Abstract
The beta-rhizobium Cupriavidus taiwanensis forms indeterminate nodules on Mimosa pudica. C. taiwanensis bacteroids resemble free-living bacteria in terms of genomic DNA content, cell size, membrane permeability, and viability, in contrast to bacteroids in indeterminate nodules of the galegoid clade. Bacteroid differentiation is thus unrelated to nodule ontogeny.
Bacteria known as rhizobia cooperate with legumes in a mutualistic endosymbiosis of major ecological importance that accounts for about 25% of the global nitrogen cycling. Rhizobia induce the formation of root nodules on host plants, in which intracellular bacteria fix nitrogen for the benefit of the plant (2). Diversity characterizes the rhizobium-legume symbiosis. This symbiosis involves most of the 18,000 legume species of the Papilionoideae, Mimosoideae, and Caesalpinioideae subfamilies. Rhizobia are phylogenetically disparate bacteria distributed in many genera of the alpha- and betaproteobacteria (5, 10). In addition, many phenotypic variations regarding the localization, shape, and anatomy of the nodules, as well as the infection mode and differentiation status of endosymbionts, called bacteroids, are encountered in nature (8).
Two types of nodules have been defined according to their ontogeny and development (6). Indeterminate nodules originate with dividing inner cortical cells and develop a persistent meristem at the distal end. Mature indeterminate nodules are characterized by a longitudinal gradient of plant cells and bacteroids at different stages of differentiation. Five steps in bacteroid differentiation have been defined, each being restricted to a well-defined histological region of the nodule (16). Determinate nodules instead originate with external cortical cells and differentiate in a synchronous manner, resulting in mature nodules that contain a homogenous population of nitrogen-fixing bacteria. It was shown that in indeterminate nodules of Medicago and related legumes of the galegoid clade, bacteroids of the nitrogen-fixing zone are terminally differentiated. They undergo profound cellular changes, including a size increase (mean, 5×), DNA amplification (mean, 24C), and modification in membrane permeability, and lose their capacity for reproduction (<1%) (9). In contrast, bacteroids of determinate nodules of Phaseolus, Lotus, or soybean differ little from free-living bacteria (9). Both histological types of nodules and the bacteroid differentiation level are controlled by the legume host (9, 11). It is so far unclear, however, whether nodule ontogeny and bacteroid differentiation are truly correlated.
We investigated this issue with an atypical model system. The beta-rhizobium Cupriavidus (formerly Ralstonia) taiwanensis nodulates Mimosa pudica (1, 4), a plant of the Mimosoideae subfamily that forms indeterminate nodules (3). Like most rhizobia, C. taiwanensis penetrates root tissues via root hairs from which infection threads elongate toward the emerging nodule. Bacteroids are released from infection threads in the cytoplasm of nodule cells, where they are enclosed in a symbiotic structure called the symbiosome. So far, the extent of bacteroid differentiation in M. pudica nodules has not been investigated.
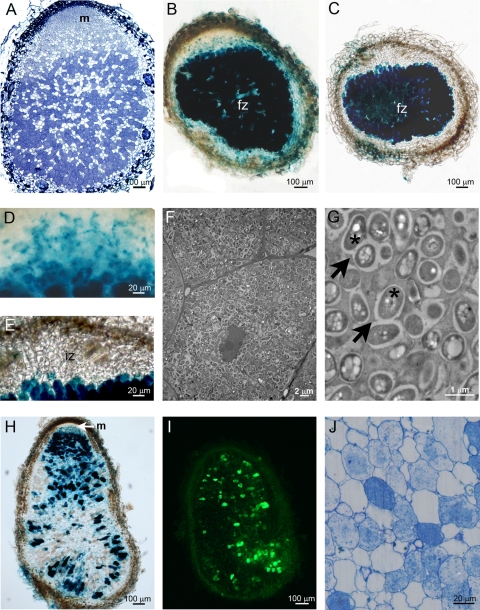
