Abstract
Fresh produce is often a high-risk food for norovirus contamination because it can become contaminated at both preharvest and postharvest stages and it undergoes minimal or no processing. Currently, there is no effective method to eliminate the viruses from fresh produce. This study systematically investigated the effectiveness of high-pressure processing (HPP) on inactivating murine norovirus (MNV-1), a surrogate for human norovirus, in aqueous medium and fresh produce. We demonstrated that MNV-1 was effectively inactivated by HPP. More than a 5-log-PFU/g reduction was achieved in all tested fresh produce when it was pressurized at 400 MPa for 2 min at 4°C. We found that pressure, pH, temperature, and food matrix affected the virus survival in foods. MNV-1 was more effectively inactivated at 4°C than at 20°C in both medium and fresh produce. MNV-1 was also more sensitive to HPP at neutral pH than at acidic pH. We further demonstrated that disruption of viral capsid structure, but not degradation of viral genomic RNA, is the primary mechanism of virus inactivation by HPP. However, HPP does not degrade viral capsid protein, and the pressurized capsid protein was still antigenic. Overall, HPP had a variable effect on the sensorial quality of fresh produce, depending on the pressure level and type of product. Taken together, HPP effectively inactivated a human norovirus surrogate in fresh produce with a minimal impact on food quality and thus can provide a novel intervention for processing fruits intended for frozen storage and related products such as purees, sauces, and juices.
Viruses account for more than 67% of food-borne illnesses worldwide (41, 45). Human norovirus (NoV) is the major food-borne virus that causes gastroenteritis outbreaks in humans. The symptoms often involve projectile vomiting, diarrhea, nausea, and low-grade fever (1, 23, 40). It is estimated that more than 90% of outbreaks of acute nonbacterial gastroenteritis are caused by human NoV, but this percentage may be underestimated due to the large number of asymptomatic NoV infections and lack of proper detection methods (23, 40, 44). Human NoVs are transmitted primarily through the fecal-oral route, either by direct person-to-person spread or by fecally contaminated food or water. Human NoV is highly contagious, and only a few particles are thought to be sufficient to cause infection (22, 23, 40). Outbreaks frequently occur in restaurants, hotels, day care centers, schools, nursing homes, cruise ships, swimming pools, hospitals, and military installations (1, 18, 23, 41, 57). However, research on human NoVs has been hampered by the lack of a suitable cell culture system or a small animal model (23, 40). Therefore, studies of human NoV must rely on proper surrogates such as murine norovirus (MNV) and feline calicivirus (FCV) (14, 32, 56, 60). A recent study demonstrated that MNV was a more suitable surrogate than FCV (14). For these reasons, human NoV and other caliciviruses have been classified as category B priority biodefense pathogens by the National Institute of Allergy and Infectious Diseases.
Fresh produce is often at risk of NoV contamination because it undergoes minimal or no processing and can become contaminated during preharvest and postharvest stages. Contamination by NoV may occur at the farm level by fecal contamination in irrigation water, soil, animals, or fertilizer and by infected workers during harvesting, processing, handling, and freezing (2, 4, 10, 29, 52, 54, 57). According to a recent compilation of U.S. outbreak data from 1998 to 2005, fresh produce has become dominant as a vehicle in food-borne virus outbreaks (20, 29, 52, 53). Disease surveillance shows that NoV is the top causative agent for fresh produce outbreaks (40%), followed by Salmonella species (18%), Escherichia coli O157:H7 (8%), Clostridium species (6%) and hepatitis A virus (HAV) (4%) (20). Fresh-produce-related outbreaks of NoVs have been reported in lettuce, salad, fruit salad, tomato, carrot, melon, strawberry, raspberry, orange juice, fresh-cut fruits, spring onion, and other fresh produce (2, 4, 7, 19, 24, 43, 47). The increasing produce-associated outbreaks suggest that there is an urgent and critical need to develop a novel intervention method to inactivate NoVs in fresh produce.
Unfortunately, none of the decontamination methods investigated thus far have been shown to effectively control human NoV in fresh produce (12, 18, 20, 22, 40, 45, 52). Sanitizers are not effective in the removal of food-borne viruses from fresh produce. Most commonly used sanitizers such as chlorine, hydrogen peroxide, peracetic acid, and sodium bicarbonate reduced human NoV surrogates in vegetables only by 1 to 1.5 logs (11, 22, 52). More importantly, recent evidence suggests that human NoV may be internalized in the leaves of vegetables and disseminated to other portions of the plants (48, 58, 59). Sanitizers may be effective in reducing the viruses on the surface but not those internalized into vegetables and fruits. Although thermal processing could efficiently inactivate viruses, it is impractical for fresh produce, since high temperatures would adversely affect the food quality and degrade vitamins and other nutritional components. Perhaps nonthermal processing technologies may provide alternative methods to improve the safety of vegetables and fruits due to the fact that these technologies have a minimal impact on organoleptic and nutritional properties (5, 15, 26). However, the survival of food-borne viruses and the mechanism of virus inactivation by nonthermal processing technologies are poorly understood.
High-pressure processing (HPP) provides a unique advantage because pressure acts uniformly throughout a food regardless of size, shape, and geometry. By destroying pathogenic and spoilage organisms without breaking the covalent bonds, HPP has minimal effects on the taste, flavor, texture, appearance, and nutritional value of food (5, 15, 17, 26, 35-37). The technology also satisfies consumers' demand for minimal processing without the use of preservatives. High pressure has been shown to effectively inactivate bacterial pathogens and spores (15, 17, 28). However, limited work on the inactivation of food-borne viruses by HPP has been reported. It has been shown that both processing parameters (pressure, temperature, and time) and nonprocessing parameters (pH value and salt concentration) affect the inactivation of FCV (16, 26, 27). It was shown that the inactivation of FCV was favored when HPP was performed at refrigeration temperature rather than at room temperature (16). In sharp contrast, Kingsley and Chen's groups showed that the inactivation of HAV in solutions was enhanced at a higher temperature (13, 35-38). These findings suggest that different viruses have different susceptibilities to and optimal parameters for HPP. Previously, the effect of temperature on pressure inactivation of MNV-1 in oyster tissue was reported by Kingsley et al. (38). However, other optimal parameters (such as pH, combination of pH and temperature, and different food matrix) for the inactivation of MNV-1 by HPP have not been reported. Also, the molecular mechanism involved in virus inactivation is still poorly understood. Although one recent study suggests that HPP affects virus binding to host cells (55), the effect of HPP on the structure of the virion and viral capsid protein was not understood. In addition, no study has been performed to inactivate MNV-1 in fresh produce and related products such as purees and juices.
In the present study, we performed a systematic study on the inactivation of human NoV using MNV-1 as a surrogate. Specifically, we determined the effect of pH, temperature, pressure, and food matrix on the survival of MNV-1 by HPP. We determined the mechanism of virus inactivation by HPP. Furthermore, we demonstrated that HPP may be a novel intervention method to eliminate viruses from foods and may be a promising technology to process fruits intended for frozen storage and fruit-related products (such as puree, sauce, and juice).
MATERIALS AND METHODS
Virus and cell culture.
Murine norovirus strain MNV-1 was kindly provided by Herbert W. Virgin IV, Washington University School of Medicine (32, 61). Working stocks of MNV-1 were propagated in confluent monolayers of murine macrophage cell line RAW 264.7 (ATCC, Manassas, VA). The cells were cultured in high-glucose Dulbecco's modified Eagle medium (DMEM) (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) (Invitrogen), at 37°C under a 5% CO2 atmosphere. Confluent RAW 264.7 cells were infected with MNV-1 at a multiplicity of infection (MOI) of 0.01. After 1 h of incubation at 37°C, 15 ml of serum-free DMEM was added. Virus was harvested after 2 days postinoculation by freeze-thawing three times.
MNV-1 plaque assay.
