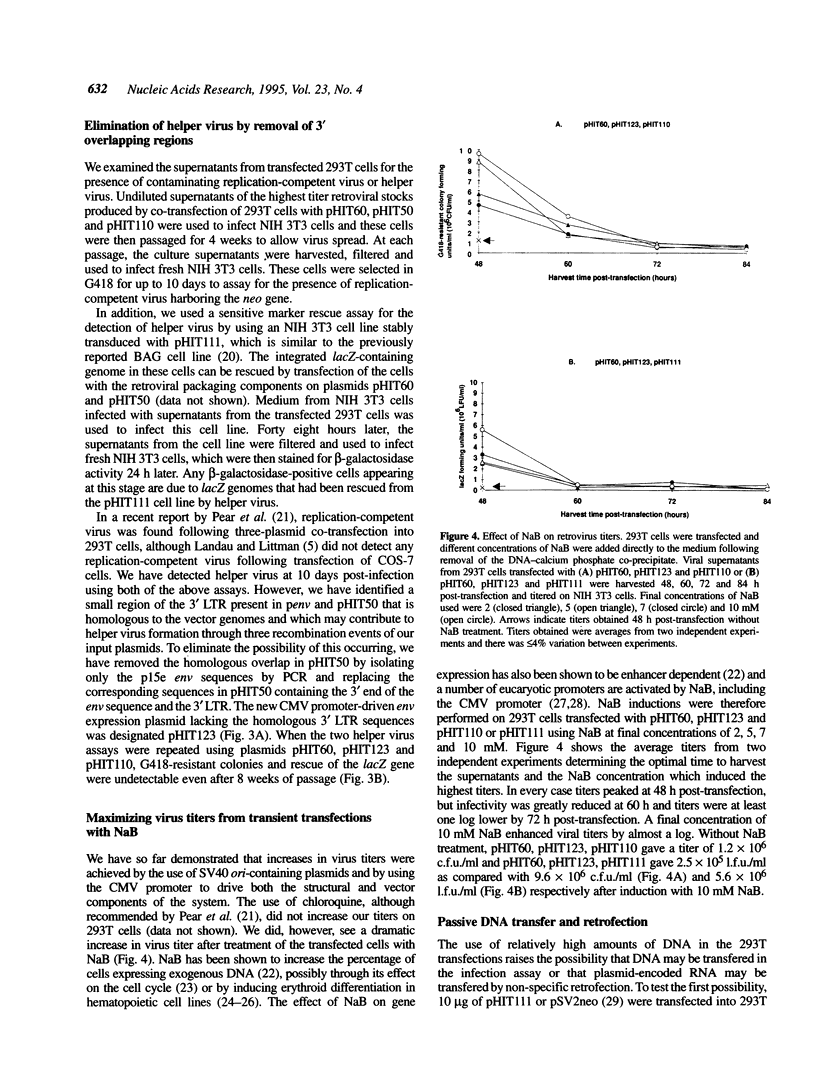
We have constructed a series of MLV-based retroviral vectors and packaging components expressed from the CMV promoter and carried on plasmids containing SV40 origins of replication. These two features greatly enhanced retroviral gene expression when introduced into cell lines carrying the SV40 large T antigen. The two packaging components, gag-pol and env, were placed on separate plasmids to reduce helper virus formation. Using a highly transfectable human cell line and sodium butyrate to further increase expression of each component, we achieved helper-free viral stocks of approximately 10(7) infectious units/ml by 48 h after transient co-transfection with the three plasmid components. This system can be used both for the generation of high titer retroviral stocks for transduction and for the rapid screening of a large number of MLV gag-pol or env mutants.