We confirmed that under our experimental conditions, M. pudica formed indeterminate nodules. Seedlings of M. pudica were grown in Gibson tubes under N-free conditions and inoculated with 107 bacterial cells grown in TY as previously described (7) except that Gibson tubes contained only quarter-strength liquid Jensen. Either of the following C. taiwanensis LMG19424 derivatives was used as an inoculum: strain 204, which constitutively expresses gfp (3); strain CBM132, which contains a plasmidic nodB-lacZ fusion (7); strain CBM722, which contains the pCBM39 plasmid harboring a nifH-lacZ fusion; and strain CBM2153, which contains the pCBM78 plasmid harboring a nifH-gfp fusion. To construct pCBM39, the nifH promoter of C. taiwanensis was amplified using 5′-CCCAAGCTTGTTAGTTGCCAAGCGACGTA-3′ and 5′-TGCACTGCAGCCATTTTGAATTGAAGGTGTAGC-3′ as primers and cloned into the HindIII-PstI restriction sites of pCZ388 (7). To construct pCBM78, the gfp gene was amplified using 5′-TGCACTGCAGTATAGGGAGACCACA-3′ and 5′-TGCACTGCAGCAGCAGCCAACTCAGC-3′ as primers and cloned at the PstI site of pCBM39 downstream the nifH promoter. Plants were harvested at different time points after inoculation and examined using confocal laser microscopy and light microscopy as described previously (7). Nodules emerged from the inner cortex (data not shown). At ca. 14 days postinoculation (dpi), nodules harbored a distinct meristem at the tip of the nodules (Fig. 1A) that persisted at 35 dpi. In addition, mature nodules contained an invasion zone where cells were invaded by infection threads (Fig. 1B and D) and a fixation zone where nodule cells were massively infected by bacteria (Fig. 1C). As expected, nifH is expressed only in the fixation zone (Fig. 1C and E). Interestingly, nodB is expressed in rhizosphere (data not shown) and infection thread colonizing bacteria as well as in bacteroids, a rare situation in the rhizobium-legume symbiosis (Fig. 1B and D) (14). Contrary to the case with Medicago indeterminate nodules, symbiosomes in M. pudica did not exhibit radial organization (Fig. 1F) (16). They contained up to four bacteria harboring polyhydroxybutyrate polymers in their cytoplasm (Fig. 1G). After 42 dpi, a degenerating zone was observed, where gene expression occurred in disparate nodule cells (Fig. 1H and I). At ca. 52 dpi, only the degenerating zone persisted, where loss of cell-to-cell contact and cytoplasmic structure degradation of nodule cells could be observed (Fig. 1J).
FIG. 1.
Imaging of indeterminate nodules formed by C. taiwanensis: fluorescence (I), light (A, B, C, D, E, H, and J), and electron (F and G) microscopy of nodules formed by C. taiwanensis genomic GFP (A, I, F, and G), plasmidic nodB-lacZ (B, D, and H), or plasmidic nifH-lacZ (C and E) or stained with toluidine blue (A and J). An apical meristem was present in nodules formed at 19 dpi (A) and 35 dpi (H). At ca. 14 dpi, nodB was strongly expressed in the fixation zone (B) and in the infection zone (D), while nifH was expressed only in the fixation zone (C and E). At 35 dpi, bacteroids were randomly organized within the nodule cell (F) and symbiosomes contained up to 4 bacteroids (G) (arrows) with poly-β-hydroxybutyrate (PHB) granules (star). At 42 dpi, the infected zone was mainly restricted to the distal part of the nodule (H). At ca. 52 dpi, gene expression occurred only in disparate cells (I) and bacteria degenerated (J). Green, GFP; m, meristem; iz, infection zone; fz, fixation zone.