RAW 264.7 cells were seeded into six-well plates (Corning Life Sciences, Wilkes-Barre, PA) at a density of 2 × 106 cells per well. After 24 h of incubation, cell monolayers were infected with 400 μl of a 10-fold dilution series of the virus and the plates were incubated for 1 h at 37°C. The cells were overlaid with 2.5 ml of Eagle minimum essential medium (MEM) containing 1% agarose, 2% FBS, 1% sodium bicarbonate, 0.1 mg of kanamycin/ml, 0.05 mg of gentamicin/ml, 15 mM HEPES (pH 7.7), and 2 mM l-glutamine (Invitrogen). After incubation at 37°C and 5% CO2 for 2 days, the plates were fixed in 10% formaldehyde and the plaques were visualized by staining with crystal violet.
Purification of murine norovirus.
Large stocks of MNV-1 were generated by inoculation of 8 to 10 confluent T150 flask RAW 264.7 cells at an MOI of 0.01 in a volume of 3 ml of DMEM. At 1 h postadsorption, 15 ml of DMEM with 2% FBS was added to the cultures, and infected cells were incubated at 37°C for 48 h. When extensive cytopathic effect (CPE) was observed, cell culture fluids were harvested and subjected to three freeze-thaw cycles to release virus particles. The purification of MNV-1 was performed using the method described by Katpally et al. (33) with minor modifications. The virus suspension was centrifuged in a Sorvall SS-34 rotor (Kendro Laboratory Products, Germany) at 8,000 × g for 15 min to remove cellular debris. The supernatant was then digested with DNase I (10 μg/ml) and MgCl2 (5 mM) at room temperature. After 1 h of incubation, 10 mM EDTA and 1% lauryl sarcosine were added to stop nuclease activity. Virus was concentrated by centrifugation at 82,000 × g for 6 h at 4°C in a Ty 50.2 rotor (Beckman Coulter, Fullerton, CA). The pellet was resuspended in phosphate-buffered saline (PBS) and further purified by centrifugation at 175,000 × g for 6 h at 4°C through a sucrose gradient (7.5 to 45%) in an SW55 Ti rotor (Beckman). The final virus-containing pellets were resuspended in 100 μl PBS. The virus titer was determined by plaque assay on RAW 264.7 cells. Viral protein was measured by Bradford reagent (Sigma Chemical Co., St. Louis, MO).
High-pressure treatment of MNV-1 in aqueous cell culture medium.
The pH values of DMEM were adjusted to 4.0 and 7.0 by citric acid because it is often used as a food additive to lower the pH of food products. One-milliliter aliquots of MNV-1 stock (108 PFU/ml) in DMEM were placed in sterile polyethylene stomacher pouches (Fisher Scientific International, Ontario, Canada) and heat sealed using an Impulse sealer (American International Electric Co., Whittier, CA). The samples were treated at 200, 250, 300, 350, 400, and 450 MPa for 2 min at 4 or 20°C using a pressure unit with temperature control (model Avure PT-1; Avure Technologies, Kent, WA). All temperatures reported in this study were initial sample temperatures. The pressure come-up rate was approximately 22 MPa/s. The pressure release was almost immediate (<4 s). Pressurization time did not include the pressure come-up or release time. After processing, the bags were removed and cooled in an ice bath, and the surviving viruses in the samples were quantified by viral plaque assay.
High-pressure treatment of MNV-1 in fresh produce.
Fresh iceberg lettuce and strawberries were purchased from a local supermarket. Strawberries were homogenized into purees. Fresh lettuce and strawberries were cut into pieces. All fresh produce was placed into sterile polyethylene pouches. The MNV-1 cocktail was inoculated into each bag to achieve a final inoculation level of approximately 107 PFU/g. The contents in each bag were mixed thoroughly in a biosafety cabinet for 1 h to allow for virus attachment, binding, and internalization, and then the bags were vacuum sealed. All samples were pressurized at 350, 400, and 450 MPa at 4 or 20°C for 2 min. After processing, the bags were removed and cooled in an ice bath, and the surviving virus was eluted and quantified by plaque assay as described above. In addition, uninoculated blueberries, raspberries, strawberries, strawberry puree, and lettuce were pressurized at 350 and 600 MPa in 4°C for 2 min. After processing, these samples were subjected to sensory evaluation and visual assessment.
High-pressure treatment of MNV-1 in fruit purees.
Using strawberry puree as a model food matrix, the effect of pH on virus inactivation was determined. The natural pH of strawberry puree is around 3.5. The pH of strawberry puree was adjusted to 2.5, 4.5, 5.5, and 6.5 using either citric acid or sodium hydroxide. The samples with pH values of 2.5 to 6.5 were inoculated with MNV-1 to a final inoculation level of approximately 106 PFU/g and subjected to HPP for 2 min at 400 MPa and 4°C. The surviving virus was determined by plaque assay. In addition, to determine the role of food matrix in virus inactivation, a number of other fruit and vegetable purees and juices with different natural pH values were also used in pressure treatment. These purees included lemon (pH 2.5), strawberry (pH 3.5), tomato (pH 4.5), watermelon (pH 5.3), and carrot (pH 5.8) in addition to carrot juice (pH 6.3). Two grams of each food sample was placed in a stomacher pouch, and the MNV-1 cocktail was inoculated to achieve a final inoculation level of approximately 107 PFU/g. The samples were pressurized at 400 MPa and 450 MPa for 2 min at 4°C, and the surviving viruses were determined by plaque assay.
Transmission electron microscopy.
Negative-staining electron microscopy of purified virions was performed to determine whether HPP damages the virus particles. Sixty microliters of highly purified MNV-1 suspension was treated by HPP at 350, 500, and 600 MPa at 4°C for 2 min. A viral plaque assay was conducted to confirm the inactivation of virus. Aliquots (20 μl) of either HPP-treated or untreated samples were fixed in copper grids (Electron Microscopy Sciences, Hatfield, PA) and negatively stained with 1% ammonium molybdate. Virus particles were visualized by an FEI Tecnai G2 Spirit transmission electron microscope (TEM) at 80 kV at the Microscopy and Imaging Facility at the Ohio State University. Images were captured on a MegaView III side-mounted charge-coupled-device (CCD) camera (Soft Imaging System, Lakewood, CO), and figures were processed using Adobe Photoshop software (Adobe Systems, San Jose, CA).
RT-PCR.
Viral RNA was extracted from MNV-1 suspensions using an RNeasy minikit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Reverse transcription-PCR (RT-PCR) was performed using a One Step RT-PCR kit (Qiagen). Two primers (5′-ATGAGGATGAGTGATGGCGC-3′ and 5′-TTATTGTTTGAGCATTCGGCC-3′) were designed to target the viral capsid VP1 gene. The amplified products were analyzed by 1% agarose gel electrophoresis.
Analysis of viral proteins by SDS-PAGE.
Two micrograms of highly purified MNV-1 suspensions (either HPP treated at 600 MPa at 4°C for 2 min or untreated) was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Samples were boiled for 5 min in loading buffer containing 1% SDS, 2.5% β-mercaptoethanol, 6.25 mM Tris-HCl (pH 6.8), and 5% glycerol and loaded onto a 12% polyacrylamide gel. Viral proteins were visualized by Coomassie blue staining.
Western blotting.
Two micrograms of highly purified MNV-1 suspensions (either HPP treated at 600 MPa at 4°C for 2 min or untreated) was separated by 15% SDS-PAGE and transferred to a Hybond ECL nitrocellulose membrane (Amersham) in a Mini Trans-Blot electrophoretic transfer cell (Bio-Rad). The blot was probed with rabbit anti-MNV VP1 polyclonal antibody (a generous gift from H. W. Virgin IV) at a dilution of 1:10,000 in blocking buffer (5% skim milk), followed by horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG secondary antibody (Santa Cruz Biotechnology) at a dilution of 1:20,000. The blot was developed with SuperSignal West Pico chemiluminescent substrate (Thermo Scientific) and exposed to Kodak BioMax MR film (Kodak).
Statistical analysis.
All experiments were carried out in triplicate. Virus survival was expressed as mean log titer ± standard deviation. Statistical analysis was performed by one-way multiple comparisons using SPSS 8.0 statistical analysis software (SPSS Inc., Chicago, IL). A P value of <0.05 was considered statistically significant.
RESULTS
Pressure inactivation of MNV-1 in aqueous medium.