Full text
PDF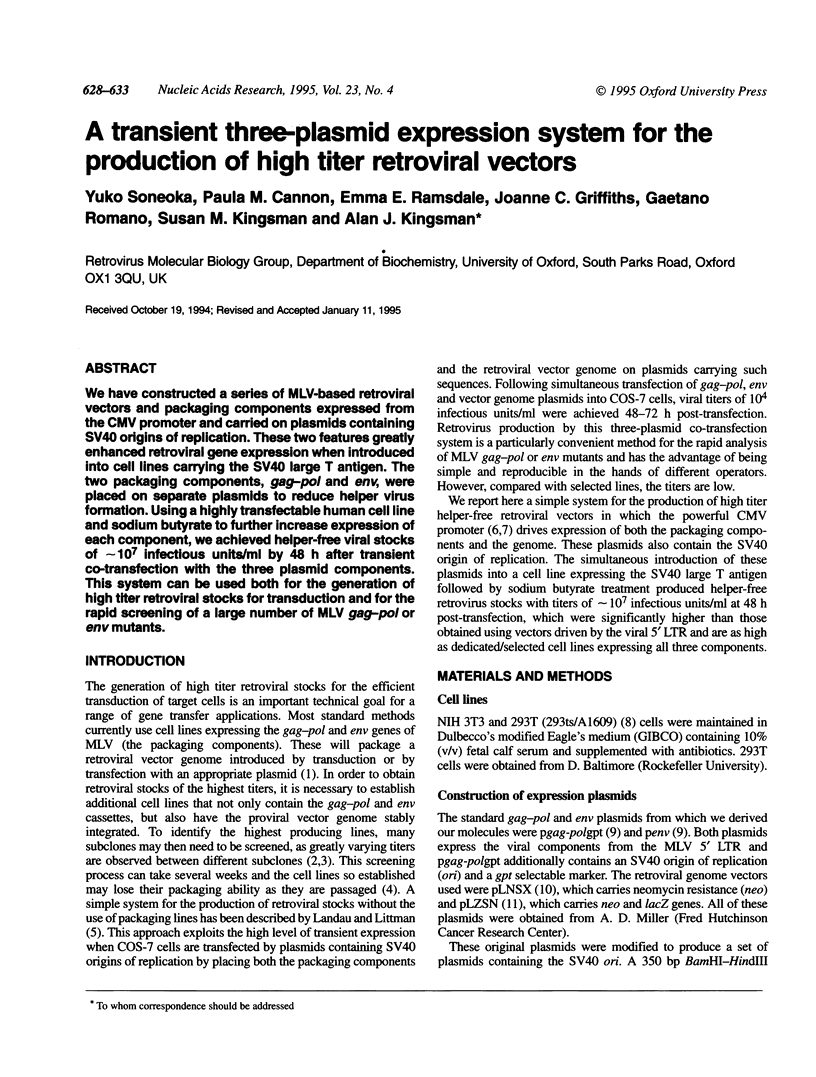


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam M. A., Ramesh N., Miller A. D., Osborne W. R. Internal initiation of translation in retroviral vectors carrying picornavirus 5' nontranslated regions. J Virol. 1991 Sep;65(9):4985–4990. doi: 10.1128/jvi.65.9.4985-4990.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams S. E., Johnson I. D., Braddock M., Kingsman A. J., Kingsman S. M., Edwards R. M. Synthesis of a gene for the HIV transactivator protein TAT by a novel single stranded approach involving in vivo gap repair. Nucleic Acids Res. 1988 May 25;16(10):4287–4298. doi: 10.1093/nar/16.10.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender M. A., Palmer T. D., Gelinas R. E., Miller A. D. Evidence that the packaging signal of Moloney murine leukemia virus extends into the gag region. J Virol. 1987 May;61(5):1639–1646. doi: 10.1128/jvi.61.5.1639-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard A. D., Powell R., Braddock M., Kingsman A. J., Kingsman S. M. An adenosine at position 27 in the human immunodeficiency virus type 1 trans-activation response element is not critical for transcriptional or translational activation by Tat. J Virol. 1992 Nov;66(11):6769–6772. doi: 10.1128/jvi.66.11.6769-6772.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshart M., Weber F., Jahn G., Dorsch-Häsler K., Fleckenstein B., Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985 Jun;41(2):521–530. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- Boulter C. A., Wagner E. F. A universal retroviral vector for efficient constitutive expression of exogenous genes. Nucleic Acids Res. 1987 Sep 11;15(17):7194–7194. doi: 10.1093/nar/15.17.7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braddock M., Thorburn A. M., Chambers A., Elliott G. D., Anderson G. J., Kingsman A. J., Kingsman S. M. A nuclear translational block imposed by the HIV-1 U3 region is relieved by the Tat-TAR interaction. Cell. 1990 Sep 21;62(6):1123–1133. doi: 10.1016/0092-8674(90)90389-v. [DOI] [PubMed] [Google Scholar]
- Chapman B. S., Thayer R. M., Vincent K. A., Haigwood N. L. Effect of intron A from human cytomegalovirus (Towne) immediate-early gene on heterologous expression in mammalian cells. Nucleic Acids Res. 1991 Jul 25;19(14):3979–3986. doi: 10.1093/nar/19.14.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Anna J. A., Tobey R. A., Gurley L. R. Concentration-dependent effects of sodium butyrate in Chinese hamster cells: cell-cycle progression, inner-histone acetylation, histone H1 dephosphorylation, and induction of an H1-like protein. Biochemistry. 1980 Jun 10;19(12):2656–2671. doi: 10.1021/bi00553a019. [DOI] [PubMed] [Google Scholar]
- DuBridge R. B., Tang P., Hsia H. C., Leong P. M., Miller J. H., Calos M. P. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol Cell Biol. 1987 Jan;7(1):379–387. doi: 10.1128/mcb.7.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finer M. H., Dull T. J., Qin L., Farson D., Roberts M. R. kat: a high-efficiency retroviral transduction system for primary human T lymphocytes. Blood. 1994 Jan 1;83(1):43–50. [PubMed] [Google Scholar]