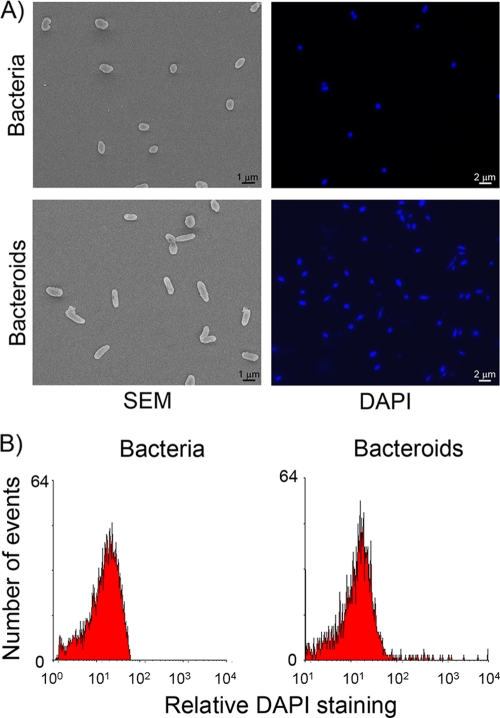
We first evaluated morphological and DNA content changes undergone by C. taiwanensis strain 204 bacteroids compared to free-living bacteria grown in TY medium. Bacteroids were recovered at 35 dpi from nodules that had been previously surface sterilized, crushed in a phosphate-buffered saline (PBS) buffer, and centrifuged to eliminate most vegetal debris. Bacteria and bacteroids were fixed in glutaraldehyde (4%) in cacodylate buffer, washed, dehydrated, and metallized (1.2 V, 10 mA). Scanning electron microscopy observation (MAB Hitachi S450 microscope) showed that bacteroids exhibited little morphological change (Fig. 2A), which was confirmed by Nomarski direct observation (data not shown). Bacteroids were indeed only slightly more elongated than free-living bacteria, being up to 2 times longer (1.7 to 2 μm, compared to 1 μm). Bacterial cells stained with the fluorescent DNA dye 4′,6-diamidino-2-phenylindole (DAPI) at 5 μg/ml were analyzed using fluorescence microscopy (Fig. 2A) and flow cytometry (Facscalibur) (Fig. 2B). No change in DNA content was observed, showing that C. taiwanensis bacteroids did not undergo genome amplification.
FIG. 2.
Cell morphology and DNA content of C. taiwanensis free-living bacteria and bacteroids isolated from M. pudica nodules. (A) Scanning electron microscopy (SEM) of free-living bacteria and bacteroids isolated from 35-dpi nodules showed a 2-fold increase in bacterial size (left panel). Fluorescence microscopy of bacteria and bacteroids stained with DAPI is similar (right panel). (B) DNA content of DAPI-stained bacteria and bacteroids recovered from 35-dpi nodules as measured by flow cytometry showed no DNA amplification.
Bacteroid membrane integrity was evaluated using 2 μg/ml propidium iodide (PI), a DNA stain that enters cells with alteration of membrane permeability. No staining was observed for free-living bacteria or for bacteroids, while penetration of the dye was very rapid in bacteria killed at 95°C for 10 min (data not shown). Membrane permeability is thus not altered in C. taiwanensis bacteroids.
To evaluate the viability of intracellular bacteria, M. pudica plants were inoculated with C. taiwanensis (CBM2153) harboring a nifH-gfp fusion. gfp-positive bacteroids (5 × 103) were sorted by flow cytometry (FacsARIA II-SORP; BD) and subsequently counted using dilution series plated on selective TY medium supplemented with tetracycline at 10 μg/ml. Twenty percent of gfp-expressing cells were able to resume growth on TY medium.
Altogether these results showed that C. taiwanensis bacteroids are not terminally differentiated in M. pudica nodules, in sharp contrast with the profound and irreversible bacteroid differentiation observed in Medicago and other galegoid legumes of the Papilionoideae subfamily (9). We have here demonstrated that all of the characters associated with bacteroid differentiation in galegoid nodules, i.e., cell enlargement, polyploidy, membrane permeability modification, and loss of viability, are actually unrelated to nodule ontogeny.
In a recent study, Oono et al. (12), in a literature- and experimentally based overview of overall bacteroid morphology (swollen versus nonswollen) in the Papilionoideae subfamily, similarly concluded there was no correlation between bacteroid differentiation and nodule ontogeny.
One of the key genes for terminal bacteroid differentiation in galegoid nodules is bacA, which is involved in very long chain fatty acid (VLCFA) modification of the outer membrane and possibly peptide transport. It has been suggested that BacA may be involved, directly or indirectly, in the import of nodule-specific cysteine-rich (NCR) plant peptides (15) with antimicrobial activity, which indeed are extremely abundant in and specific to galegoid nodules (6a, 9). C. taiwanensis lacks any bacA gene, which is in agreement with the fact that this beta-rhizobium does not undergo terminal bacteroid differentiation in symbiosis with Mimosa pudica.