In order to determine the effectiveness of the inactivation of MNV-1 by HPP, we first aimed to investigate the survival of MNV-1 in aqueous cell culture medium after pressurization. DMEM was inoculated with MNV-1 and was treated at different pressures, pHs, and temperatures. The kinetics of MNV-1 inactivation by HPP is shown in Fig. 1. Clearly, pressure magnitude, pH value, and treatment temperature affected the effectiveness of virus pressure inactivation. As expected, MNV-1 was efficiently inactivated at higher pressure levels. At each pressure level, it became easier for MNV-1 to be inactivated at 4°C than at 20°C (P < 0.05). For instance, an 8.1-log virus reduction was observed at 4°C when the sample was treated under 350 MPa at pH 7.0. However, only 4.1-log virus reductions were achieved at 20°C under the same treatment condition. To determine the effect of pH on pressure inactivation of virus, the pH of DMEM was adjusted to 4.0 using citric acid. MNV-1 was more efficiently inactivated in a neutral environment (pH 7.0) than in an acidic environment (pH 4.0) at both 4 and 20°C throughout the pressure range. For example, at pH 7.0, the virus was almost completely killed (>8-log reductions) by 350 MPa of pressure at 4°C, whereas only a 6.0-log virus reduction was observed at pH 4.0 when other conditions were the same. Taken together, these results clearly demonstrated that the effectiveness of virus inactivation in the medium was significantly affected by pressure, pH, and temperature. It can be concluded that the optimal conditions for virus inactivation are a minimum pressure level of 350 MPa, pH 7.0, and a temperature of 4°C in cell culture medium. These results also suggested that HPP may be a promising technology to inactivate viruses in foods.
FIG. 1.
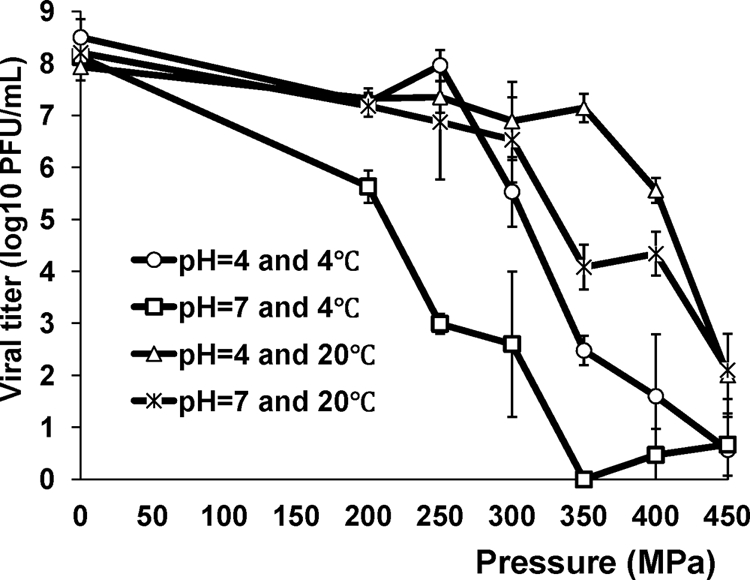
Effect of temperature and pH on the pressure inactivation of MNV-1 in aqueous cell culture medium. MNV-1 stock (108 PFU/ml) in DMEM was adjusted to pH 4.0 or 7.0 using citric acid. Samples were subjected to pressure treatment ranging from 200 MPa to 450 MPa for 2 min at either 4°C or 20°C. The survival of MNV-1 was determined by plaque assay. Data are the means of three replicates. Error bars represent ±1 standard deviation.
Pressure inactivation of MNV-1 in fresh produce.
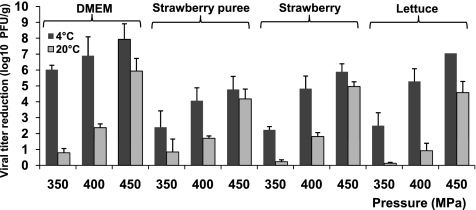
Fresh vegetables and fruits were chosen as the food matrices, since they are the major vehicles to transmit NoVs (2, 4, 7, 11, 19, 24, 43, 47). Available evidence suggests that food-borne viruses can attach tightly and be internalized in fresh vegetables and fruits (48, 58, 59). The internalized viruses cannot be removed by traditional washing and sanitization strategies. To determine whether HPP can effectively inactivate MNV-1 in fresh produce, fresh lettuce, fresh-cut strawberries, and strawberry puree were inoculated with MNV-1 to a final concentration of approximately 107 PFU/g and then subjected to HPP at pressures ranging from 350 to 450 MPa at either 4 or 20°C for 2 min. These pressures were chosen since MNV-1 can effectively be inactivated in the medium under these conditions. As shown in Fig. 2, significant inactivation of MNV-1 was observed in all three fresh produce types. At 4°C and 350 MPa, 2.4-, 2.2-, and 2.4-log virus reductions were achieved in fresh lettuce, fresh-cut strawberry, and strawberry puree, respectively. When the pressure was increased to 400 and 450 MPa, virus inactivation was significantly improved. At 450 MPa and 4°C, 4.7- to 7.0-log virus reductions were achieved in all samples. Statistics analysis showed there was no significant difference in the efficiency of virus inactivation across the three types of fresh produce (P > 0.05). However, MNV-1 was more easily inactivated in aqueous medium than fresh produce (P < 0.05). At 350 MPa of pressure, 6.0- and 8.1-log reductions were observed in aqueous medium at pH 4.0 and pH 7.0, respectively (Fig. 1). These results suggested that some components (as yet undefined) of fresh produce may provide a protective effect for MNV-1 inactivation.
FIG. 2.
Effectiveness of MNV-1 inactivation in DMEM, strawberry puree, and fresh-cut strawberries and lettuces by HPP. Fresh-cut iceberg lettuce and strawberries and strawberry puree were inoculated with MNV-1 to a final inoculation level of approximately 107 PFU/g and pressure treated at 350 MPa, 400 MPa, and 450 MPa at either 4°C or 20°C for 2 min. The surviving viruses were determined by plaque assay. Log virus reduction = log (titers of untreated samples) − log (titers of pressurized samples). Data are the means of three replicates. Error bars represent ±1 standard deviation.
The inactivation efficiency was also dependent on the treatment temperature in fresh produce samples. MNV-1 was significantly more sensitive to HPP at 4°C than at 20°C in fresh produce (P < 0.05) across the pressure spectrum, which is consistent with the results for aqueous medium. For example, at 4°C and 450 MPa, approximately 4.7- to 7.0-log virus reductions were found in all three products. This was significantly more efficient than a higher temperature (20°C), which achieved only 4.1- to 4.9-log reductions under the same pressure. Taken together, these results demonstrated that MNV-1 can be more effectively killed at a subambient temperature of 4°C when subjected to a moderate pressure level of 450 MPa.
Effect of pH on pressure inactivation of MNV-1 in strawberry puree.
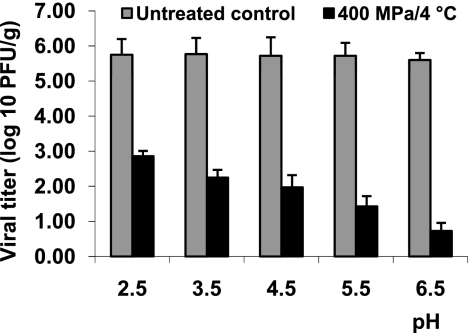
The pH of fruits and vegetables typically ranges from 2.5 to 6.5. Our results showed that MNV-1 was more sensitive to HPP at higher pH in cell culture medium (Fig. 1). To further investigate the role of pH on virus inactivation in fruits and related products, strawberry puree was selected as the model food matrix for HPP. The natural pH of strawberry puree is about 3.5. The pH values of strawberry puree were artificially adjusted from 2.5 to 6.5 using either citric acid or sodium hydroxide, and the samples were subjected to HPP for 2 min at 400 MPa and 4°C. Without pressure treatment, MNV-1 was highly stable in strawberry purees within the pH values ranging from 2.5 to 6.5. Exposure of MNV-1 under an acidic condition for 24 h did not reduce the viral titer (data not shown). As shown in Fig. 3, MNV-1 was easily inactivated at higher pH in strawberry puree samples, which is consistent with the previous observation from the aqueous medium. A 4.8-log virus reduction was achieved at pH 6.5; however, only a 2.8-log virus reduction was reached at pH 2.5. Taken together, these results demonstrated that MNV-1 was more sensitive to HPP at higher pH in both strawberry and aqueous medium.