- Foecking M. K., Hofstetter H. Powerful and versatile enhancer-promoter unit for mammalian expression vectors. Gene. 1986;45(1):101–105. doi: 10.1016/0378-1119(86)90137-x. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Howard B. H., Reeves R. Expression of recombinant plasmids in mammalian cells is enhanced by sodium butyrate. Nucleic Acids Res. 1983 Nov 11;11(21):7631–7648. doi: 10.1093/nar/11.21.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hock R. A., Miller A. D., Osborne W. R. Expression of human adenosine deaminase from various strong promoters after gene transfer into human hematopoietic cell lines. Blood. 1989 Aug 1;74(2):876–881. [PubMed] [Google Scholar]
- Landau N. R., Littman D. R. Packaging system for rapid production of murine leukemia virus vectors with variable tropism. J Virol. 1992 Aug;66(8):5110–5113. doi: 10.1128/jvi.66.8.5110-5113.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder A., Leder P. Butyric acid, a potent inducer of erythroid differentiation in cultured erythroleukemic cells. Cell. 1975 Jul;5(3):319–322. doi: 10.1016/0092-8674(75)90107-5. [DOI] [PubMed] [Google Scholar]
- Levine K. L., Steiner B., Johnson K., Aronoff R., Quinton T. J., Linial M. L. Unusual features of integrated cDNAs generated by infection with genome-free retroviruses. Mol Cell Biol. 1990 May;10(5):1891–1900. doi: 10.1128/mcb.10.5.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linial M. Creation of a processed pseudogene by retroviral infection. Cell. 1987 Apr 10;49(1):93–102. doi: 10.1016/0092-8674(87)90759-8. [DOI] [PubMed] [Google Scholar]
- Markowitz D., Goff S., Bank A. A safe packaging line for gene transfer: separating viral genes on two different plasmids. J Virol. 1988 Apr;62(4):1120–1124. doi: 10.1128/jvi.62.4.1120-1124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D. Retrovirus packaging cells. Hum Gene Ther. 1990 Spring;1(1):5–14. doi: 10.1089/hum.1990.1.1-5. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Ogden J. E., Stanway C., Kim S., Mellor J., Kingsman A. J., Kingsman S. M. Efficient expression of the Saccharomyces cerevisiae PGK gene depends on an upstream activation sequence but does not require TATA sequences. Mol Cell Biol. 1986 Dec;6(12):4335–4343. doi: 10.1128/mcb.6.12.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pear W. S., Nolan G. P., Scott M. L., Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J., Turner D., Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1987 Jan;84(1):156–160. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radsak K., Fuhrmann R., Franke R. P., Schneider D., Kollert A., Brücher K. H., Drenckhahn D. Induction by sodium butyrate of cytomegalovirus replication in human endothelial cells. Arch Virol. 1989;107(1-2):151–158. doi: 10.1007/BF01313887. [DOI] [PubMed] [Google Scholar]
- Reeves R., Cserjesi P. Sodium butyrate induces new gene expression in Friend erythroleukemic cells. J Biol Chem. 1979 May 25;254(10):4283–4290. [PubMed] [Google Scholar]
- Sanes J. R., Rubenstein J. L., Nicolas J. F. Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 1986 Dec 1;5(12):3133–3142. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Sorge J., Wright D., Erdman V. D., Cutting A. E. Amphotropic retrovirus vector system for human cell gene transfer. Mol Cell Biol. 1984 Sep;4(9):1730–1737. doi: 10.1128/mcb.4.9.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Tanaka J., Sadanari H., Sato H., Fukuda S. Sodium butyrate-inducible replication of human cytomegalovirus in a human epithelial cell line. Virology. 1991 Nov;185(1):271–280. doi: 10.1016/0042-6822(91)90774-6. [DOI] [PubMed] [Google Scholar]