Acknowledgments
We are grateful to Ton Timmers for careful reading of the manuscript. We thank Fatima-Ezzahra L'Faqihi-Olive and Valérie Duplan-Eche from the IFR150 cytometry platform and Bruno Payré and Isabelle Fourquaux from the IFR-BMT CMEAB platform for helpful technical assistance.
This work was supported by grants from the SPE INRA department and ANR-08-BLAN-0295-01.
Footnotes
Published ahead of print on 21 January 2011.
REFERENCES
- 1.Amadou, C., et al. 2008. Genome sequence of the beta-rhizobium Cupriavidus taiwanensis and comparative genomics of rhizobia. Genome Res. 18:1472-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Batut, J., S. G. E. Andersson, and D. O'Callaghan. 2004. The evolution of chronic infection strategies in the alpha-proteobacteria. Nat. Rev. Microbiol. 2:933-945. [DOI] [PubMed] [Google Scholar]
- 3.Chen, W. M., E. K. James, A. R. Prescott, M. Kierans, and J. I. Sprent. 2003. Nodulation of Mimosa spp. by the beta-proteobacterium Ralstonia taiwanensis. Mol. Plant-Microbe Interact. 16:1051-1061. [DOI] [PubMed] [Google Scholar]
- 4.Chen, W. M., et al. 2001. Ralstonia taiwanensis sp. nov., isolated from root nodules of Mimosa species and sputum of a cystic fibrosis patient. Int. J. Syst. Evol. Microbiol. 51:1729-1735. [DOI] [PubMed] [Google Scholar]
- 5.Chen, W. M., et al. 2003. Legume symbiotic nitrogen fixation by beta-proteobacteria is widespread in nature. J. Bacteriol. 185:7266-7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Den Herder, G., and M. Parniske. 2009. The unbearable naivety of legumes in symbiosis. Curr. Opin. Plant Biol. 12:491-499. [DOI] [PubMed] [Google Scholar]
- 6a.Karunakaran, K., et al. 2010. BacA is essential for bacteroid development in nodules of galegoid, but not phaseoloid, legumes. J. Bacteriol. 192:2920-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marchetti, M., et al. 2010. Experimental evolution of a plant pathogen into a legume symbiont. PLoS Biol. 8:e1000280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masson-Boivin, C., E. Giraud, X. Perret, and J. Batut. 2009. Establishing nitrogen-fixing symbiosis with legumes: how many rhizobium recipes? Trends Microbiol. 17:458-466. [DOI] [PubMed] [Google Scholar]
- 9.Mergaert, P., et al. 2006. Eukaryotic control on bacterial cell cycle and differentiation in the Rhizobium-legume symbiosis. Proc. Natl. Acad. Sci. U. S. A. 103:5230-5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moulin, L., A. Munive, B. Dreyfus, and C. Boivin-Masson. 2001. Nodulation of legumes by members of the beta-subclass of Proteobacteria. Nature 411:948-950. [DOI] [PubMed] [Google Scholar]
- 11.Oke, V., and S. R. Long. 1999. Bacteroid formation in the Rhizobium-legume symbiosis. Curr. Opin. Microbiol. 2:641-646. [DOI] [PubMed] [Google Scholar]
- 12.Oono, R., I. Schmitt, J. I. Sprent, and R. F. Denison. 2010. Multiple evolutionary origins of legume traits leading to extreme rhizobial differentiation. New Phytol. 187:508-520. [DOI] [PubMed] [Google Scholar]
- 13.Reference deleted.
- 14.Schlaman, H. R. M., B. Horvath, E. Vijgenboom, R. J. H. Okker, and B. J. J. Lugtenberg. 1991. Suppression of nodulation gene-expression in bacteroids of Rhizobium leguminosarum biovar viceae. J. Bacteriol. 173:4277-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van de Velde, W., et al. 2010. Plant peptides govern terminal differentiation of bacteria in symbiosis. Science 327:1122-1126. [DOI] [PubMed] [Google Scholar]
- 16.Vasse, J., F. Debilly, S. Camut, and G. Truchet. 1990. Correlation between ultrastructural differentiation of bacteroids and nitrogen-fixation in alfalfa nodules. J. Bacteriol. 172:4295-4306. [DOI] [PMC free article] [PubMed] [Google Scholar]