FIG. 3.
Effect of pH on pressure inactivation of MNV-1 in strawberry purees. The pH values of strawberry purees (natural pH is around 3.5) were adjusted to between 2.5 and 6.5 by either citric acid or sodium hydroxide, inoculated with MNV-1 to a final inoculation level of approximately 106 PFU/g, and treated at 400 MPa for 2 min at 4°C. The surviving viruses were determined by plaque assay. Data are the means of three replicates. Error bars represent ±1 standard deviation.
Effectiveness of MNV-1 inactivation by HPP in different purees.
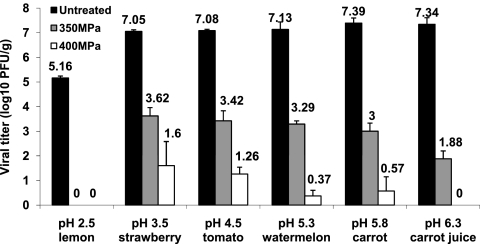
It is necessary to determine the effectiveness of MNV-1 inactivation in different purees, since they have different pH values and the food matrix may play a role in virus inactivation. Therefore, the MNV-1 survival was determined in a number of purees and juices at two pressures (350 and 400 MPa) at 4°C for 2 min. These food samples included lemon (pH 2.5), tomato (pH 4.5), strawberry (pH 3.5), watermelon (pH 5.3), carrot (pH 5.8), and carrot juice (pH 6.3). With the exception of lemon puree, the effectiveness of virus inactivation appeared to be correlated with the natural pH in foods (Fig. 4). The higher the pH of the food, the greater the log reduction of the virus. At 350 MPa, 4.3- and 4.7-log virus reductions were achieved in carrot puree (pH 5.8) and carrot juice (pH 6.3), respectively, while only 3.4 logs of reduction were observed in strawberry puree (pH 3.5) (P < 0.05). At 400 MPa, 6.8-log PFU/ml of virus was inactivated in carrot puree, which is significantly higher than the result for strawberry puree (5.4-log reduction) (P < 0.05). Surprisingly, a 5.4-log virus reduction was achieved in lemon puree at 350 MPa even at extremely low pH (2.5) (Fig. 4). It should be noted that the viral titer reduction (5.4 log) in lemon puree was higher than that of strawberry puree in which the pH was artificially modified to 2.5, after the same pressure processing (Fig. 3). These results provided strong evidence that the food matrix plays an important role in protecting virus from inactivation. Collectively, the results suggested that both pH and food matrix affected MNV-1 inactivation in purees.
FIG. 4.
Pressure inactivation of MNV-1 in different purees. Purees were inoculated with MNV-1 to a final inoculation level of approximately 107 PFU/g and pressure treated at either 350 MPa or 400 MPa at 4°C for 2 min. The purees used include lemon (pH 2.5), strawberry (pH 3.5), tomato (pH 4.5), watermelon (pH 5.3), carrot (pH 5.8), and carrot juice (pH 6.3). High-pressure treatments of inoculated purees were performed at either 350 MPa or 400 MPa at 4°C for 2 min. The surviving viruses were determined by plaque assay. Data are the means of three replicates. Error bars represent ±1 standard deviation.
Visual assessment of pressure-treated fresh produce.
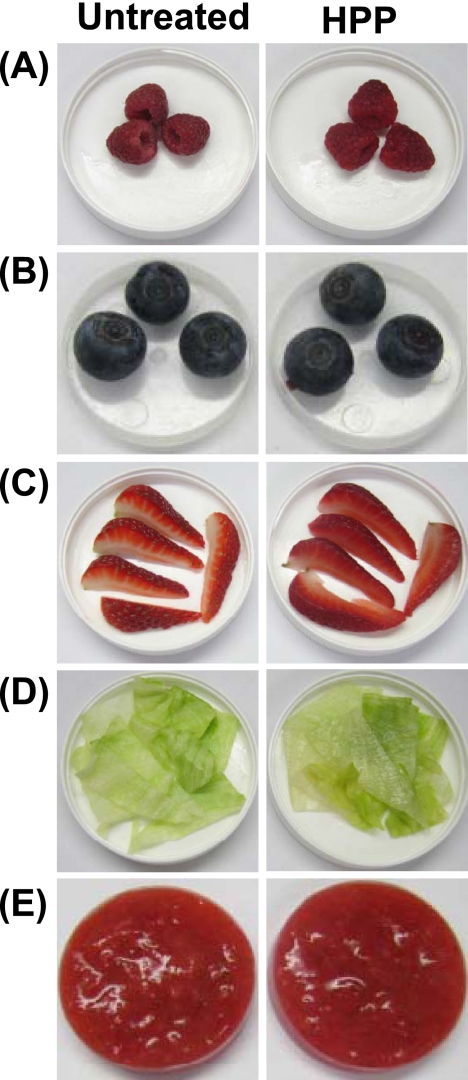
Fresh strawberries, raspberries, blueberries, lettuce, and strawberry puree were selected as models to evaluate the possible change in quality attributes of foods after HPP. Samples were treated at 350 MPa and 4°C for 2 min. Visual examination showed that processed products retained the freshness and the original color (Fig. 5). Strawberries displayed a slight color change. Strawberries had partially lost the opacity of the inner white tissue, appearing more translucent. In addition, the color of their seeds changed from green to tan. The influence of treatment on the food texture was product dependent. For example, blueberries, raspberries, and strawberries underwent a minor textural change, with slight softening of the tissue. In the case of lettuce, although the crunchiness and crispiness were mostly preserved after pressure treatment, the leaves had partially lost their opacity, acquiring a more glassy or translucent appearance. Thus, HPP may affect the quality of leafy greens. There were no perceived changes in the appearance of strawberry purees after processing, but they appeared to have a lighter consistency and thickness than the untreated purees. These observations suggested that HPP had a minimal impact on the quality of the tested fresh produce (except lettuce) but that different foods may have different responses to processing. At 600 MPa, HPP did not significantly affect the color, freshness, and texture of blueberries and strawberry puree although lettuce, strawberries, and raspberries underwent considerable textural loss due to pressure softening of the tissue (see Fig. S1 in the supplemental material).
FIG. 5.
Visual assessment of fresh produce processed by HPP. Raspberries (A), blueberries (B), fresh-cut strawberries (C), lettuce (D), and strawberry puree (E) were used as models to evaluate the effect of HPP on food quality. Samples were processed at 350 MPa at 4°C for 2 min.
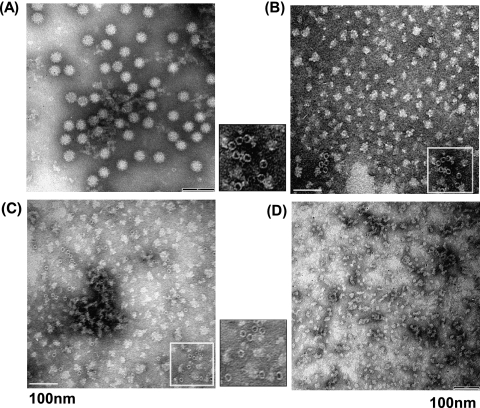
HPP disrupts virus particles.
To gain insight into the mechanism of virus inactivation by HPP, highly purified MNV-1 was treated at 350, 500, and 600 MPa at 4°C for 2 min. At 350 and 500 MPa, there were approximately 1-log virus survivors. Under these conditions, a mixture of inactivated and intact viruses may be observed. We chose a pressure of 600 MPa since MNV-1 was completely killed under this condition, thus allowing us to precisely determine the mechanism of virus inactivation by HPP. The virus particles were visualized by electron microscopy. As shown in Fig. 6 A, unpressurized MNV-1 particles were small round-structured virions approximately 30 to 38 nm in diameter. At a pressure of 350 MPa, we found a large amount of irregularly shaped particles with sizes ranging from 5 to 30 nm (Fig. 6B). In addition, we saw some empty particles with sizes of approximately 10 to 15 nm in diameter. These particles still retained a round-structured shape but were smaller. Most likely, the viral genomic RNA was spilled out by HPP, which resulted in empty particles. At a pressure of 500 MPa, we also saw a large amount of irregularly shaped particles, protein debris, and some small empty particles (Fig. 6C). At 600 MPa, only small protein debris, but very few empty particles, was observed (Fig. 6D). In all three pressure treatments, we failed to observe any small round-structured virions with a size of 30 to 38 nm (Fig. 6B to D). Because electron microscopy (EM) analysis requires a high number of particles (usually 104 to 106 per ml), it is not surprising that we failed to find any intact virus particles since the majority of viruses were killed at 350 MPa. Therefore, this result demonstrated that the structure of the virion and the capsid was gradually disrupted when pressure was increased.
FIG. 6.
HPP disrupts virus particles. Purified MNV-1 was completely inactivated at 350, 500, and 600 MPa at 4°C for 2 min. Treated and untreated virus particles were negatively stained with 1% ammonium molybdate and visualized by a transmission electron microscope. (A) Untreated MNV-1 virion. (B) MNV-1 particles treated with 350 MPa. (C) MNV-1 particles treated with 500 MPa. (D) MNV-1 particles treated with 600 MPa.
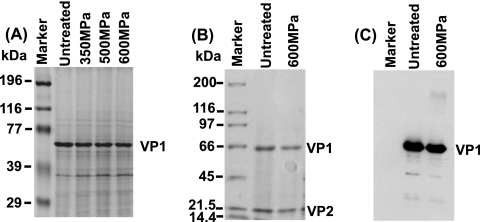
HPP disrupts the structure of the viral capsid but not the primary and secondary structures of the VP1 protein.
Norovirus is a nonenveloped positive-sense RNA virus. The virus particle is composed of extremely stable capsid proteins that protect the viral genetic material, RNA. It was known that MNV possesses two capsid proteins, the major capsid protein (VP1) and the minor capsid protein (VP2) (6, 31). VP1 plays many essential roles in viral infection, including attachment, entry, packaging of mature virion, and release, whereas VP2 protein plays a role in stabilizing the VP1 capsid structure by preventing the virus particle from degradation and disassembly (6, 31). To investigate whether HPP treatment destroyed the viral capsids, equal amounts of viral proteins (before and after HPP) were analyzed by SDS-PAGE. As shown in Fig. 7 A, we observed a 58-kDa VP1 protein band in the untreated sample in a 10% SDS-PAGE gel. Surprisingly, we detected an equivalent amount of VP1 protein from 350-, 500-, and 600-MPa-pressure-treated samples, demonstrating that the viral major capsid protein was not degraded by HPP. To visualize the minor capsid protein (VP2), we analyzed the virus samples in a 12% SDS-PAGE gel. As expected, two protein bands (VP1 and VP2) with molecular sizes of approximately 58 and 18 kDa, respectively, were visualized (Fig. 7B, lane 2). Neither of the viral capsid proteins (VP1 and VP2) was degraded (Fig. 7B, lane 3) after 600-MPa pressure treatment despite the fact that viruses were completely inactivated and virus structure was completely disrupted under this condition (Fig. 6D). These results suggested that HPP treatment may not affect the primary and secondary protein structures of the viral capsid proteins but damages the tertiary and quaternary structures of the viral capsids, which results in the disruption of the virion.
FIG. 7.
HPP does not degrade viral capsid proteins. (A) Visualization of MNV-1 capsid protein by 12% SDS-PAGE. The purified MNV-1 was pressurized at 350, 500, and 600 MPa at 4°C for 2 min. Total viral proteins were analyzed by 12% SDS-PAGE followed by Coomassie staining. VP1, major capsid protein. (B) Visualization of MNV-1 capsid protein by 15% SDS-PAGE. The purified MNV-1 was pressurized at 600 MPa at 4°C for 2 min. Total viral proteins were analyzed by 15% SDS-PAGE followed by Coomassie staining. VP2, minor capsid protein. (C) Western blot analysis of VP1 protein. Identical samples from panel B were separated by SDS-PAGE and subjected to Western blotting using rabbit anti-MNV VP1 polyclonal antibody.
To determine whether pressurized viral proteins were still antigenic, we performed Western blot analysis using a polyclonal antibody against the VP1 protein. Briefly, total viral proteins before and after pressure (600 MPa for 2 min) treatment were analyzed by SDS-PAGE, followed by Western blotting. As shown in Fig. 7C, similar amounts of VP1 from untreated and treated samples were detected by Western blotting. This result confirmed that pressure-treated VP1 still reacted with antibody despite the complete disruption of the three-dimensional structure of VP1.
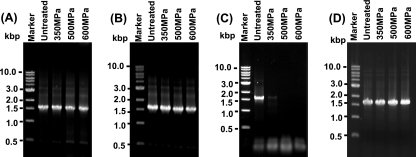
HPP does not degrade viral genomic RNA.
It would be lethal to the virus if the viral genome was damaged. Therefore, RT-PCR was performed to determine whether HPP damages the viral RNA. Two primers were designed to amplify the VP1 gene from viral genomic RNA. Since viral genomic RNA was extremely sensitive to environmental RNase, all experiments were done under RNase-free conditions. As expected, a strong 1.5-kb DNA band (VP1 gene) was observed in RNA extracted from untreated viruses (Fig. 8 A, lane 2). Similar amounts of the VP1 gene were detected in RNA extracted from viruses treated with 350, 500, and 600 MPa (Fig. 8A, lanes 2 to 4). This result suggests that viral genomic RNA was not degraded by HPP, at least under the conditions of 600 MPa for 2 min. To further confirm this result, we first extracted the viral genomic RNA from the virus and then subjected it to 350-, 500-, and 600-MPa treatment. As shown in Fig. 8B, similar amounts of VP1 were amplified from each sample, suggesting that viral genomic RNA was not degraded by HPP. Next, we purified some virus samples for which the final elution buffer (PBS) was not treated with diethyl pyrocarbonate (DEPC), a reagent that inactivates the RNase. Presumably, these virus samples were contaminated by environmental RNase. All other remaining steps were done under RNase-free conditions. These viruses were treated by three pressure levels, and viral RNA was extracted, followed by RT-PCR. As shown in Fig. 8C, only a weak VP1 band was observed at a pressure level of 350 MPa, and no VP1 gene was detected at a pressure level of 500 or 600 MPa (Fig. 8C). This result suggests that genomic RNA was liberated when the capsid was disrupted by HPP, which in turn was degraded by endogenous RNase. For virus-contaminated foods and environmental samples, we may not be able to detect any viral RNA genome after viruses are completely inactivated by HPP, since many endogenous RNases exist in foods and the environment. Finally, we also treated the VP1 gene (double-stranded DNA) with three pressure levels. As shown in Fig. 8D, HPP does not degrade the VP1 gene. Altogether, these experiments demonstrated HPP that does not degrade viral nucleic acids.
FIG. 8.
HPP does not degrade viral genomic RNA. (A) Detection of VP1 gene from pressure-treated MNV by RT-PCR. Viral genomic RNA was extracted from either HPP-treated or untreated MNV-1. The VP1 gene of MNV-1 was amplified by one-step RT-PCR, and PCR products were visualized by 1% agarose gel electrophoresis. (B) Detection of VP1 gene from pressure-treated viral genomic RNA by RT-PCR. Viral genomic RNA was extracted from MNV, and an equal amount of RNA was treated by HPP followed by RT-PCR. (C) Detection of VP1 gene from pressure-treated MNV contaminated by environmental RNases. MNV was purified and eluted in PBS buffer (not treated by DEPC). All other remaining steps were done under RNase-free conditions. These viruses were treated with three pressure levels, and viral RNA was extracted; this process was followed by RT-PCR. (D) Direct treatment of VP1 gene by HPP. The VP1 gene was amplified by RT-PCR, and an equal amount of purified VP1 gene was treated by HPP.
DISCUSSION
Fresh produce is one of the high-risk foods for NoV contamination. Available evidence suggests that NoV becomes internalized and disseminated in fresh produce (48, 58, 59). None of the decontamination methods investigated thus far has been shown to effectively inactivate NoV in fresh produce (18, 20, 22, 40, 45, 52). In this study, we systematically investigated the survival of a human NoV surrogate (MNV-1) by HPP. We found that MNV-1 was efficiently inactivated at optimized HPP parameters with minimal impact on the quality of certain fruits or fruit products. We further provided new mechanistic insight into virus inactivation by HPP. Our results suggest that HPP may be a novel intervention technology appropriate for processing select fruits and vegetables intended for frozen storage as well as related products such as purees, sauce, and juices.
Susceptibility of MNV-1 to high pressure and the optimal inactivation condition.
Pressure, holding time, temperature, pH, and food matrix affect the effectiveness of virus inactivation by HPP. Generally speaking, the higher the pressure and the longer the holding time, the more effective inactivation becomes. However, consumer perception of food safety depends not only on microbial safety but also on food quality. Based on our results, MNV-1 can be effectively inactivated by HPP. The optimal conditions for inactivation of MNV-1 are refrigeration temperature and neutral pH with a treatment pressure of 450 MPa and a holding time of 2 min. Under this condition, 7.4-log and 4.7- to 7.0-log virus reductions were achieved in DMEM and fresh produce, respectively, without significantly altering the physical quality of the food samples.
It should be noted that the susceptibilities of different viruses to HPP were significantly different. For instance, FCV can be efficiently inactivated (5-log reductions) at 300 MPa at ambient temperature for 3 min (16, 27). For rotavirus, it has been reported that an 8-log virus reduction was achieved at 300 MPa and 25°C for 2 min in cell culture medium (34). HAV can achieve more than 6 logs of reduction at 450 MPa at ambient temperature for 5 min in cell culture medium (36, 37). Surprisingly, no virus reduction was observed for poliovirus or Aichi virus even at 600 MPa at ambient temperature for 5 min in cell culture medium (39, 60). Therefore, human NoV surrogates (such as MNV-1 and FCV) and the two cultivable food-borne viruses (HAV and rotavirus) can be efficiently inactivated by HPP between 500 and 600 MPa. Under this pressure range, bacterial vegetative cells, yeasts, and molds were also killed (15, 17), while bacterial spores can still survive above 1,000 MPa (16, 17, 25). Taken together, these results suggest that HPP is capable of inactivating most common bacterial and viral agents with a minimal impact on the quality of certain food products.
Effect of food matrix on virus inactivation.
The food matrix and type of substrate have a dramatic effect on the survival of pathogens during pressure treatment. Carbohydrates, proteins, ions, lipids, and other food constituents can offer a protective effect (3, 5, 25, 36). Under 450 MPa at pH 4.0 and 4°C, there were almost 8-log virus reductions in DMEM, while only 5.8- and 4.7-log reductions were observed in strawberries and strawberry puree, respectively. In another example, we found that MNV-1 was more sensitive to HPP at higher pH in aqueous medium than in all food samples. However, lemon puree is a special case. Specifically, a large virus reduction was detected in lemon puree at 350 MPa even at an extremely low pH (2.5). In contrast, MNV-1 was more difficult to inactivate in strawberry puree when the pH was adjusted to 2.5 (Fig. 3). Perhaps food constituents of lemon puree confer lesser protection on MNV-1 than those of other types of purees. Another possibility is that some food components in lemon may directly inactivate MNV-1. These results demonstrated that the food matrix plays an important role in virus inactivation by HPP. It has been reported that sucrose, Ca2+, and other cations protect bacteria from inactivation (3, 17). Recently, Kingsley and Chen reported that a supplement of sodium chloride in liquid solution protected HAV and FCV from high-pressure inactivation (16, 36, 38). It is possible that salts may stabilize viral capsid proteins at high pressures.
Effect of temperature on virus inactivation.
In both aqueous medium and food samples, we found that MNV-1 was more susceptible to HPP at a refrigerated temperature of 4°C than at an ambient temperature of 20°C. Under 350 MPa at pH 7.0, an 8.1-log virus reduction was observed at 4°C in aqueous medium. However, only a 4.1-log virus reduction was achieved at 20°C under the same pressure and pH. Under 450 MPa at 4°C, approximately 5- to 7-log virus reductions were found in all fresh produce, but only 4- to 5-log reductions were observed at 20°C (Fig. 2). Similar phenomena were observed for FCV (16, 26, 27). Perhaps low temperature may promote the exposure of nonpolar side chains to water and increase the density of water molecules in a protein solvation cage to maximize protein denaturation. In contrast, HAV, a picornavirus, is more resistant to HPP at a lower temperature than at room temperature (13, 36, 37). Thus, different viruses may have different responses to temperature during HPP.
Effect of pH on virus inactivation.
Our results demonstrated that pH is a critical parameter that influences the survival of MNV-1 after HPP. Specifically, we found that MNV-1 was more efficiently inactivated in a neutral pH environment than an acidic environment either at 4°C or at 20°C across the pressure range studied. In aqueous medium, MNV-1 was completely killed (8.1-log reductions) by 350 MPa of pressure at 4°C and pH 7.0, whereas only a 6.0-log virus reduction was observed at pH 4.0. In the same food matrix (strawberry puree), a 4.8-log virus reduction was achieved at pH 6.5; however, only a 2.8-log virus reduction was observed at pH 2.5. For different purees, a 4.3-log virus reduction was achieved in carrot puree (pH 5.8) at 350 MPa, whereas only a 3.4-log reduction was observed in strawberry puree (pH 3.5). This finding will be helpful to determine an optimal pressure to treat food products with different pH values. Our observation is consistent with the previous reports for FCV (16) and tobacco mosaic virus (8, 51), which were also more resistant to HPP at lower pH. In contrast, a significantly enhanced inactivation of HAV was found in acidic environments (35-37). It is unclear why pH has an opposite effect on pressure inactivation for different viruses. It is likely that the nature of viral capsid protein determines the susceptibility of virus to pH.
Mechanism of virus inactivation by HPP.
Our study also provided a mechanistic insight into virus inactivation by HPP. For bacterial pathogens, it was reported that HPP denatured bacterial proteins and lipids, destroyed the integrity of bacterial membranes, inhibited key enzymes, and disrupted transcription and translation and cellular functions responsible for survival and binary fission (3, 15, 17). However, the mechanism of virus inactivation by HPP is poorly understood. Although it has been suggested that virus inactivation by HPP may include the damage of viral proteins (13, 16, 36, 37, 46), the detailed mechanism of inactivation has not been experimentally demonstrated.
HPP affects only noncovalent bonds, such as ionic, hydrophobic, and hydrogen bonds (9, 25, 30), which suggests that primary and secondary protein structures remain intact. However, HPP may alter quaternary and tertiary structures and/or conformation of a protein that results in a misfolding or unfolding. In our study, we found that the two structural proteins of MNV-1 (VP1 and VP2) were not degraded by HPP from a 350- to 600-MPa pressure range (Fig. 7A and B), although MNV-1 was completely inactivated at 600 MPa for 2 min. However, the structure of the virion and viral capsid was completely destroyed, as shown by EM analysis (Fig. 6B). It is worth noting that pressure-treated VP1 protein still reacted with antibody, suggesting that VP1 was antigenic. This evidence supports the hypothesis that the primary and secondary structures of viral proteins remained intact but that the quaternary and tertiary structures of viral proteins were damaged. It has been known that expression of VP1 capsid protein resulted in virus-like particles that are morphologically and structurally similar to native virions (31). The minor capsid protein stabilizes the structure of VP1 (6). HPP may have affected the proper folding of VP1 and VP2, which in turn destroyed the three-dimensional structure of the proteins and thus disrupted the small round-structured virions. Most recently, it has been shown that HPP affected the receptor binding of the MNV-1 capsid, which led to the loss of viral infectivity (55). However, our study clearly demonstrated that the damage of the viral capsid structure is the primary mechanism of virus inactivation. Disruption of the viral capsid would likely impair the functions of capsid such as attachment, receptor binding, and virus entry.
It is known that HPP usually does not affect covalent bonds. Thus, it may not break down viral genomic RNA. Previously, Tang et al. (55) showed that there was a significant concentration of the VP1 gene detected from HPP-treated MNV-1. This is not surprising, since they reported a 3-log virus survival post-HPP treatment. Sánchez et al. used a quantitative RT-PCR (RT-qPCR) method to evaluate the survival of MNV and human NoV genogroup II.4 and showed that the RNA of human NoV was more resistant to HPP (up to 500 MPa) than the RNA of MNV (50). However, it is not clear whether their experiments were performed under RNase-free conditions. In contrast, our RT-PCR was performed using RNA samples from the completely inactivated viruses after 600 MPa for 2 min. There were equivalent amounts of VP1 gene amplified from untreated and treated viruses. A similar result was obtained when viral genomic RNA was directly treated by HPP. Thus, these results demonstrated that the viral genomic RNA was not degraded by HPP at a pressure range from 350 to 600 MPa for 2 min. Taken together, these results suggest that the primary mechanism of MNV-1 inactivation by HPP is the damage of viral capsid structure but not genomic RNA.
HPP may be a novel intervention to enhance the safety of fresh produce.
A number of commercial HPP applications have been successful, including raw bivalve shellfish, yogurt, avocado products, chopped onions, and ready-to-eat meat products (3, 13, 17, 27, 28). Our results suggest that HPP may be a practical processing intervention for the control of food-borne viruses in fresh-produce-related products, such as purees, sauces, and juices, as well as fruits intended for frozen storage. With the exception of lettuce, HPP at 350 MPa did not affect the sensory qualities of the tested fresh produce such as appearance, color, and aroma; a more severe textural loss was observed in lettuce, raspberries, and strawberries at 600 MPa. In the last two decades, there have been many NoV outbreaks associated with frozen fruits, including blueberries, raspberries, strawberries, and grapes (2, 4, 7, 11, 19, 24, 42, 47). These fruits are important ingredients for salads, cakes, purees, juices, smoothies, creams, ice creams, and other related products. Furthermore, viruses survive longer during frozen and chilled storage (21, 49). Measures used to control bacterial levels, such as decreasing temperature and lowering water activity, have no effect on viruses (4, 11, 23, 40). No reduction of MNV-1 was observed in frozen onions and spinach even after 6 months of freezing (4). Since HPP can efficiently inactivate both surface and internalized viruses, application of HPP in the fresh produce industry will significantly improve the safety of fresh produce.
In summary, our study demonstrated that human NoV surrogate MNV-1 is effectively inactivated by HPP under optimum processing conditions of moderate pressure level, refrigeration temperature, and neutral pH. HPP may be a promising intervention to eliminate and minimize the NoV risk in fresh produce and related products, with a minimal impact on food quality.
Supplementary Material
Acknowledgments
This study was partially supported by a special emphasis grant (2010-01498) from the USDA National Integrated Food Safety Initiative (J.L. is the principal investigator [PI] of this project, and H.C. is one of the co-PIs) and an award from the Center for Advanced Processing and Packaging Studies (CAPPS) to J.L.
We thank Herbert W. Virgin IV for his generous gift of MNV-1 and antibody. We thank members of J.L.'s laboratory for critical reviews of the manuscript.
Footnotes
Published ahead of print on 30 December 2010.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Adler, J. L., and R. Zickl. 1969. Winter vomiting disease. J. Infect. Dis. 119:668-673. [DOI] [PubMed] [Google Scholar]
- 2.Allwood, P. B., et al. 2004. Occurrence of Escherichia coli, noroviruses, and F-specific coliphages in fresh market-ready produce. J. Food Prot. 67:2387-2390. [DOI] [PubMed] [Google Scholar]
- 3.Baert, L., J. Debevere, and M. Uyttendaele. 2009. The efficacy of preservation methods to inactivate foodborne viruses. Int. J. Food Microbiol. 131:83-94. [DOI] [PubMed] [Google Scholar]
- 4.Baert, L., M. Uyttendaele, M. Vermeersch, E. Van Coillie, and J. Debevere. 2008. Survival and transfer of murine norovirus 1, a surrogate for human noroviruses, during the production process of deep-frozen onions and spinach. J. Food Prot. 71:1590-1597. [DOI] [PubMed] [Google Scholar]
- 5.Balny, C., P. Masson, and K. Heremans. 2002. High pressure effects on biological macromolecules: from structural changes to alteration of cellular processes. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1595:3-10. [DOI] [PubMed] [Google Scholar]
- 6.Bertolotti-Ciarlet, A., S. E. Crawford, A. M. Hutson, and M. K. Estes. 2003. The 3′ end of Norwalk virus mRNA contains determinants that regulate the expression and stability of the viral capsid protein VP1: a novel function for the VP2 protein. J. Virol. 77:11603-11615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beuchat, L. R. 1996. Pathogenic microorganisms associated with fresh produce. J. Food Prot. 59:204-216. [DOI] [PubMed] [Google Scholar]
- 8.Bonafe, C. F., et al. 1998. Tobacco mosaic virus disassembly by high hydrostatic pressure in combination with urea and low temperature. Biochemistry 37:11097-11105. [DOI] [PubMed] [Google Scholar]
- 9.Buckow, R., S. Isbarn, D. Knorr, V. Heinz, and A. Lehmacher. 2008. Predictive model for inactivation of feline calicivirus, a norovirus surrogate, by heat and high hydrostatic pressure. Appl. Environ. Microbiol. 74:1030-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butot, S., T. Putallaz, and G. Sánchez. 2007. Procedure for rapid concentration and detection of enteric viruses from berries and vegetables. Appl. Environ. Microbiol. 73:186-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butot, S., T. Putallaz, and G. Sánchez. 2008. Effects of sanitation, freezing and frozen storage on enteric viruses in berries and herbs. Int. J. Food Microbiol. 126:30-35. [DOI] [PubMed] [Google Scholar]
- 12.Butot, S., T. Putallaz, R. Amoroso, and G. Sánchez. 2009. Inactivation of enteric viruses in minimally processed berries and herbs. Appl. Environ. Microbiol. 75:4155-4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calci, K. R., G. K. Meade, R. C. Tezloff, and D. H. Kingsley. 2005. High-pressure inactivation of hepatitis A virus within oysters. Appl. Environ. Microbiol. 71:339-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cannon, J. L., et al. 2006. Surrogates for the study of norovirus stability and inactivation in the environment: a comparison of murine norovirus and feline calicivirus. J. Food Prot. 69:2761-2765. [DOI] [PubMed] [Google Scholar]
- 15.Chen, H., and D. G. Hoover. 2003. Pressure inactivation kinetics of Yersinia enterocolitica ATCC 35669. Int. J. Food Microbiol. 87:161-171. [DOI] [PubMed] [Google Scholar]
- 16.Chen, H., D. G. Hoover, and D. H. Kingsley. 2005. Temperature and treatment time influence high hydrostatic pressure inactivation of feline calicivirus, a norovirus surrogate. J. Food Prot. 68:2389-2394. [DOI] [PubMed] [Google Scholar]
- 17.Chen, H., D. Guan, and D. G. Hoover. 2006. Sensitivities of foodborne pathogens to pressure changes. J. Food Prot. 69:130-136. [DOI] [PubMed] [Google Scholar]
- 18.Cliver, D. O. 1997. Virus transmission via food. Food Technol. 51:71-78. [Google Scholar]
- 19.Cotterelle, B., et al. 2005. Outbreak of norovirus infection associated with the consumption of frozen raspberries, France, March 2005. Euro Surveill. 10:E050428.1. [DOI] [PubMed] [Google Scholar]
- 20.Doyle, M. P., and M. C. Erickson. 2008. Summer meeting 2007—the problems with fresh produce: an overview. J. Appl. Microbiol. 105:317-330. [DOI] [PubMed] [Google Scholar]
- 21.D'Souza, D. H., et al. 2006. Persistence of caliciviruses on environmental surfaces and their transfer to food. Int. J. Food Microbiol. 108:84-91. [DOI] [PubMed] [Google Scholar]
- 22.Duizer, E., et al. 2004. Inactivation of caliciviruses. Appl. Environ. Microbiol. 70:4538-4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Estes, M. K., B. V. Prasad, and R. L. Atmar. 2006. Noroviruses everywhere: has something changed? Curr. Opin. Infect. Dis. 19:467-474. [DOI] [PubMed] [Google Scholar]
- 24.Falkenhorst, G., et al. 2005. Imported frozen raspberries cause a series of norovirus outbreaks in Denmark, 2005. Euro Surveill. 10:E050922.2. [DOI] [PubMed] [Google Scholar]
- 25.Gross, M., and R. Jaenicke. 1994. Proteins under pressure: the influence of high hydrostatic pressure on structure, function and assembly of proteins and protein complexes. Eur. J. Biochem. 221:617-630. [DOI] [PubMed] [Google Scholar]
- 26.Grove, S. F., et al. 2006. Inactivation of foodborne viruses of significance by high pressure and other processes. J. Food Prot. 69:957-968. [DOI] [PubMed] [Google Scholar]
- 27.Grove, S. F., et al. 2008. Inactivation of hepatitis A virus, poliovirus and a norovirus surrogate by high pressure processing. Innov. Food Sci. Emerg. Technol. 9:206-210. [Google Scholar]
- 28.He, H., R. M. Adams, D. F. Farkas, and M. T. Morrissey. 2002. Use of high-pressure processing for oyster shucking and shelf-life extension. J. Food Sci. 67:640-645. [Google Scholar]
- 29.Heaton, J. C., and K. Jones. 2008. Microbial contamination of fruit and vegetables and the behaviour of enteropathogens in the phyllosphere: a review. J. Appl. Microbiol. 104:613-626. [DOI] [PubMed] [Google Scholar]
- 30.Heremans, R., and L. Smeller. 1998. Protein structure and dynamics at high pressure. Biochim. Biophys. Acta 1386:353-370. [DOI] [PubMed] [Google Scholar]
- 31.Jiang, X., M. Wang, D. Y. Graham, and M. K. Estes. 1992. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J. Virol. 66:6527-6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karst, S. M., C. E. Wobus, M. Lay, J. Davidson, and H. W. Virgin IV. 2003. STAT 1-dependent innate immunity to a Norwalk-like virus. Science 299:1575-1578. [DOI] [PubMed] [Google Scholar]
- 33.Katpally, U., C. E. Wobus, K. Dryden, H. W. Virgin IV, and T. J. Smith. 2008. Structure of antibody-neutralized murine norovirus and unexpected differences from viruslike particles. J. Virol. 82:2079-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khadre, M. A., and A. E. Yousef. 2002. Susceptibility of human rotavirus to ozone, high pressure, and pulsed electric field. J. Food Prot. 65:1441-1446. [DOI] [PubMed] [Google Scholar]
- 35.Kingsley, D. H., D. Guan, and D. G. Hoover. 2005. Pressure inactivation of hepatitis A virus in strawberry puree and sliced green onions. J. Food Prot. 68:1748-1751. [DOI] [PubMed] [Google Scholar]
- 36.Kingsley, D. H., and H. Chen. 2009. Influence of pH, salt, and temperature on pressure inactivation of hepatitis A virus. Int. J. Food Microbiol. 130:61-64. [DOI] [PubMed] [Google Scholar]
- 37.Kingsley, D. H., D. Hoover, E. Papafragkou, and G. P. Richards. 2002. Inactivation of hepatitis A virus and a calicivirus by high hydrostatic pressure. J. Food Prot. 65:1605-1609. [DOI] [PubMed] [Google Scholar]
- 38.Kingsley, D. H., D. R. Holliman, K. R. Calci, H. Chen, and G. J. Flick. 2007. Inactivation of a norovirus by high-pressure processing. Appl. Environ. Microbiol. 73:581-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kingsley, D. H., H. Chen, and D. Hoover. 2004. Inactivation of selected picornaviruses by high hydrostatic pressure. Virus Res. 102:221-224. [DOI] [PubMed] [Google Scholar]
- 40.Koopmans, M., and E. Duizer. 2004. Foodborne viruses: an emerging problem. Int. J. Food Microbiol. 90:23-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larson, J., R. Vender, and P. Camuto. 1994. Cholestatic jaundice due to ackee fruit poisoning. Am. J. Gastroenterol. 89:1577-1578. [PubMed] [Google Scholar]
- 42.Le Guyader, F. S., et al. 2004. Detection of noroviruses in raspberries associated with a gastroenteritis outbreak. Int. J. Food Microbiol. 97:179-186. [DOI] [PubMed] [Google Scholar]
- 43.Lynch, M. F., R. V. Tauxe, and C. W. Hedberg. 2009. The growing burden of foodborne outbreaks due to contaminated fresh produce: risks and opportunities. Epidemiol. Infect. 137:307-315. [DOI] [PubMed] [Google Scholar]
- 44.Mead, P. S., et al. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mormann, S., M. Dabisch, and B. Becker. 2010. Effects of technological processes on the tenacity and inactivation of norovirus genogroup II in experimentally contaminated foods. Appl. Environ. Microbiol. 76:536-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oliveira, A. C., et al. 1999. Low temperature and pressure stability of picornaviruses: implication for virus uncoating. Biophys. J. 76:1270-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pönkä, A., L. Maunula, C. H. von Bonsdorff, and O. Lyytikäinen. 1999. An outbreak of calicivirus associated with consumption of frozen raspberries. Epidemiol. Infect. 123:469-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rawsthorne, H., T. G. Phister, and L. A. Jaykus. 2009. Development of a fluorescent in situ method for visualization of enteric viruses. Appl. Environ. Microbiol. 75:7822-7827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rzezutka, A., and N. Cook. 2004. Survival of human enteric viruses in the environment and food. FEMS Microbiol. Rev. 28:441-453. [DOI] [PubMed] [Google Scholar]
- 50.Sánchez, G., R. Aznar, A. Martínez, and D. Rodrigo. Inactivation of human and murine norovirus by high-pressure processing. Foodborne Pathog. Dis., in press. [DOI] [PubMed]
- 51.Santos, J. L., J. A. Bispo, G. F. Landini, and C. F. Bonafe. 2004. Proton dependence of tobacco mosaic virus dissociation by pressure. Biophys. Chem. 111:53-61. [DOI] [PubMed] [Google Scholar]
- 52.Seymour, I. J., and H. Appleton. 2001. Foodborne viruses and fresh produce. J. Appl. Microbiol. 91:759-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sivapalasingam, S., C. R. Friedman, L. Cohen, and R. V. Tauxe. 2004. Fresh produce: a growing cause of outbreaks of foodborne illness in the United States, 1973 through 1997. J. Food Prot. 67:2342-2353. [DOI] [PubMed] [Google Scholar]
- 54.Steele, M., and J. Odemeru. 2004. Irrigation water as a source of foodborne pathogens on fruit and vegetables. J. Food Prot. 67:2839-2849. [DOI] [PubMed] [Google Scholar]
- 55.Tang, Q., et al. 2010. Mechanism of inactivation of murine norovirus-1 by high pressure processing. Int. J. Food Microbiol. 137:186-189. [DOI] [PubMed] [Google Scholar]
- 56.Thurston-Enriquez, J. A., C. N. Haas, J. Jacangelo, and C. P. Gerba. 2005. Inactivation of enteric adenovirus and feline calicivirus by chlorine dioxide. Appl. Environ. Microbiol. 71:3100-3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tuan Zainazor, C., et al. 2010. The scenario of norovirus contamination in food and food handlers. J. Microbiol. Biotechnol. 20:229-237. [PubMed] [Google Scholar]
- 58.Urbanucci, A., M. Myrmel, I. Berg, C. H. von Bonsdorff, and L. Maunula. 2009. Potential internalisation of caliciviruses in lettuce. Int. J. Food Microbiol. 135:175-178. [DOI] [PubMed] [Google Scholar]
- 59.Wei, J., Y. Jin, T. Sims, and K. E. Kniel. 2010. Manure- and biosolids-resident murine norovirus 1 attachment to and internalization by Romaine lettuce. Appl. Environ. Microbiol. 76:578-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilkinson, N., A. S. Kurdzeil, S. Langton, E. Needs, and N. Cook. 2001. Resistance of poliovirus to inactivation by high hydrostatic pressures. Innov. Food Sci. Emerg. Technol. 2:95-98. [Google Scholar]
- 61.Wobus, C. E., L. B. Thackray, and H. W. Virgin IV. 2006. Murine norovirus: a model system to study norovirus biology and pathogenesis. J. Virol. 80:5104-5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.